Abstract
Phosphatidylinositol 3-kinase (PI3K)-dependent signaling couples to receptors for many different ligands in diverse cellular systems. Recent findings suggest that PI3K-dependent signaling also mediates inhibition of odorant responses in rat olfactory receptor neurons (ORNs). Here, we present evidence that murine ORNs show PI3K-dependent calcium responses to odorant stimulation, they express 2 G protein-coupled receptor (GPCR)-activated isoforms of PI3K, PI3Kβ and PI3Kγ, and they exhibit odorant-induced PI3K activity. These findings support our use of a transgenic mouse model to begin to investigate the mechanisms underlying PI3K-mediated inhibition of odorant responses in mammalian ORNs. Mice deficient in PI3Kγ, a class IB PI3K that is activated via GPCRs, lack detectable odorant-induced PI3K activity in their olfactory epithelium and their ORNs are less sensitive to PI3K inhibition. We conclude that odorant-dependent PI3K signaling generalizes to the murine olfactory system and that PI3Kγ plays a role in mediating inhibition of odorant responses in mammalian ORNs.
Keywords: calcium imaging, complex odorants, ELISA, inhibitory input, transgenic
Introduction
As our understanding of olfaction progresses, it becomes increasingly clear that the organization of the olfactory periphery is more complex than formerly appreciated (see Breer et al. 2006; Ma 2007; Munger et al. 2009). A long standing, yet controversial, aspect of organizational complexity in the main olfactory epithelium (OE) of mammals has been the potential involvement of phosphoinositide (PI) signaling in addition to the well understood role of cyclic nucleotide signaling in olfactory transduction in canonical olfactory receptor neurons (ORNs) (Schandar et al. 1998; Schild and Restrepo 1998; Noé and Breer 1998; Gold 1999; Lin et al. 2007). Recent evidence shows that phosphatidylinositol 3-kinase (PI3K)-mediated activity, leading to the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), can modulate odorant-activated increases in the intracellular calcium concentration in acutely dissociated rat ORNs (Spehr et al. 2002). Also, exogeneous PIP3 negatively regulates the olfactory cyclic nucleotide gated channel (Zhainazarov et al. 2004) and does so through complex interaction between PIP3 and Ca2+/calmodulin at the N-terminus of the channel (Brady et al. 2006). Together, these findings suggest the need to reconsider the potential involvement of PI signaling in mammalian ORNs, and in particular to better understand the role that PI3K-mediated signaling plays in these cells.
PI3Ks phosphorylate the hydroxyl group in the D3 position in the inositol ring of phosphatidylinositol. They are divided into 3 main classes on the basis of their in vitro lipid substrate specificity, structure, and likely mode of regulation (Rameh and Cantley 1999). Class I PI3Ks are involved in rapid cellular signaling activated by extracellular stimuli (Coelho and Leevers 2000) and predominantly catalyze the synthesis of PIP3 from phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2], resulting in a transient rise in PIP3 close to the cell membrane. Class I PI3Ks are further classified based on their preferred pathway of activation into class IA comprised PI3K α, β, and δ, and class IB, with a single known member, PI3Kγ. Although class IA PI3Ks are primarily activated by receptor tyrosine kinases (RTKs), PI3Kγ is activated exclusively by binding to the Gβγ subunit of heterotrimeric G proteins. Despite its classification in class IA, PI3Kβ can be activated through both RTKs and G protein signaling (Hazeki et al. 1998; Murga et al. 2000).
PI3K-dependent signaling regulates processes as diverse as proliferation, growth, survival, and intracellular trafficking (Fruman et al. 1998; Vanhaesebroeck et al. 2001), including the survival of mammalian ORNs (Moon et al. 2009). Thus, it is important to establish the functional context of any PI3K-mediated signaling of interest. If PI3K-dependent signaling generalizes to mouse, the availability of genetically manipulated mice lacking one or more isoforms of PI3K can facilitate a better understanding of the role of PI3K signaling in mammalian olfactory transduction.
Here, we show that both G protein-activated isoforms of PI3K, PI3Kβ and PI3Kγ, are expressed in mouse ORNs, odorant-induced PI3K activity can be detected in the mouse OE, and odorant-responsive mouse ORNs are sensitive to PI3K inhibition. Furthermore, we show that ORNs from PI3Kγ-deficient mice show an almost complete lack of odorant-induced PI3K activity and reduced sensitivity to PI3K inhibition in calcium imaging. We conclude that odorant-dependent PI3K signaling generalizes to the murine olfactory system and that PI3Kγ plays a role in mediating inhibition of odorant responses in mammalian ORNs.
Materials and methods
Animals
All live tissue experiments were performed using adult wild type (wt) C57BL6 and PI3Kγ knock out (KO) mice (genetic background C57BL6; Li et al. 2000) from 3 to 6 months of age. All procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Florida. Euthanization of all animals was performed by inhalation of CO2 and subsequent decapitation. For immunohistochemistry, heads from transgenic mice expressing the green fluorescent protein under the promoter of the olfactory marker protein (OMP-GFP) (Potter et al. 2001) and PI3Kγ KO-LacZ (Hirsch et al. 2000) mice were generously provided as fixed specimens by F. Margolis (Baltimore, USA) and E. Hirsch (Turin, Italy), respectively.
Chemicals
For odorant stimulation, Henkel 100 (H100; Wetzel et al. 1999), a complex odorant mixture (generous gift of H. Hatt, Bochum, Germany), was used at 1:50000 (stimulation) and 1:5000 (to establish odor responsiveness) dilutions. Forskolin and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma-Aldrich, Wortmannin and LY294002 from Alomone Labs, and AS252424 and TGX-221 from Cayman Chemicals.
Ca2+ imaging
For Ca2+ imaging, acutely dissociated ORNs were prepared following the procedure described in Spehr et al. (2002). Mouse heads were opened, keeping the septum and the underlying olfactory turbinates intact, the OE was dissected free of the heads, and then the tissue was maintained in a petri dish filled with ice-cold modified artificial cerebrospinal fluid (ACSF). The tissue was incubated with 1 mg/mL papain in ethylene glycol tetraacetic acid (EGTA)-buffered Ringer's solution (0.1 μM Ca2+) for 10 min at 37 °C and dissociated by gentle trituration with a fire-polished glass pipette. An aliquot of the cell suspension was mixed with 3 μM Fura-2AM (Molecular Probes) containing 0.04% Pluronic F127 and placed on a glass coverslip coated with concanavalin A in a recording chamber. Oxygenated ACSF was continuously superfused over the cells at 2 mL/min and transiently switched to ACSF supplemented with drugs/odorants for stimulation of the cells. Both the illumination and image acquisition were controlled by Imaging Workbench 5.2 software (INDEC BioSystems). Only functional ORNs activated by a mixture of 100 μM IBMX and 10 μM forskolin were used in the experiments. All experiments were performed at room temperature (RT) of 22 ± 2 °C.
PI3K inhibitor dependent enhancement of the response to odorants was analyzed by determining the incidence of cells showing an effect and the magnitude of the effect for each cell. The incidence rate was determined by first counting the number of cells potentially able to show an increase in response, that is, cells that were capable of responding to a higher concentration of odorant (Henkel 100 1:5000 dilution) than the test concentration (Henkel 100 1:50000 dilution) but were not saturated by the test concentration. The number of cells showing a drug-dependent increase was normalized to the number of cells potentially capable of showing an increase for each preparation (animal). The mean and standard error of the mean (SEM) were calculated between preparations (animals) tested. The magnitudes of response enhancement were calculated by normalizing the calcium response amplitude to odorant mix plus PI3K inhibitors to the amplitude of response to the odorant mix alone in the same cell and means and SEM of the effect were calculated over all cells showing response enhancement.
Western blotting and immunohistochemistry
Membrane fractions enriched in mouse olfactory cilia were prepared as previously described (Washburn et al. 2002). Protein concentrations of membrane or whole tissue preparations were determined using a Coomassie Plus Bradford assay (Pierce) and equal amounts were loaded per well for separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (4–12% Bis–Tris NuPAGE; Invitrogen). The proteins were transferred to nitrocellulose membranes, and the membranes were blocked with either 5% milk (anti-PI3Kpan [H-239, sc-7189; Santa Cruz Biotechnology] and anti-PI3Kβ [S-19, sc-602; Santa Cruz]) or 5% bovine serum albumin (anti-PI3Kγ [no. 4252; Cell Signaling]) in phosphate-buffered saline (PBS)-T (PBS pH 7.4 with 0.1% Tween 20) for 1 h at RT prior to incubation with the primary antibodies. Mouse spleen homogenate was used as a positive control for PI3K expression due to the high expression of all class I isoforms in cells of the immune system (Li et al. 2000).
For immunostaining, heads from OMP-GFP and PI3Kγ KO-LacZ mice were fixed in 4% paraformaldehyde, decalcified for 5 days in 0.5 M EGTA, and embedded in optimal cutting temperature medium. For fluorescent staining of PI3Kβ coronal sections (12 μm) of the nose of OMP-GFP mice were subjected to an antigen retrieval procedure, incubating them in 0.1 mM citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) for 1.5 h at 65 °C. Nonspecific binding was blocked with 10% normal goat serum (NGS) diluted in PBS with 0.1% Triton-X 100 for 1 h at RT prior to incubation with primary antibody (anti-PI3Kβ S-19, sc-602, Santa Cruz) in PBS with 0.1% Triton-X 100 and 1% NGS overnight at 4 °C. The primary antibody was detected with an Alexa-543 conjugated goat anti-rabbit secondary antibody (A-11010, Invitrogen). Due to the lack of highly specific antibodies against PI3Kγ indirect detection of PI3Kγ was performed in the OE of PI3Kγ KO-LacZ mice with an anti-β-galactosidase (β-gal) antibody (55978, Cappel, MPBiomedicals). Nonspecific binding was blocked with 10% horse serum, and then sections were incubated with primary antibodies against β-gal (55978 rabbit anti-β-gal, Cappel, MPBiomedicals, 1:500; Rothman et al. 2005) and OMP (goat anti-OMP no. 544-10001, WAKO, 1:500) overnight at 4 °C. The primary antibodies were detected by consecutive incubation with secondary antibodies against goat (donkey anti-goat fluorescein isothiocyanate sc-2024, Santa Cruz) and rabbit (Alexa goat anti-rabbit 543 nm (A-11010, Invitrogen) for 45 min at RT.
Sections were analyzed with an Olympus IX70 fluorescent inverted microscope (Olympus Imaging America Inc.) at ×40 magnification.
PI3K enzyme-linked immunosorbent assay
Dissociated OE cells were stimulated with H100, and the enzyme reactions were stopped by immediate freezing in liquid N2 followed by the addition of ice-cold 0.5 M trichloroacetic acid. PIs were extracted and detected with a PIP3 mass ELISA Kit, which can detect both PIP3 and PI(3,4)P2, following the manufacturer's protocol (Echelon Bioscience). The plates were read on a multiwell plate reader (Bio-Rad Laboratories) at 450 nm. The data were quantified with standards (0.625 to 20 pmol per well) and analyzed with Microplate Manager 4.0 software (Bio-Rad Laboratories).
Data analysis
All data are expressed as mean ± SEM. Statistical significance was assessed using Student's t-test.
Results
PI3K-dependent signaling occurs in ORNs of wt mice
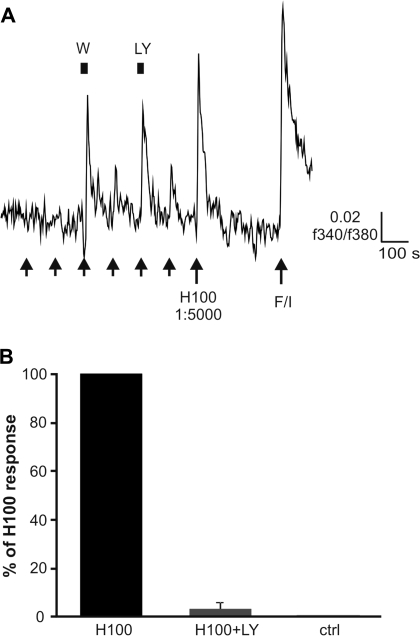
In order to determine if inhibiting PI3K signaling can enhance the amplitude of calcium transients elicited by stimulation with complex odorants, as initially shown in rat ORNs (Spehr et al. 2002), we imaged ORNs acutely dissociated from the OE of wt mice. Cells were identified as canonical ORNs by their ability to respond to a mixture of 10 μM forskolin and 100 μM IBMX. Cells that responded to forskolin/IBMX were stimulated with a 1:5000 dilution of H100 (Wetzel et al. 1999), an odorant mixture of sufficient complexity to contain excitatory as well as inhibitory compounds for a workable percentage of ORNs in the population. H100 evoked an increase in intracellular calcium in 18.8% (92/489 cells from 3 animals) of all ORNs screened. Reducing the concentration of H100 to 1:50000 decreased the incidence of activation to 11.2% (55/489 cells from 3 animals). Inhibiting PI3K-dependent activity by preincubating the cells with the pan-specific PI3K inhibitors wortmannin (1 μM) and LY294009 (10 μM) (Figure 1A) increased the peak magnitude of the calcium signal to 213.6 ± 27.7% (n = 16 cells) and 234.6 ± 20.3% (n = 13 cells), respectively, of that evoked by H100 alone prior to incubation. The effect was evident in only some of the cells that were capable of showing an increase in the magnitude of their response, that is, that did not show a saturated response to H100 in 1:50000 dilution and were responsive to a higher concentration of the odorant mixture (1:5000). Of 54 such capable cells from 3 preparations (animals, 18 ± 3.8 cells per animal), wortmannin (1 μM) increased the peak magnitude of the calcium signal in 36.9 ± 4.5% of the cells, whereas LY294009 (10 μM) did so in 32.4 ± 5.2% of the cells.
Figure 1.
Odorant-induced PI3K-dependent signaling in mouse ORNs. (A) ORNs from wt mice show enhanced responses to complex odorant stimulation following inhibition of PI3K by wortmannin and LY294002. Cells for analysis were selected by responsiveness to H100 in higher concentration and to forskolin and IBMX in combination. Arrows show application of H100 (1:50000 dilution), H 1:5000 = H100 in 1:5000 dilution, F/I = forskolin (107 M) + IBMX (1007 M); PI3K inhibitors: Wortmannin (W, 1μM) and LY294002 (LY, 10μM). (B) Odorant stimulation (H100 in 1:10000 dilution) of OE cells elicits PI3K activity as measured by ELISA that can be inhibited by LY294002. As control for PI3K activity in the absence of odorant stimulation, cells were treated with DMSO (0.02%). Bars denote the mean ± SEM of 4 independent repetitions and are presented as the percentage of the response to H100 in 1:10000 dilution.
Odorants increase PI3K activity in ORNs of wt mice
To confirm that odorant stimulation of mouse ORNs is indeed accompanied by a change in PI3K activity, we measured PI3K activation in the dissociated OE using a PIP3 mass enzyme-linked immunosorbent assay (ELISA), which measures class I PI3K activation. Cells mock treated with 0.02% dimethyl sulfoxide failed to show any measurable change in PI3K activity. Stimulation with H100 (1:10000 dilution) increased the concentration of detected phospholipids to an equivalent of 21.4 ± 4.1 pmol PIP3 per μg protein within 10 s of odorant stimulation (n = 5). The increase in PI3K activity was reduced by the pan-specific PI3K inhibitor LY294002 to 5.9 ± 1.9% of the mean level of activation evoked by odorant stimulation alone (n = 4, Figure 1B). These results indicate that the ELISA used to measure odorant-induced PI3K activity reflects changes in PIP3 because the signal was inhibited with PI3K-specific inhibitors.
Both the β and γ isoforms of PI3K are expressed in the OE of adult wt mice
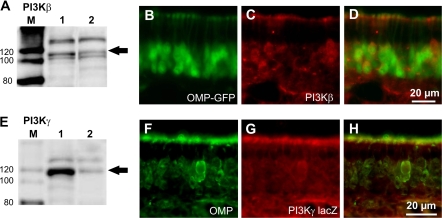
We then examined whether one or both of the isoforms of PI3K known to couple through G protein-coupled receptors (GPCRs), PI3Kβ and PI3Kγ, are expressed in the murine OE. Western blots of OE proteins from wt mice revealed bands of appropriate molecular weight for the catalytic subunits of PI3Kβ (Figure 2A) and PI3Kγ (Figure 2E). These antibodies have been previously shown to be specific for p110 protein detection in mice and rats (Murga et al. 2000; Ciraolo et al. 2008; Guillermet-Guibert et al. 2008; Ukhanov et al. 2010). Expression of both isoforms could be localized to the ORNs immunohistochemically. As shown in representative images (Figure 2B–D), immunofluorescent labeling of PI3Kβ expression colocalized with GFP fluorescence in the somata, dendrite, and knob layers of a majority of the ORNs within the OE of OMP-GFP transgenic mice (Potter et al. 2001). Because an antibody against PI3Kγ suitable for immunohistochemistry is not currently available, we visualized β-galactosidase (β-gal) under the promoter of PI3Kγ in PI3Kγ KO-LacZ mice. Similar to PI3Kβ, immunohistochemistry with an anti-β-gal antibody (Figure 2F–H) showed that PI3Kγ is also expressed in a majority of the ORNs of these mice. Control staining in wt mice showed no labeled ORNs (Supplementary Figure 1). Although β-gal is expressed under the same promotor as PI3Kγ, it is not necessarily localized to the same subcellular compartments, so we were unable to localize PI3Kγ expression to a particular compartment of the ORNs.
Figure 2.
PI3Kβ and γ are expressed in the OE. (A) and (E) Western blot analysis of the catalytic p110 subunits of PI3Kβ and γ using specific antibodies against p110β (A) and p110γ (E), respectively (M: Marker; 1: mouse spleen; 2: mouse whole OE homogenate). (B–D) Representative images of immunofluorescence in the OE of OMP-GFP transgenic mice: intrinsic OMP-GFP fluorescence (green, B) labeled with an anti-p110β antibody (red, C). Overlay of (B) and (C) is shown in (D). (F–H) Colocalization of OMP immunofluorescence (green, F) with β-gal immunofluorescence (red, G) in PI3Kγ KO-LacZ transgenic mice. Overlay of (F) and (G) is shown in (H).
PI3Kγ and β specific inhibitors both affect odorant-activated PI3K-dependent signaling in the mouse OE
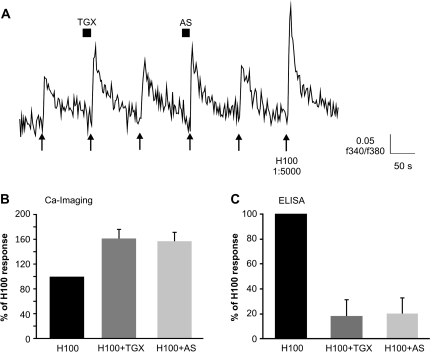
In order to implicate PI3Kβ and/or γ functionally in signal transduction, we tested the effect of isoform-specific inhibitors for PI3Kβ (TGX-221, 200 nM) and PI3Kγ (AS252424, 200 nM) on the odorant-evoked response of ORNs from wt mice. Both inhibitors increased the magnitude of the calcium signal evoked by H100 in ORNs, and could do so in the same ORN (Figure 3A, n = 11). TGX-221 and AS252424 enhanced calcium responses evoked by H100 to 161.2 ± 15.6% and 157.1 ± 15.2%, respectively, of the response to H100 alone, suggesting that both isoforms contribute equally to PI3K-mediated signaling in these cells (Figure 3B). Consistent with this finding, both inhibitors also reduced H100-induced PIP3 to the same extent in the OE as shown by ELISA (Figure 3C).
Figure 3.
Effects obtained by pan-specific inhibitors for PI3K are mimicked by the PI3K isoform-specific inhibitors TGX-221 (PI3Kβ, 200 nM) and AS252424 (PI3Kγ, 200 nM). (A) ORNs from wt mice show enhanced responses to complex odorant stimulation following inhibition of PI3K by TGX-221 or AS252424 in calcium imaging. Arrows indicate H 100 (1: 50000 dilution) application, H 1:5000 = H100 in 1:5000 dilution. Cells were preincubated with inhibitors for 10 s prior to odorant application when indicated. (B) TGX-221 and AS252424 elicit the same level of enhancement of responses to complex odorant stimulation in calcium imaging (n = 11 cells). Bars denote the mean ± SEM of amplitudes normalized to H 100 alone. (C) PI3K activity measured in ELISA can be inhibited by TGX-221 and AS252424 to the same level. Bars denote the mean ± SEM of 2 independent repetitions and are presented as the percentage of the response to H100 in 1:10000 dilution.
PI3Kγ contributes to odorant-activated PI3K-dependent signaling in the mouse OE
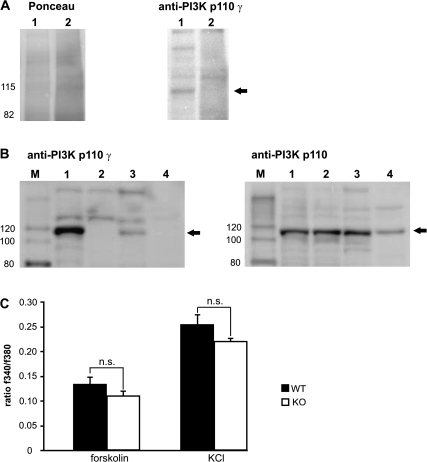
To better understand the relative roles of the PI3K isoforms in mouse olfactory signal transduction, we compared the odorant-evoked responses of ORNs from PI3Kγ KO mice with those of wt mice, as has been done to establish the role of PI3Kγ in macrophages and neutrophils (Sasaki et al. 2000; Hirsch et al. 2000; Li et al. 2000; Patrucco et al. 2004; Suire et al. 2006; Costa et al. 2007). In comparison with wt mice, the OE of the PI3Kγ KO mice is normal with respect to its thickness and OMP expression (data not shown). Despite the absence of PI3Kγ protein in the OE of PI3Kγ KO mice (Figure 4A), western blot analysis with a pan-specific PI3K antibody shows residual expression of PI3K in the ciliary membranes of ORNs (Figure 4B), consistent with coexpression of at least 2 isoforms of PI3K.
Figure 4.
PI3Kγ KO mice lack p110γ in olfactory cilia but retain normal responsiveness to non-PI3K dependent input. (A) Western blot analysis of the catalytic p110γ subunit (anti-PI3K p110γ) in wt (lane 1) and PI3Kγ KO (lane 2) mouse OE. Ponceau staining of the blot shows the total protein present in both samples. (B) Western blot analysis of the catalytic p110 subunit using a pan-specific PI3K antibody (anti-PI3K p110) in mouse spleen and cilia extract shows a residual PI3K expression in cilia of PI3Kγ KO mice. (M: Marker; 1: wt mouse spleen; 2: KO mouse spleen; 3: wt mouse cilia homogenate; 4: KO mouse cilia homogenate). (C) Amplitudes of calcium transients in response to different stimuli are not significantly altered between wt and PI3Kγ KO mice.
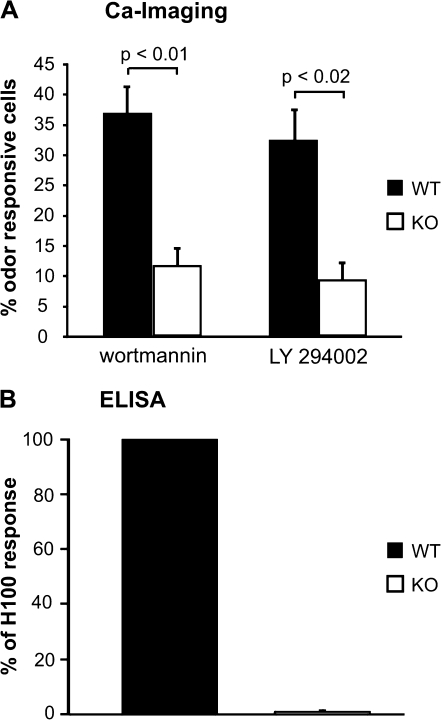
Functionally, ORNs from these mice show calcium responses to stimulation with KCl (45 mM) and forskolin (10 μM) that are not significantly different in amplitude compared with cells from wt mice (Figure 4C), suggesting that PI3Kγ KO ORNs are excitable and normal with respect to cyclic nucleotide-mediated signaling. However, in contrast to ORNs from wt mice, the pan-specific PI3K inhibitors wortmannin and LY294002 had a significantly lower incidence of effect on ORNs from the PI3Kγ KO mice compared with those from wt mice (Figure 5A). Only 11.8 ± 3% of the cells per animal from the PI3Kγ KO mice capable of showing an enhanced response (n = 50 cells from 3 animals) were sensitive to wortmannin treatment and only 9.5 ± 2.8% of the cells were sensitive to LY294002, compared with 36.9 ± 4.5% and 32.4 ± 5.2%, respectively, of the cells from wt mice. Furthermore, H100 failed to elicit a measurable increase in PI3K activity in cells dissociated from the OE of PI3Kγ KO mice (Figure 5B). Together these data suggest that PI3Kγ has a significant role in mediating PI3K-dependent signaling in mouse ORNs.
Figure 5.
PI3Kγ KO mice show a large reduction in PI3K-mediated signaling upon odorant stimulation. (A) PI3Kγ KO mice show effects of odorant response enhancement to the pan-specific PI3K inhibitors wortmannin and LY294002 in a reduced number of cells. Values have been normalized to the number of cells with the potential to show this effect. Values represent mean between preparations ±SEM. (B) PI3K activity upon odorant stimulation is almost completely abolished in PI3Kγ KO mice. Bars denote the mean ± SEM of 5 independent repetitions and are presented as the percentage of the response to H100 in 1:10000 dilution in wt mice.
Discussion
Notwithstanding known differences in the olfactory receptor reservoir (Gloriam et al. 2007), the electrophysiological properties of the ORNs (Ma et al. 1999), and olfactory behavior (Doty 1986) of mice and rats, PI3K-dependent signaling appears to generalize to both species. As in rat ORNs (Spehr et al. 2002), pharmacological inhibition of PI3K can enhance the magnitude of the calcium response of mouse ORNs to complex odorants. Furthermore, as in rats (Klasen et al. 2010), odorant stimulation induces a rise in PI3K activity in the mouse OE.
Mouse ORNs express 2 known GPCR-coupled isoforms of PI3K, PI3Kβ and PI3Kγ. Interestingly, both PI3Kβ and γ are expressed in a majority of mature ORNs, suggesting they are not confined to a particular subset of ORNs, such as the TRPM5-postitive ORNs (Lin et al. 2007) or the GC-D ORNs (Fülle et al. 1995), but rather that they play a role in canonical ORNs. This finding would appear to conflict with our finding that only some cells showed a physiological response to PI3K inhibition, but we assume the latter reflects the restricted odorant specificity of individual mammalian ORNs (Saito et al. 2009) and the limited array of odorants used to challenge the cells. If, as suggested earlier (Spehr et al. 2002), an odorant mixture needs to be sufficiently complex to contain at least one excitatory and one inhibitory ligand for the particular cell in question in order to see a physiological effect, H100 has only a limited probability of doing so considering that each cell expresses only one of about 1000 ORs predicted to be functional in mice (Zhang and Firestein 2002). We assume, therefore, that PI3K signaling is functional in many, if not most, canonical mouse ORNs rather than just a specialized subset of cells, arguing that it serves a fundamental role in murine, and presumably all mammalian, ORNs.
Due to methodological constraints, we cannot confirm whether there is an overlap in the expression of both isoforms of PI3K that are located in the transduction compartment of ORNs. The antigen retrieval used for PI3Kβ immunostaining could have affected the tissue differentially, making exact localization difficult, and the PI3Kγ KO-LacZ transgenic mouse strain has the PI3Kγ gene disrupted by insertion of an IRES-LacZ and a neomycin resistance cassette in the first coding exon (Hirsch et al. 2000), which allows localization of PI3Kγ expression to specific cell types but not to subcellular regions. However, given that PI3Kγ clearly is expressed in cilia based on western blots and that isoform-specific inhibitors affected odorant-induced calcium responses and odorant-dependent PI3K activity, our results argue in favor of localization of both PI3Kγ and β to the signal transduction compartment of ORNs.
ORNs dissociated from the OE of PI3Kγ KO mice are significantly less responsive to PI3K inhibition, but they still have a residual sensitivity to PI3K inhibitors, suggesting that PI3Kγ is not exclusively responsible for PI3K-dependent signaling in these cells. Questions then arise as to the relative roles of PI3Kβ and γ in murine ORNs. Because both isoforms are expressed in most ORNs and both isoform-specific inhibitors can act in the same cell, we assume PI3Kβ and γ are coexpressed and function together in most cells. Such redundant expression is known in bone marrow-derived macrophages (Guillermet-Guibert et al. 2008) and blood platelets (Canobbio et al. 2009) where both PI3Kβ and γ signal downstream from the same GPCR to mediate the same effect. However, the functional significance of such redundancy is still unclear. In macrophages, it is argued that PI3Kβ is the predominant isoform that mediates GPCR-coupled PI3K signaling while PI3Kγ provides reserve signaling capacity when needed (Guillermet-Guibert et al. 2008). Alternately, the isoforms could work in parallel but with slightly different kinetics, for example, to mediate different temporal aspects of odorant responses.
Overall, our results set the stage for using genetically manipulated mice to explore both the cellular mechanisms and behavioral role(s) of PI3K-mediated signal transduction in mammalian ORNs. Although they argue that PI3Kγ-mediated signaling plays an important role in transduction, they also implicate a role for PI3Kβ. The availability of isoform-specific mutant mice (PI3Kγ: Hirsch et al. 2000; Li et al. 2000; Sasaki et al. 2000; Patrucco et al. 2004; Suire et al. 2006; Costa et al. 2007 and PI3Kβ: Ciraolo et al. 2008; Guillermet-Guibert et al. 2008) will allow more careful dissection of the functional roles of the 2 isoforms of PI3K in mammalian olfactory transduction.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders (DC05512 and DC009730); Deutsche Forschungsgemeinschaft Postdoctoral Fellowship to K.K.; and Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation to D.B.
Supplementary Material
Acknowledgments
We thank Drs Emilio Hirsch and Frank Margolis for providing fixed specimen of transgenic mice strains. We also thank Drs Kirill and Maria Ukhanov for help with staining of the PI3Kγ KO-LacZ mice.
References
- Brady JD, Rich ED, Martens JR, Karpen JW, Varnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2006;103:15635–15640. doi: 10.1073/pnas.0603344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M Forthcoming. Genetic evidence for a predominant role of PI3K{beta} catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114(10):2011–2012. doi: 10.1182/blood-2009-03-208074. [DOI] [PubMed] [Google Scholar]
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110β activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CM, Leevers SJ. Do growth and cell division rates determine cell size in multicellular organisms? J Cell Sci. 2000;113(Pt 17):2927–2934. doi: 10.1242/jcs.113.17.2927. [DOI] [PubMed] [Google Scholar]
- Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, Neilsen PO, Ciraolo E, Altruda F, Prestwich GD, et al. Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci U S A. 2007;104:14354–14359. doi: 10.1073/pnas.0703175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Odor-guided behavior in mammals. Experientia. 1986;42(3):257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Fülle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloriam DE, Fredriksson R, Schiöth HB. The G protein-coupled receptor subset of the rat genome. BMC Genomics. 2007;8:338. doi: 10.1186/1471-2164-8-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold GH. Controversial issues in vertebrate olfactory transduction. Annu Rev Physiol. 1999;61:857–71. doi: 10.1146/annurev.physiol.61.1.857. [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki O, Okada T, Kurosu H, Takasuga S, Suzuki T, Katada T. Activation of PI 3-kinase by G protein betagamma subunits. Life Sci. 1998;62:1555–1559. doi: 10.1016/s0024-3205(98)00106-4. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Klasen K, Corey EA, Kuck F, Wetzel CH, Hatt H, Ache BW. Odorant-stimulated phosphoinositide signaling in mammalian olfactory receptor neurons. Cell Signal. 2010;22:150–157. doi: 10.1016/j.cellsig.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Moon C, Liu BQ, Kim SY, Kim EJ, Park YJ, Yoo JY, Han HS, Bae YC, Ronnett GV. Leukemia inhibitory factor promotes olfactory sensory neuronal survival via phosphoinositide 3-kinase pathway activation and Bcl-2. J Neurosci Res. 2009;87:1098–1106. doi: 10.1002/jnr.21919. [DOI] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Ann Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J Biol Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- Noé J, Breer H. Functional and molecular characterization of individual olfactory neurons. J Neurochem. 1998;71:2286–2293. doi: 10.1046/j.1471-4159.1998.71062286.x. [DOI] [PubMed] [Google Scholar]
- Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Potter SM, Zheng C, Koos DS, Feinstein P, Fraser SE, Mombaerts P. Structure and emergence of specific olfactory glomeruli in the mouse. J Neurosci. 2001;21:9713–9723. doi: 10.1523/JNEUROSCI.21-24-09713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- Rothman A, Feinstein P, Hirota J, Mombaerts P. The promoter of the mouse odorant receptor gene M71. Mol Cell Neurosci. 2005;28:535–546. doi: 10.1016/j.mcn.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Schandar M, Laugwitz KL, Boekhoff I, Kroner C, Gudermann T, Schultz G, Breer H. Odorants selectively activate distinct G protein subtypes in olfactory cilia. J Biol Chem. 1998;273:16669–16677. doi: 10.1074/jbc.273.27.16669. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–434. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Spehr M, Wetzel CH, Hatt H, Ache BW. 3-phosphoinositides modulate cyclic nucleotide signaling in olfactory receptor neurons. Neuron. 2002;33:731–739. doi: 10.1016/s0896-6273(02)00610-4. [DOI] [PubMed] [Google Scholar]
- Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, et al. Gβγs and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signaling in neutrophils. Nat Cell Biol. 2006;8:1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- Ukhanov K, Corey EA, Brunert D, Klasen K, Ache BW Forthcoming. Inhibitory odorant signaling in mammalian olfactory receptor neurons. J Neurophysiol. 2010;103(2):1114–1122. doi: 10.1152/jn.00980.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Washburn KB, Turner TJ, Talamo BR. Comparison of mechanical agitation and calcium shock methods for preparation of a membrane fraction enriched in olfactory cilia. Chem Senses. 2002;27:635–642. doi: 10.1093/chemse/27.7.635. [DOI] [PubMed] [Google Scholar]
- Wetzel CH, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus Laevis oocytes. J Neurosci. 1999;19:7426–7433. doi: 10.1523/JNEUROSCI.19-17-07426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by Ptdlns(3,4,5)P3. J Membr Biol. 2004;201(1):51–57. doi: 10.1007/s00232-004-0707-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.