Abstract
The multi-endpoint cytokinesis-blocked micronucleus assay is used for assessing chromosome aberrations. We have recently reported that this assay is extremely sensitive to genetic damage caused by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyr-idyl)-1-butanone (NNK) and that the binucleated cells with micronuclei, nucleoplasmic bridges, and nuclear buds in lymphocytes (chromosome damage endpoints measured by the assay) are strong predictors of lung cancer risk. In the current study, we refined our analysis to include toxicity endpoints (micronuclei in mononucleated cells, apoptosis, necrosis, and nuclear division index) to investigate the benefit of including these variables on improving the predictive value of the assay. Baseline and NNK-induced micronuclei in mononucleated cells were significantly higher in patients (n = 139) than controls (n = 130; P < 0.001). Baseline apoptosis was higher among cases; however, the controls showed a significant higher fold increase in NNK-induced apoptosis compared with baseline (P < 0.001). Principal components analysis was used to derive a summary measure for all endpoints and calculate the positive predictive value (PPV) and negative predictive value (NPV) for disease status. First principal component for NNK-induced chromosome damage endpoints (binucleated cells with micronuclei, nucleoplasmic bridges, and nuclear buds) had an area under the curve = 97.9 (95% confidence interval, 95.9-99.0), PPV = 94.8, and NPV = 92.6. The discriminatory power improved when micronuclei in mononucleated cells were included: area under the curve = 99.1 (95% confidence interval, 97.9- 100.0), PPV = 98.7 and NPV = 95.6. The simplicity, rapidity, and sensitivity of the assay together with potential for automation make it a valuable tool for screening and prioritizing potential cases for intensive screening.
Introduction
Lung cancer is the leading cause of cancer mortality in the United States, and there is an urgent need to improve outcome by identifying and validating markers to predict risk and facilitate earlier diagnosis (1). Cancer results from an accumulation of multiple genetic changes that lead to genetic instability. Such instability can be mediated through chromosomal changes and therefore has the potential to be cytogenetically detectable (2). Evidence that cytogenetic biomarkers are positively correlated with cancer risk has been strongly validated in recent results from both cohort and nested case-control studies, leading to the conclusion that chromosome aberrations are a marker of cancer risk (3-7), reflecting the outcome of both the genotoxic effects of carcinogens and individual cancer susceptibility.
The cytokinesis-blocked micronucleus assay (CBMN) in human lymphocytes is one of the most commonly used methods for measuring DNA damage (8). The inhibition of cytokinesis by cytochalasin B allows one to discriminate between cells that did not divide after treatment and cells that did divide, thus preventing the confounding effects caused by differences in cell division kinetics (9, 10). The CBMN assay, more recently known as the CBMN “cytome” or CBMN Cyt assay (11), is a multi-endpoint assay for identifying chromosome fragments or whole chromosomes that fail to engage with the mitotic spindle and therefore lag behind when the cell divides. Because cells are blocked in the binucleated stage, it is also possible to measure nucleoplasmic bridges originating from asymmetrical chromosome rearrangements and/or telomere end fusions (12, 13) as well as nuclear buds that represent a mechanism by which cells remove amplified DNA and that is therefore considered a marker of possible gene amplification (14).
For the most comprehensive analysis, the frequency of micronuclei in mononucleated cells can also be determined. Mononucleated cells result from cells that did not divide ex vivo due to DNA damage—induced cell cycle checkpoint arrest or cells that completed DNA repliction but did not divide due to mitotic slippage, or micronuclei originating from nuclear buds that may be produced during S phase during nuclear elimination of amplified DNA or DNA repair complexes (10, 11). Therefore, micronuclei in mononucleated cells and micronuclei in binucleated cells are different but complementary measures. In addition to micronuclei in mononucleated or binucleated cells, the CBMN Cyt assay also allows for scoring of other critical events, such as cell death (both apoptosis and necrosis) as well as cell division and cell cytotoxicity indices, leading to a better understanding of the mechanisms involved in sensitivity to chemical exposures (15).
Host susceptibility to carcinogenic exposures plays an important role in modifying an individual’s risk for development of cancer. This notion is supported by the fact that only a fraction of long-term smokers (~15%) will develop lung cancer in their lifetimes (16). Cigarette smoke contains over 60 carcinogens (17). The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a strong pulmonary carcinogen and a potent inducer of lung adenocarcinoma, now the leading lung cancer subtype in the United States (18). NNK induces lung cancer independent of route of administration in both susceptible and resistant strains of mice (19). The estimated NNK dose of lifetime smokers (2 packs per day for 40 years) is 1.6 mg NNK/ kg body weight close to the lowest dose shown to induce lung tumors in rats 1.8 mg (20). The total level of NNK in mainstream smoke is 3 to 15 times that of benzo[a]-pyrene (21). Studies on the metabolism of NNK have shown that it induces cross-links in DNA; interacts with DNA, forming different types of adducts; and increases the frequency of chromosome aberrations (22, 23). DNA adducts are generated by NNK through the methylation or the pyridyloxobutylation pathway.
Such adducts have been detected in cells and tissues susceptible to NNK carcinogenesis in rodents and humans (24). Bulky adducts are repaired by nucleotide excision repair; unrepaired DNA damage can block DNA replication and thereby result in the generation of double-strand DNA breaks. As for the methylated adducts, it has been shown that the resultant strand breaks are due to the creation of AP sites that subsequently lead to strand breaks. It has been proposed that inhibition of replication fork movement due to methylation lesions causes nuclease attack at stalled replication forks that result in double-strand DNA breaks, which cause chromatid-type aberrations (25). Various N-methylation lesions, such as N3-methyladenine, are supposed to block DNA replication.
Also, apurinic sites resulting from spontaneous hydrolysis of N-methylpurines, such as N7-methylguanine and N3-methyladenine, are likely critical candidates in blocking replication and thus leading to double-strand DNA breaks. If not repaired, or illegitimately recombined, double-strand DNA breaks will be expressed as chromatid-type aberrations in M1. Likewise, a similar process may give rise to aberrations in M2 with O6MeG/ T-derived secondary lesions being the trigger (26, 27). Hecht (28) reported that DNA adducts derived from NNK are present at a higher level in lung tissues from lung cancer patients than controls, and metabolites of NNK are found in the urine of people who use tobacco products or are exposed to environmental tobacco smoke. The repair kinetics for NNK-induced genetic damage has not been clearly elucidated but may involve several DNA repair pathways, including base excision and nucleotide excision repair pathways (29).
We have recently conducted a proof-of-principle case-control study to evaluate whether NNK-induced damage, as assessed by the CBMN assay, was associated with lung cancer risk. Using only three (micronuclei, nucleoplasmic bridges, and nuclear buds in binucleated cells) of the potential eight endpoints [micronuclei, nucleoplasmic bridges, and nuclear buds in binucleated cells, micronuclei in mononucleated cells, apoptosis, necrosis, nuclear division index (NDI), and nuclear division cytotoxicity index (NDCI)] assessed by the CBMN Cyt assay, our results indicated that lung cancer cases (n = 139) and controls (n = 130) had differential sensitivity to the genotoxic effects of NNK. The lymphocytes from patients with lung cancer were significantly more sensitive to NNK clastogenic effects than were lymphocytes from the controls (30). We concluded that this assay is sensitive to NNK-induced genetic damage and that the three endpoints used serve as a strong predictor of lung cancer risk. In the current study, we evaluated the added advantage of including in the analysis the remaining five endpoints on improving the predictive value of the CBMN Cyt assay, thus allowing an even more sensitive and specific characterization of high-risk smokers.
Materials and Methods
Study Population
Cases and controls for this analysis were accrued from an ongoing molecular epidemiologic study on susceptibility markers for lung cancer. The 139 cases were consecutive patients with newly diagnosed, previously untreated, histologically confirmed lung cancer who were self-reported ever-smokers. All cases were recruited from The University of Texas M. D. Anderson Cancer Center, with no age, sex, ethnicity, tumor histology, or disease stage restrictions. The 130 healthy controls were recruited from the Kelsey-Seybold Clinic, Houston’s largest private multispecialty physician group. Controls were frequency matched to the cases by age (±5 years), sex, ethnicity, and smoking status (current and former). Data related to the subjects’ medical history, family history of cancer, smoking habits, and occupational history were obtained through an interviewer-administered risk factor questionnaire and by review of an institutional electronic patient history database. The institutional review boards at both The University of Texas M. D. Anderson Cancer Center and Kelsey-Seybold Clinic approved this study. After giving informed consent, all study participants donated a 10 mL blood sample, which was drawn into coded heparinized tubes.
Lymphocyte Cultures for CBMN Assay
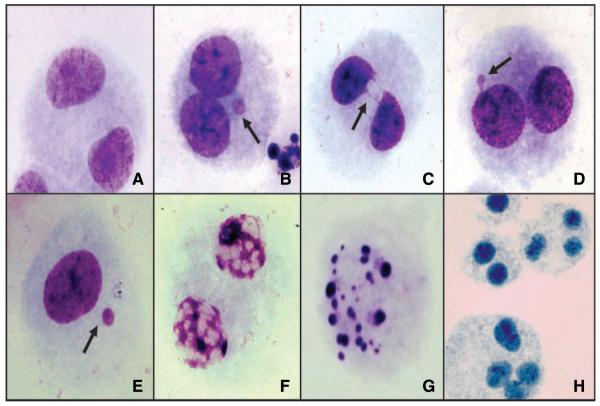
The CBMN Cyt assay was done using the cytochalasin B technique described by Fenech and Morley (31) and following recommendations from the International Collaborative Project on Micronucleus Frequency in Human Populations (HUMN Project) to measure the different endpoints in untreated cells and NNK-treated cells. Duplicate lymphocyte cultures were prepared for each study subject. Each culture contained 2.0 × 106 cells in 5 mL RPMI 1640 supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 10% fetal bovine serum, 2 mmol/L L-glutamine (Life Technologies/Invitrogen), and 1% phytohemagglutinin (Remel). For the cultures treated with NNK, 24 h after initiation, the peripheral blood lymphocytes were centrifuged, and the supernatant growth medium was removed and reserved. The lymphocytes were resuspended in 5 mL serum-free RPMI 1640 supplemented with 0.24 mmol/L NNK, a concentration used previously by us and others (National Cancer Institute, Midwest Carcinogen Repository; purity, >98%; refs. 32, 33) and incubated at 37°C in the presence of 5% CO2 for 2 h. Next, the peripheral blood lymphocytes were washed twice with serum-free RPMI 1640, transferred to clean tubes, and reincubated for 48 h in the reserved supernatant. At 44 h after initiation, cells were blocked from entering cytokinesis by the addition of cytochalasin B (Sigma; final concentration, 4 μg/mL). Cultures for the determination of spontaneous damage (untreated cells) were handled in the same manner, with the exception of treatment with NNK. The total incubation time for all cultures was 72 h. After incubation, the cells were fixed in 3:1 methanol/glacial acetic acid, dropped onto clean microscopic slides, air dried, and stained with Giemsa stain. Slides were scored blindly using a Nikon E-400 light optical microscope following the scoring criteria outlined by the HUMN Project (31, 34). For each sample, 1,000 binucleated cells were evaluated for the frequency of micronuclei (MN-BN), nucleoplasmic bridges (NPB-BN), and nuclear buds (Bud-BN) in binucleated cells. In addition, micronuclei in 1,000 mononucleated cells were evaluated (MN-Mono). Five hundred cells were assessed for cell proliferation and cell death by measuring the frequency of mononucleated, binucleated, and multinucleated cells as well as necrotic and apoptotic cells following the recommendations of the HUMN Project (11, 31, 34); Fig. 1 illustrates the different endpoints assessed using the CBMN Cyt assay.
Figure 1.
Representative images of cells scored using the CBMN Cyt assay: (A) normal binucleated cell, (B) binucleated cell with micronuclei, (C) binucleated cell with nucleoplasmic bridge, (D) binucleated cell with bud, (E) mononucleated cell with micronucleus, (F) cell in necrosis, (G) cell in apoptosis, and (H) polynucleated cells.
Statistical Analysis
All analyses were done using the Intercooled Stata 8.0 statistical software package (Stata). Pearson’s χ2 test was used to test for differences between the cases and controls in terms of sex, alcohol consumption, and family history of cancer. Student’s t test was used to test differences in mean age and average number of cigarettes smoked per day. To compare the distribution frequency of spontaneous and NNK-induced micronuclei in mononucleated cells, apoptosis and necrosis between the cases and controls, we used the nonparametric Wilcoxon rank-sum test (continuous) and Pearson’s χ2 test (categorical). Odds ratios and 95% confidence intervals (95% CI) were calculated to provide an estimate of the risk of lung cancer associated with the number of spontaneous and NNK-treated micronuclei in mononucleated cells. Unconditional multivariable logistic regression analysis was used to control for confounding by age, sex, alcohol consumption, smoking status, and smoking years.
To comprehensively evaluate the overall data (MN-BN, NPB-BN, Bud-BN, MN-Mono, apoptosis, necrosis, NDI, and NDCI), we used principal components analysis to derive a summary measure for all endpoints (spontaneous, NNK treated, and combined). Principal components analysis aims to explain the variance-covariance structure in a set with p number of variables (X1, X2, ...,Xp) and involves a mathematical procedure that transforms a number of (possibly) correlated variables into a (smaller) number (k) of uncorrelated variables called principal components. Although the entire amount of variability is explained in the total set of p principal components, the majority of this variability is explained in only k components (where k ≤ p). The first principal component (FPC) is the linear combination with maximum variance, and each succeeding component is the linear combination with remaining maximum variance.
In this study, we retained only the FPC. We randomly split the participants into two groups: one to derive the FPC (training set) and the other to evaluate the ability of FPC to discriminate between lung cancer cases and controls. The training set involved 62 cases and 62 controls, whereas the testing set contained the remaining 77 cases and 68 controls. Using the test set, we calculated specificity and sensitivity, constructed receiver operator characteristic curves, and calculated the area under the curve statistic to estimate the ability of each FPC to discriminate between cases and controls. We also calculated positive predictive value (PPV) and negative predictive value (NPV) for each FPC.
Results
Demographics and Study Population
The demographic characteristics of the 139 cases and 130 controls are summarized in Table 1. Cases and controls did not differ significantly in terms of sex. The cases were on average 2.5 years younger than the controls (P < 0.001); however, the difference was still within the 5-year matching criterion. Twenty-four percent of the patients self-reported a family history of cancer in first-degree relatives compared with 22% of the controls (P = 0.780). Cases were more likely to report alcohol use (P = 0.003) and had on average smoked cigarettes for 42.1 years compared with 37.7 years for controls (P < 0.001) with a median of 32 years. However, both groups reported smoking about the same number of cigarettes per day.
Table 1.
Demographic characteristics of the study population
| Variable | Cases (n = 139) | Controls (n = 130) | P* |
|---|---|---|---|
| Age, mean (SE), y | 58.4 (0.41) | 60.9 (0.32) | <0.001 |
| Sex, n (%) | |||
| Men | 97 (69.8) | 93 (71.5) | |
| Women | 42 (30.2) | 37 (28.5) | 0.75 |
| Family history of cancer, n (%) | |||
| No | 106 (76.3) | 101 (77.7) | |
| Yes | 33 (23.7) | 29 (22.3) | 0.78 |
| History of alcohol use, n (%) | |||
| Yes | 91 (65.5) | 65 (50.0) | |
| No | 47 (34.5) | 59 (45.4) | 0.003 |
| Cigarette smoking, mean (SE) | |||
| No. years smoked | 42.1 (0.5) | 37.7 (9.6) | <0.001 |
| No. cigarettes smoked per day | 30.2 (1.38) | 29.2 (1.21) | 0.651 |
NOTE: All study subjects were self-reported Caucasians and current smokers.
P values were derived from the χ2 test for categorical variables and Student’s t test for continuous variables. All P values are two-sided.
Summary of the Frequencies of Spontaneous and NNK-Induced Micronuclei in Binucleated Cells
We have reported previously that the three most commonly used measures of the CBMN assay (MN-BN, NPB-BN, and Bud-BN) may serve as a strong predictor of lung cancer risk (30). The mean levels of both spontaneous and NNK-induced chromosomal damage observed in 1,000 binucleated cells were significantly higher in cases compared with controls. The probability of being a cancer patient were 96%, 98%, and 100% when using the 95th percentiles of spontaneous and NNK-induced micronuclei, nucleoplasmic bridges, and nuclear buds, respectively, in combination. In the following section, we present the results generated by using the CBMN Cyt assay as a comprehensive biomarker assay for assessing chromosomal damage, cell death, and cell proliferation.
Frequencies of Spontaneous and NNK-Induced MN-Mono Cells
Data on the frequency of spontaneous MN-Mono cells are summarized in Table 2. The mean number of spontaneous MN-Mono was significantly higher in the cases (mean ± SE, 2.47 ± 0.10) than in the control subjects (mean ± SE, 1.28 ± 0.12; P < 0.001). Women had slightly higher levels of MN-Mono than did men among both cases (mean ± SE, 2.55 ± 0.18 compared with 2.43 ± 0.13) and controls (1.41 ± 0.25 compared with 1.23 ± 0.14). There was no difference in the number of MN-Mono by age (median, 62 years) or by years of smoking (median, 32 years).
Table 2.
Overall spontaneous and NNK-induced micronuclei/1,000 mononucleated cells and cell death (apoptosis and necrosis) in 500 cells
| Spontaneous (mean ± SE) | NNK induced (mean ± SE) | Percent mean increase | |
|---|---|---|---|
| Cases | |||
| Micronuclei in mononucleated/1,000 | 2.47 ± 0.10 | 5.68 ± 0.14 | 130.0 |
| Cell death/500 cells | |||
| Apoptosis | 122.17 ± 2.22 | 152.35 ± 2.78 | 24.7 |
| Necrosis | 104.12 ± 2.02 | 127.05 ± 2.26 | 22.0 |
| Controls | |||
| Micronuclei in mononucleated/1,000 | 1.28 ± 0.12 | 2.10 ± 0.09 | 64.1 |
| Cell death/500 cells | |||
| Apoptosis | 32.89 ± 1.60 | 67.58 ± 1.33 | 105.0 |
| Necrosis | 28.94 ± 1.38 | 58.46 ± 1.15 | 102.0 |
NOTE: All spontaneous and NNK-induced mean levels were significantly different between cases and controls at the 0.01% level.
With regard to NNK-induced MN-Mono, the mean number was also statistically significantly higher in cases (mean ± SE, 5.68 ± 0.14) than in controls (mean ± SE, 2.10 ± 0.09; P < 0.001;Table 2). Men had slightly higher levels of MN-Mono than did women among both cases (mean ± SE, 5.75 ± 0.17 compared with 5.50 ± 0.23) and controls (2.14 ± 0.12 compared with 2.00 ± 0.14). There was no difference in the number of MN-Mono by median age or by median years of smoking.
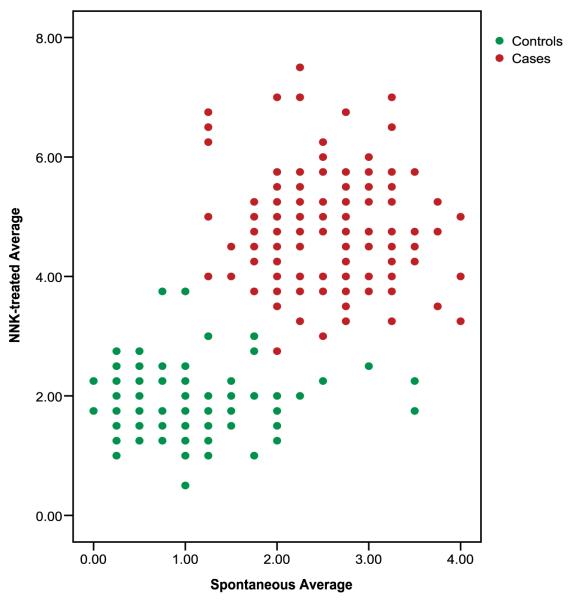
Approximately 42% of the cases had ≥3 MN-Mono compared with only 14% of control subjects, and 86% of the control subjects had <3 MN-Mono compared with 58% of the cases (P < 0.001; Table 3). When the spontaneous MN-Mono data were analyzed as a continuous variable using multivariate logistic regression, there was a 2.10-fold increase in lung cancer risk (95% CI, 1.59-2.76) for each unit increase in MN-Mono frequency. Substantially more cases exhibited ≥3 NNK-induced MN-Mono than did controls (91% versus 28%); conversely, far fewer cases than controls had <3 NNK-induced MN-Mono (3% versus 72%; P < 0.001;Table 3). When the NNK-induced MN data were analyzed as a continuous variable, there was a 5.43-fold increase in lung cancer risk (95% CI, 3.46-8.52) for each unit increase in NNK-induced MN-Mono. No substantial differences were detected when the frequencies of spontaneous or induced MN-Mono were stratified by gender, age, number of years smoked, number of cigarettes per day, alcohol intake, family history of cancer, tumor histology, or disease stage. Figure 2 shows the distribution of the average spontaneous and NNK-treated CBMN Cyt DNA damage endpoints (MN-BN, NPB-BN- Bud-BN, and MN-Mono) in cases compared with controls.
Table 3.
Distributions and risk estimates of lung cancer for spontaneous and NNK-induced micronuclei (mononucleated)
| Case patients, n (%) |
Control subjects, n (%) |
Odds ratio (95% CI)* |
|
|---|---|---|---|
| MN as a dichotomy Pearson’s χ2 test |
|||
| Spontaneous | |||
| 0-2 | 81 (58.3) | 112 (86.2) | 1.0 |
| ≥3 | 58 (41.7) | 18 (13.8) | 3.96 (1.98-7.92) |
| PPV | 76.3 | ||
| NNK induced | |||
| 0-2 | 4 (2.9) | 94 (72.4) | 1.0 |
| ≥3 | 135 (91.1) | 36 (27.7) | 75.12 (24.46-230.68) |
| PPV | 78.9 | ||
| Micronuclei as continuous variable (multivariate logistic regression analysis) | |||
| Spontaneous | 2.10 (1.59-2.76) | ||
| NNK-induced | 5.43 (3.46-8.52) | ||
Abbreviation: PPV, Positive predictive value.
Adjusted by age, sex, history of alcohol use, number of years smoked, and number of cigarettes smoked per day.
Figure 2.
Bivariate distribution of average spontaneous CBMN Cyt DNA damage endpoints (micronuclei in binucleated cells, nucleoplasmic bridges, nuclear buds, and micronuclei in mononucleated cells) by average NNK-treated endpoints.
Cell Division and Cytotoxicity
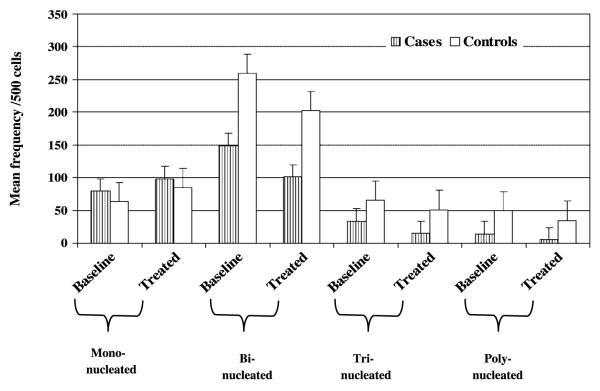
The proportion of mononucleated, binucleated, trinucleated, and multinucleated cells per 500 cells was scored for each study subject for both baseline and NNK-treated cultures. Cases had a significantly higher mean number of mononucleated cells than did controls in both baseline and treated cultures (mean ± SE, 79.9 ± 1.49 compared with 63.8 ± 0.54 and 98.5 ± 2.16 compared with 85.3 ± 0.81 for baseline and NNK-treated cultures, respectively; P < 0.001). On the other hand, the controls showed a significantly higher mean number of binucleated, trinucleated, and polynucleated cells than did the cases for both baseline and NNK-treated cultures (P < 0.001;Fig. 3).
Figure 3.
Comparison of mean frequency of cell proliferation/500 cells in baseline and NNK-induced cultures among cases and controls. Cases showed a higher number of mononucleated cells than controls (baseline and treated), whereas the reverse was observed for the binucleated, trinucleated, and polynucleated cells.
Using this information, we calculated the NDI according to the following equation:
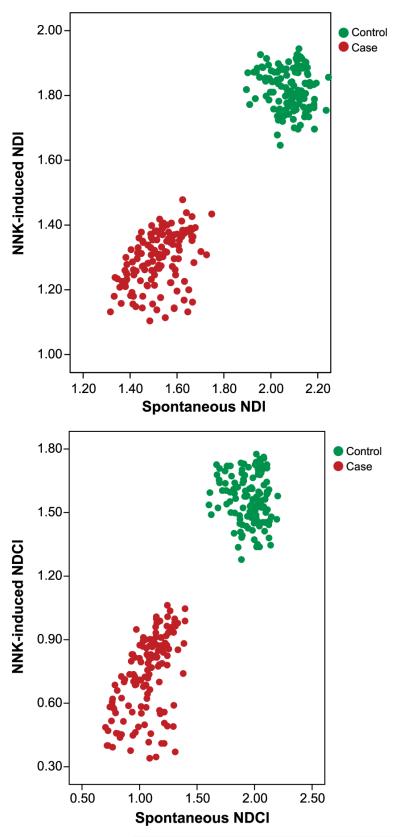
where M1 to M4 are the numbers of cells with one to four nuclei and N is the total number of viable cells scored. The NDI and the proportion of binucleated cells are biomarkers of mitogen response and immune function in lymphocytes as well as cytostatic effects of agents examined in the assay (35). At baseline, lymphocytes from cases had a significantly lower mean NDI than did lymphocytes, from controls (mean ± SE, 1.52 ± 0.01 compared with 2.08 ± 0.01, respectively; P < 0.001). Similarly, after NNK treatment, lymphocytes from cases had a significantly lower mean NDI than did lymphocytes from controls (1.30 ± 0.01 compared with 1.82 ± 0.01, respectively; P < 0.001). Figure 4A shows the clear-cut bivariate distribution between the cases and controls for the average spontaneous NDI by average NNK-treated endpoints, with the cases showing slower nuclear division.
Figure 4.
Bivariate distribution of average spontaneous NDI (A) and NDCI (B) by average NNK-treated endpoints.
Frequencies of Spontaneous and NNK-Induced Cell Death (Apoptosis or Necrosis)
Spontaneous Apoptosis and Necrosis
The frequency of spontaneous apoptotic cells was significantly higher in the cases (mean ± SE, 122.17 ± 2.22) than in the controls (32.89 ± 1.60; P < 0.001;Table 2). Approximately 89% of the controls had a low number of spontaneous apoptotic cells (<60) compared with none of the cases. Almost half (44%) of the cases had a high number (>120) of spontaneous apoptotic cells compared with none of the controls. With regard to frequency of necrotic cells, the average number of spontaneous necrotic cells was significantly higher in the cases than in the controls (mean ± SE, 104.12 ± 2.02 and 28.94 ± 1.38, respectively; P < 0.001;Table 2). Approximately 81% of the controls had a low number (<60) of spontaneous necrotic cells compared with none of the cases. About 15% of the cases had a high number (>120) of spontaneous necrotic cells compared with none of the controls.
NNK-Induced Apoptosis and Necrosis
The number of NNK-induced apoptotic cells was also significantly higher in cases (mean ± SE, 152.35 ± 2.78) than in controls (67.58 ± 1.33; P < 0.001; Table 2). An interesting observation was that although the cases had a significantly higher spontaneous level of apoptosis than the controls (median, 118 compared with 28.5, respectively), the level of NNK-induced apoptosis was lower in cases than in controls. Significantly different percent mean increase was observed between both spontaneous and NNK-induced apoptosis among cases (24.7%) and controls (105%).
With regard to NNK-induced necrosis, the extent of necrotic cells was also significantly higher in cases (mean ± SE, 127.35 ± 2.26) than in controls (58.46 ± 1.15; P < 0.001;Table 2). Similar to the results from apoptosis, although the cases had a significantly higher level of spontaneous necrosis than did the controls (median, 97 compared with 25.5, respectively), the extent of NNK-induced necrosis was significantly lower in cases than in controls (22% compared with 102%, respectively). No substantial differences, within cases or controls, were detected when the frequencies of spontaneous or induced apoptotic or necrotic cells were stratified by gender, age, number of years smoked, number of cigarettes per day, alcohol intake, family history of cancer, tumor histology, or disease.
In addition, and to ensure a more accurate assessment of nuclear division status and cell division kinetics, the number of cells in apoptosis and necrosis was also taken into consideration, generating a NDCI. The NDCI takes account of viable as well as necrotic and apoptotic cells:
where APO is the number of apoptotic cells, NEC is the number of necrotic cells, M1 to M4 are the numbers of viable cells with one to four nuclei, and N is the total number of cells scored. At baseline, lymphocytes from cases had a significantly lower mean NDCI than did those from controls (mean ± SE, 1.07 ± 0.01 compared with 1.95 ± 0.01, respectively; P < 0.001). Similarly, after NNK treatment, lymphocytes from cases had a significantly lower mean NDCI than did those from controls (mean ± SE, 0.74 ± 0.02 compared with 1.57 ± 0.01, respectively; P < 0.001). Figure 4B shows the bivariate distribution of average spontaneous NDCI by average NNK-treated endpoints.
Principal Components Analyses
The FPC for the NNK-induced chromosome damage endpoints, including MN-BN, NPB-BN, and Bud-BN, had an area under the curve = 97.9 (95% CI, 95.9-99.0), PPV = 94.8, and NPV = 92.6. When the additional endpoint of NNK-induced MN-Mono was included in the principal components analysis, the discriminatory power of the resulting FPC was higher: area under the curve = 99.1 (95% CI, 97.9-100.0), PPV = 98.7, and NPV = 95.6, showing that the addition of NNK-induced MN-Mono leads to even better discrimination between cases and controls Table 4.
Table 4.
Area under the curve, PPV, and NPV for each FPC
| FPC | Area under the curve (95% CI) | PPV | NPV |
|---|---|---|---|
| Spontaneous | |||
| MN-BN, NPB-BN, Bud-BN | 95.5 (91.7-99.3) | 96.1 | 89.7 |
| MN-BN, NPB-BN, Bud-BN + MN-Mono | 95.9 (92.1-99.6) | 96.1 | 89.7 |
| NNK induced | |||
| MN-BN, NPB-BN, Bud-BN | 97.9 (95.9-99.0) | 94.8 | 92.6 |
| MN-BN, NPB-BN, Bud-BN + MN-Mono | 99.1 (97.9-100.0) | 98.7 | 95.6 |
| Spontaneous and NNK induced | |||
| MN-BN, NPB-BN, Bud-BN | 95.8 (92.1-99.6) | 92.6 | 89.6 |
| MN-BN, NPB-BN, Bud-BN + MN-Mono | 93.5 (89.2-97.9) | 92.2 | 93.5 |
Discussion
The CBMN assay is a genotoxicity assay that provides simultaneous information on a variety of chromosomal damage endpoints that reflect chromosomal breakage, chromosome rearrangements, and gene amplification. In a recent study, we reported that lung cancer cases and matched controls had differential sensitivity to the genotoxic effects of the tobacco-specific nitrosamine NNK. The lymphocytes from patients with lung cancer were significantly more sensitive to NNK than were those from controls, with 1.6-, 3.4-, and 8.9-fold increases in micronuclei, nucleoplasmic bridges, and nuclear bud frequencies, respectively. Our results also indicated that these cytogenetic endpoints appear to be highly predictive of lung cancer status (30). Over the past few years, the CBMN assay has been modified to measure not only chromosome breakage, chromosome loss, and nondis-junctions but also other cellular events, such as apoptosis and necrosis (8), and is now known as the CBMN Cyt assay (11). To our knowledge, our current report is the first case-control study to evaluate the added benefit of including these additional variables (that could be simultaneously evaluated as part of the CBMN Cyt assay) to test their effect on improving the predictive value of this assay and also to provide insight into the underlying mechanisms in lung carcinogenesis.
Our results showed that the cases had significantly higher levels of baseline micronuclei in mononucleated cells than did the controls, indicating a significantly higher level of in vivo genetic damage and cytotoxicity. It is possible that some of the lymphocytes already harbor micronuclei before they are stimulated to divide in culture (10, 11). This is especially plausible in situations of increased genomic instability or chronic exposure to genotoxins. Micronuclei in mononucleated cells may also represent cells in which the replicated DNA escaped nuclear division or cells that divided but escaped cytochalasin B block. Micronuclei in mononucleated cells may also represent cells that are at a very early stage of induced cell death (either apoptosis or necrosis;ref. 10). In our study, the cases had significantly higher levels of NNK-induced micronuclei in mononucleated cells, which may reflect an increase in the number of damaged cells that failed to divide. This possibility is supported by the cell division data showing a significantly reduced number of cells proceeding to the second and subsequent cell divisions in the cases compared with controls. Our data did not reveal any substantial differences when the frequencies of spontaneous or induced micronuclei in mononucleated cells were stratified by patient age or smoking history.
It is important to note that all the cases and controls were heavy smokers and within the same age group and were heavy smokers; therefore, the effect of age or smoking could not be evaluated. In addition, our data showed no substantial differences when the frequencies of the endpoints (spontaneous or induced) were stratified by disease stage or tumor histology, thus excluding the probability of being a disease marker.
The extent of the observed DNA damage is dependent on the extent of other cellular events, such as necrosis and/or apoptosis. Necrosis leads to the release of degradative enzymes that cause partial digestion of the DNA during the early stages (12). Our results indicate that the lung cancer cases had a significantly higher level of spontaneous necrotic cells than did the controls (P < 0.001). This could be due to the chronic inflammatory milieu in the cases with increased necrosis (36). Similarly, the cases showed significantly higher levels of NNK-induced necrosis compared with controls, reflecting a higher differential sensitivity of blood lymphocytes in the cases not only to the genotoxic effect of the NNK but also to the cytotoxic effects of the chemical. This observation is supported by the significantly reduced NDCI in cases compared with controls.
The lung cancer cases in our study had a higher baseline level of apoptotic cells than did the controls. Although intriguing, this observation could potentially be explained by the increased oxidative burden in the cancer cases. Oxidative stress generates reactive oxygen species that are known to induce cellular senescence and apoptosis (37, 38). The tobacco carcinogen NNK has been reported to induce apoptosis, either through induction of reactive oxygen species (39) or through a mechanism that involves β-adrenergic-mediated release of arachidonic acid (40). Although the cancer cases had a higher frequency of apoptotic cells than did the controls, the induction of apoptosis by NNK was lower in cases than in controls, indicating the possibility of defective apoptotic machinery in the cases, thus allowing for the survival of damaged cells. NNK is considered to be a major contributor to lung carcinogenesis in smokers (41, 42). It has been reported that NNK induces DNA damage (29), DNA adduct formation, increased oxidative stress (43), and p53 and Ras mutations (29, 41). It has been recently reported that NNK induces phosphorylation of Bcl2, facilitating the interaction between Bcl2 and c-Myc, which in turn stabilizes c-Myc protein and enhances its global inhibition of apoptosis, thereby contributing to lung cancer development and proliferation of tumor cells (44).
One of the added advantages to the application of the CBMN Cyt assay is that it could be applied to large populations, because only a small amount of blood is needed, a wide spectrum of genetic damage in cells could potentially be assessed at a relatively low cost, and it is technically not as demanding as the conventional chromosome aberrations assays (45). To comprehensively evaluate the added benefit of using the overall data, we used FPC analysis to identify the measures that are most important for distinguishing among cases and controls and to predict case status. Our data indicated that including the MN-Mono data in the analysis improved the PPV from 94.8% [PPV derived from NNK-induced MN-BN, NPB-BN, and Buds-BN] to 98.7% [NNK-induced MN-BN, NPB-BN, Buds-BN, and MN-Mono]. Similarly, the best NPV were observed when MN-Mono data were included in the analysis: 95.6% [NPV derived from NNK-induced MN-BN, NPB-BN, Buds-BN, and MN-Mono] versus 92.6% [NNK-induced MN-BN, NPB-BN, and Buds-BN]. Unfortunately, owing to the relatively small sample sizes that led to model convergence problems, we were not able to simultaneously evaluate the discriminatory power of the FPC (including MN-Mono) and NDCI. Larger sample sizes are therefore needed to comprehensively evaluate the added effect of cellular events (such as apoptosis, necrosis, and cell proliferation) on improving the predictive values of this biomarker assay.
In summary, our study showed that the CBMN Cyt assay is an exquisitely sensitive and specific predictor of lung cancer risk. The addition to the assay of micronuclei in the mononucleated cells not only improves the PPV and the NPV but also provides an added advantage to evaluating the level of in vivo genetic instability and delay in cell proliferation that may in turn shed light on the underlying mechanisms of the carcinogenic process. The simplicity, rapidity, and sensitivity of the CBMN test make it a valuable tool for screening and possibly for prioritizing potential cases for intensive surveillance. This assay appears to provide results that yield more accurate predictions than other phenotypic assays currently undergoing assessment in this population of lung cancer cases and controls. Lung cancer is the leading cause of cancer mortality in the United States, and there is an urgent need to improve outcome by identifying and validating markers to predict risk (1). Prevention of even 10% of annual deaths from lung cancer would save an estimated 17,000 lives, equivalent to all the annual deaths in the United States from ovarian cancer and almost all the annual deaths from brain cancers. Clinically, the ability to identify high-risk subgroups is imperative, where such individuals might benefit from increased screening surveillance that is not appropriate for low-risk individuals. Additionally, the high-risk subgroups could be targeted for prevention trials.
Acknowledgments
Grant support: National Cancer Institute grants CA55769, CA70907, CA98549, DMDD17-02-10706, FAMRI, NIEHS ES07784, and CJE CA093592.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Hirsch FR, Fischer JR, Niklinski J, Zochbauer-Muller S. Future developments in the treatment of lung cancer. Lung Cancer. 2002;38:S81–5. doi: 10.1016/s0169-5002(02)00277-5. [DOI] [PubMed] [Google Scholar]
- 2.Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. Science. 1991;254:1153–60. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- 3.Liou SH, Lung JC, Chen YH, et al. Increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area. Cancer Res. 1999;59:1481–4. [PubMed] [Google Scholar]
- 4.Bonassi S, Hagmar L, Stromberg U, et al. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. Cancer Res. 2000;60:1619–25. [PubMed] [Google Scholar]
- 5.Bonassi S, Znaor A, Norppa H, Hagmar L. Chromosomal aberrations and risk of cancer in humans: an epidemiologic perspective. Cytogenet Genome Res. 2004;104:376–82. doi: 10.1159/000077519. [DOI] [PubMed] [Google Scholar]
- 6.Smerhovsky Z, Landa K, Rossner P, et al. Risk of cancer in an occupationally exposed cohort with increased level of chromosomal aberrations. Environ Health Perspect. 2001;109:41–5. doi: 10.1289/ehp.0110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker JD, Preston RJ. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges, and cancer risk assessment. Mutat Res. 1996;365:147–59. doi: 10.1016/s0165-1110(96)90018-4. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Fenech M. Chromosomal biomarkers of genomic instability relevant to cancer. Drug Discov Today. 2002;7:1128–37. doi: 10.1016/s1359-6446(02)02502-3. [DOI] [PubMed] [Google Scholar]
- 10.Kirsh-Volders M, Fenech M. Inclusion of micronuclei in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis block micronucleus assay for biomonitoring purposes. Mutagenesis. 2001;16:51–8. doi: 10.1093/mutage/16.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 12.Umegaki K, Fenech M. Cytokinesis-block micronucleus assay in WIL2-NS cells: a sensitive system to detect chromosomal damage induced by reactive oxygen species and activated human neutrophils. Mutagenesis. 2000;15:261–9. doi: 10.1093/mutage/15.3.261. [DOI] [PubMed] [Google Scholar]
- 13.Stewenius Y, Gorunova L, Jonson T, et al. Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity. Proc Natl Acad Sci U S A. 2005;102:5541–6. doi: 10.1073/pnas.0408454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenech M. Biomarkers of genetic damage for cancer epidemiology. Toxicology. 2002;181 – 2:411–6. doi: 10.1016/s0300-483x(02)00480-8. [DOI] [PubMed] [Google Scholar]
- 15.Fenech M, Crott J, Turner J, Brown S. Necrosis, apoptosis, cytostasis and DNA damage in human lymphocytes measured simultaneously within the cytokinesis-block micronucleus assay: description of the method and results for hydrogen peroxide. Mutagenesis. 1999;14:605–12. doi: 10.1093/mutage/14.6.605. [DOI] [PubMed] [Google Scholar]
- 16.Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12:689–98. [PubMed] [Google Scholar]
- 17.Hoffmann D, Hoffmann I, El Bayoumy K. Less harmeful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–90. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 18.Thun MJ, Lally CA, Flannery JT., Jr. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SS, Hoffmann D. The relevance of tobacco specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–94. [PubMed] [Google Scholar]
- 20.Adams JD, O’Mara-Adams, Hoffmann D. Toxic and carcinogenic agents in undiluted mainstream smoke and side-stream smoke of different types of cigarettes. Carcinogenesis. 1987;8:729–31. doi: 10.1093/carcin/8.5.729. [DOI] [PubMed] [Google Scholar]
- 21.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 22.Weitberg AB, Corvese D. Oxygen radicals potentiate the genetic toxicity of tobacco-specific nitrosamines. Clin Genet. 1993;43:88–91. doi: 10.1111/j.1399-0004.1993.tb04455.x. [DOI] [PubMed] [Google Scholar]
- 23.Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J Natl Cancer Inst. 2000;92:874–97. doi: 10.1093/jnci/92.11.874. [DOI] [PubMed] [Google Scholar]
- 24.Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat Res. 1999;424:127–42. doi: 10.1016/s0027-5107(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 25.Kaina B. Critical steps in alkylation-induced aberration formation. Mutat Res. 1998;404:119–24. doi: 10.1016/s0027-5107(98)00103-1. [DOI] [PubMed] [Google Scholar]
- 26.Kaina B, Ziouta A, Ochs K, Coqurell T. Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex-, Mex+ and methylation-tolerant mismatch repair compromised cells: facts and models. Mutat Res. 1997;381:227–41. doi: 10.1016/s0027-5107(97)00187-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res. 2004;104:77–86. doi: 10.1159/000077469. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 29.Cloutier JF, Drouin R, Weinfeld M, O’Connor TR, Castonguay A. Characterization and mapping of DNA damage induced by reactive metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) at nucleotide resolution in human genomic DNA. J Mol Biol. 2001;313:539–57. doi: 10.1006/jmbi.2001.4997. [DOI] [PubMed] [Google Scholar]
- 30.El-Zein RA, Schabath MB, Etzel CJ, Lopez MS, Franklin JD, Spitz MR. Cytokinesis-blocked micronucleus assay as a novel biomarker for lung cancer risk. Cancer Res. 2006;66:6449–56. doi: 10.1158/0008-5472.CAN-06-0326. [DOI] [PubMed] [Google Scholar]
- 31.Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Rahman SZ, El Zein RA. The 399Gln polymorphism in the DNA repair gene XRCC1 modulates the genotoxic response induced in human lymphocytes by the tobacco-specific nitrosamine NNK. Cancer Lett. 2000;159:63–71. doi: 10.1016/s0304-3835(00)00532-2. [DOI] [PubMed] [Google Scholar]
- 33.Hill CE, Affatato AA, Wolfe KJ, et al. Gender differences in genetic damage induced by the tobacco-specific nitrosamine NNK and the influence on the Thr241Met polymorphism in the XRCC3 gene. Environ Mol Mutagen. 2005;4:22–9. doi: 10.1002/em.20128. [DOI] [PubMed] [Google Scholar]
- 34.Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN Project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 35.Eastmond DA, Tucker JD. Kinetochore localization in micronu-cleated cytokinesis-blocked Chinese hamster ovary cells: a new and rapid assay for identifying aneuploidy-inducing agents. Mutat Res. 1989;224:517–25. doi: 10.1016/0165-1218(89)90079-7. [DOI] [PubMed] [Google Scholar]
- 36.Brown R, Pinkerton R, Tuttle M. Respiratory infections in smokers. Am Fam Physician. 1987;36:133–40. [PubMed] [Google Scholar]
- 37.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Fruehauf JP, Meyskens FL., Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 39.Baskaran K, Laconi S, Reddy MK. Transformation of hamster pancreatic duct cells by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), in vitro. Carcinogenesis. 1994;15:2461–6. doi: 10.1093/carcin/15.11.2461. [DOI] [PubMed] [Google Scholar]
- 40.Tithof PK, Elgayyar M, Schuller HM, Barnhill M, Andrews R. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, a nicotine derivative, induces apoptosis of endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:1946–54. doi: 10.1152/ajpheart.2001.281.5.H1946. [DOI] [PubMed] [Google Scholar]
- 41.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2:455–63. doi: 10.1038/nrc824. [DOI] [PubMed] [Google Scholar]
- 42.Schuller HM, Plummer HK, Jull BA. Receptor-mediated effects of nicotine and its nitrosated derivative NNK on pulmonary neuroen-docrine cells. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:51–8. doi: 10.1002/ar.a.10019. [DOI] [PubMed] [Google Scholar]
- 43.Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–9. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 44.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 45.Fenech M, Perepetskaya G, Mikhalevich L. A more comprehensive application of the micronucleus technique for biomonitoring of genetic damage rates in human populations—experiences from the Chernobyl catastrophe. Environ Mol Mutagen. 1997;30:112–8. [PubMed] [Google Scholar]