Abstract
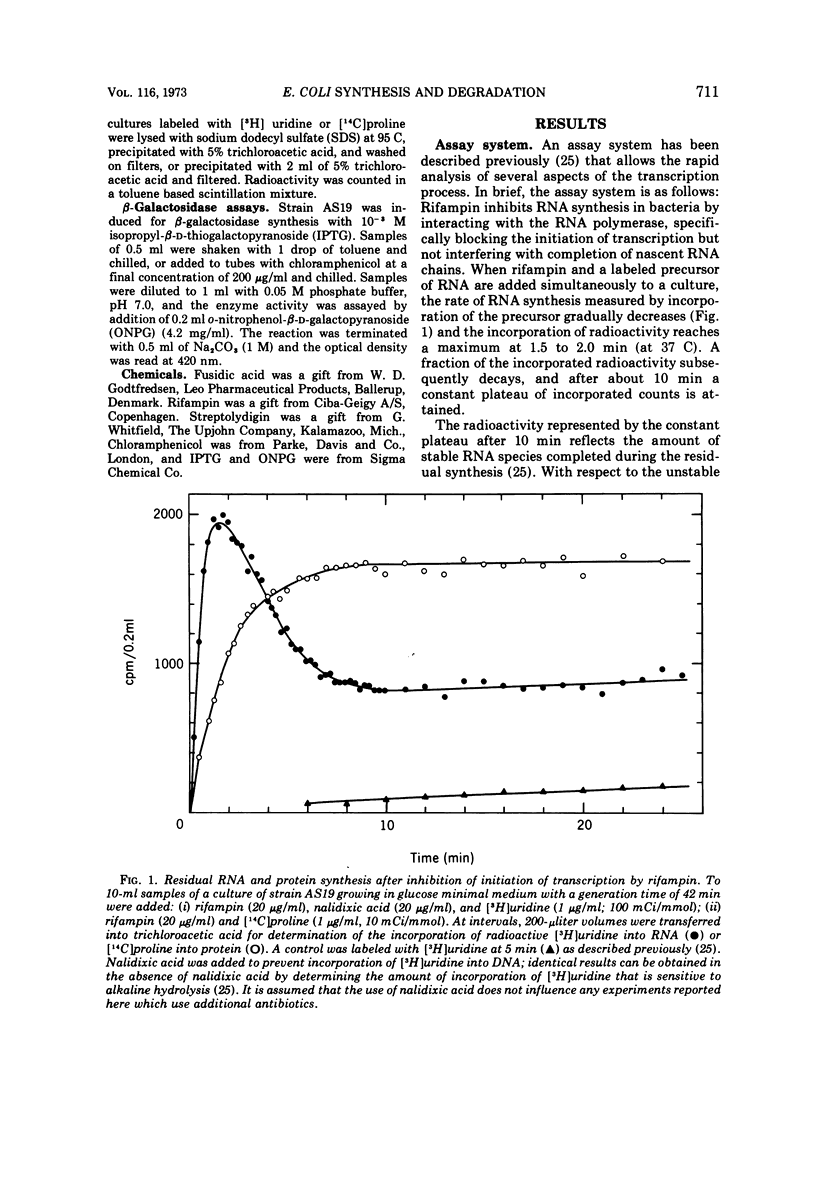
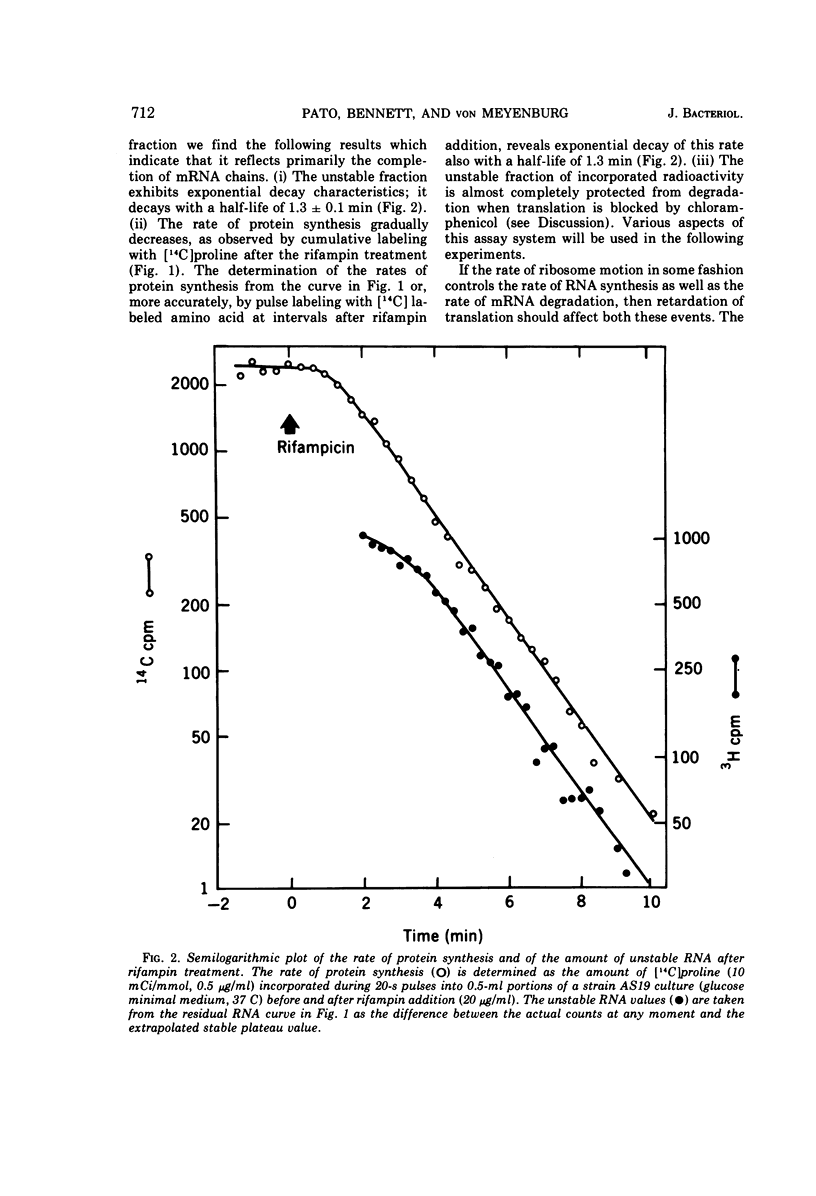
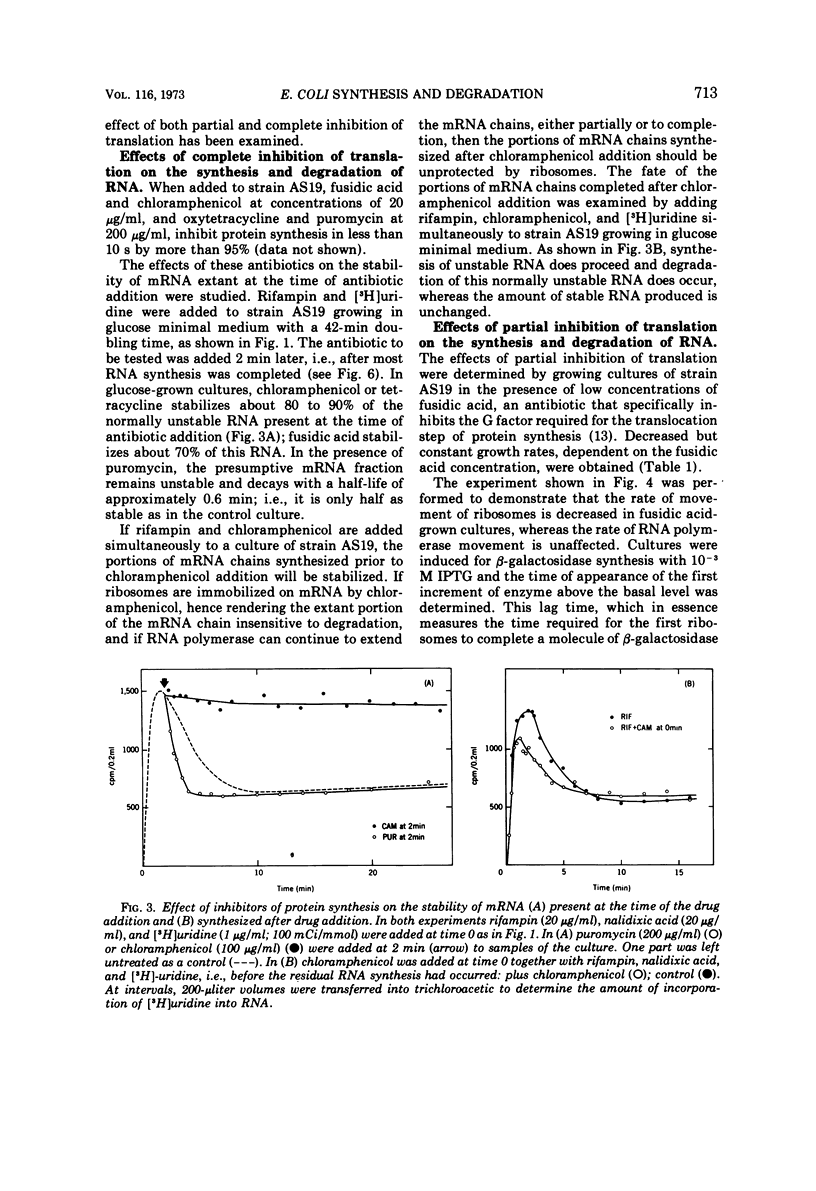
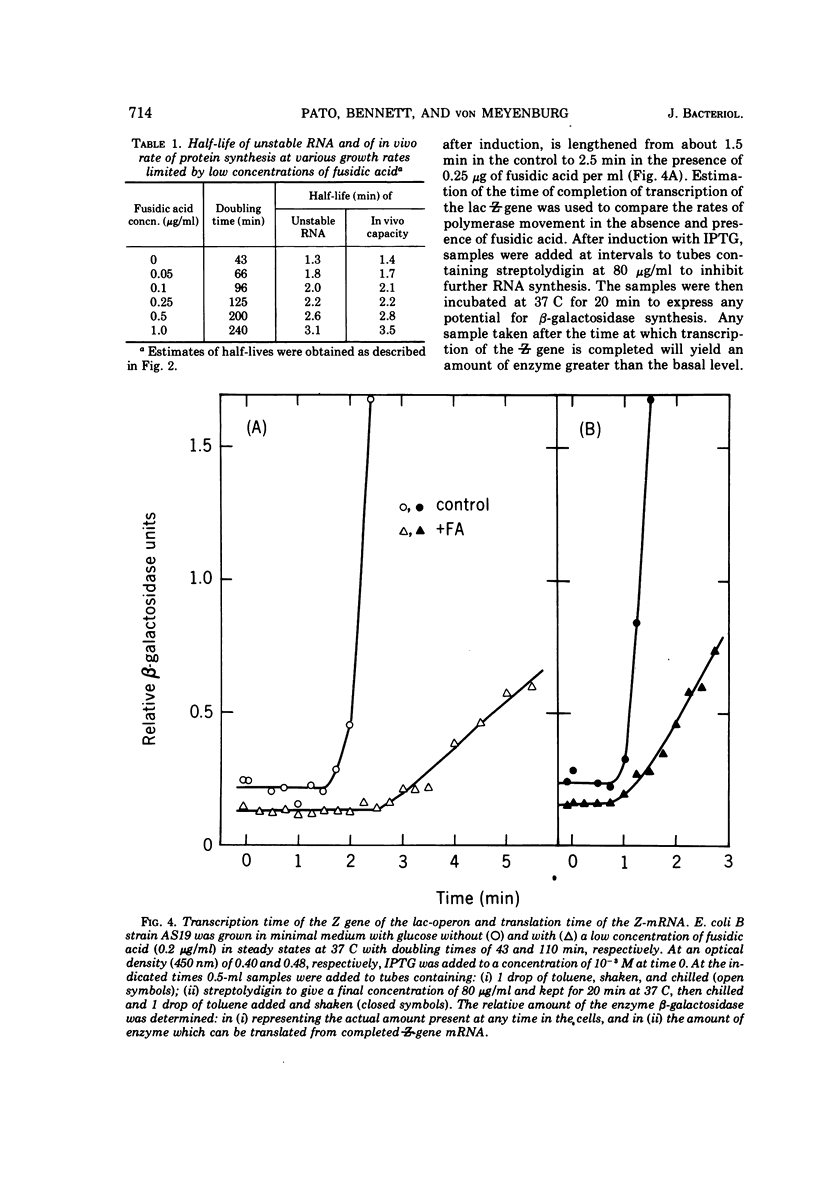
Various aspects of the coupling between the movement of ribosomes along messenger ribonucleic acids (mRNA) and the synthesis and degradation of mRNA have been investigated. Decreasing the rate of movement of ribosomes along an mRNA does not affect the rate of movement of some, and possibly most, of the RNA polymerases transcribing the gene coding for that mRNA. Inhibiting translation with antibiotics such as chloramphenicol, tetracycline, or fusidic acid protects extant mRNA from degradation, presumably by immobilizing ribosomes, whereas puromycin exposes mRNA to more rapid degradation than normal. The promoter distal (3′) portion of mRNA, synthesized after ribosomes have been immobilized by chloramphenicol on the promoter proximal (5′) portion of the mRNA, is subsequently degraded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell A. L., Apirion D. Is RNase V a manifestation of RNase II? Biochem Biophys Res Commun. 1971 Aug 20;44(4):844–851. doi: 10.1016/0006-291x(71)90788-1. [DOI] [PubMed] [Google Scholar]
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- Carter T., Newton A. New polarity suppressors in Escherichia coli: suppression and messenger RNA stability. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2962–2966. doi: 10.1073/pnas.68.12.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Gottesman M., Pastan I., Varmus H. E., Emmer M., Perlman R. L. Regulation of lac mRNA synthesis in a soluble cell-free system. Nat New Biol. 1971 Mar 10;230(10):37–40. doi: 10.1038/newbio230037a0. [DOI] [PubMed] [Google Scholar]
- Dütting D., Hübner L. The effect of antibiotics on the in vivo synthesis of messenger ribonucleic acid from the lactose operon of Escherichia coli. Mol Gen Genet. 1972;116(3):277–290. doi: 10.1007/BF00269771. [DOI] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Singer M. F. Inability to detect RNase V in Escherichia coli and comparison of other ribonucleases before and after infection with coliphage T7. Biochem Biophys Res Commun. 1971 Aug 20;44(4):837–843. doi: 10.1016/0006-291x(71)90787-x. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Kano Y. Inhibition of transcription of the tryptophan operon in Escherichia coli by a block in initiation of translation. Nat New Biol. 1971 Aug 11;232(2):169–173. doi: 10.1038/newbio232169a0. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Kepes A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog Biophys Mol Biol. 1969;19(1):199–236. doi: 10.1016/0079-6107(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Kawano G., Tanaka N. Association of fusidic acid sensitivity with G factor in a protein-synthesizing system. Biochem Biophys Res Commun. 1968 Dec 9;33(5):769–773. doi: 10.1016/0006-291x(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Kwan C. N., Apirion D., Schlessinger D. Ribonuclease V of escherichia coli. I. Dependence on ribosomes and translocation. Proc Natl Acad Sci U S A. 1969 Oct;64(2):693–700. doi: 10.1073/pnas.64.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Forchhammer J. Evidence for reduced breakdown of messenger RNA during blocked transcription or translation in Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):593–606. doi: 10.1016/0022-2836(69)90361-1. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D. Polyribosome metabolism in Escherichia coli. I. Extraction of polyribosomes and ribosomal subunits from fragile, growing Escherichia coli. J Mol Biol. 1966 Sep;20(1):123–143. doi: 10.1016/0022-2836(66)90122-7. [DOI] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]


