Abstract
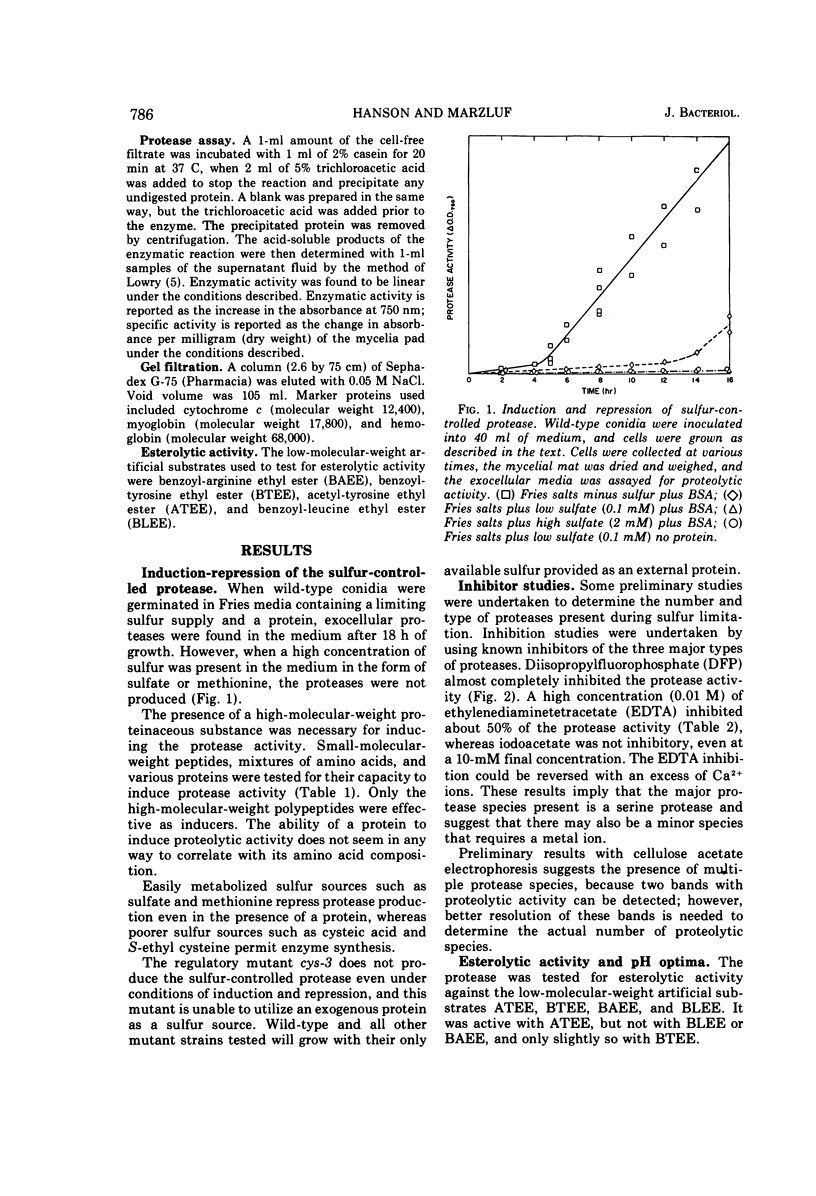
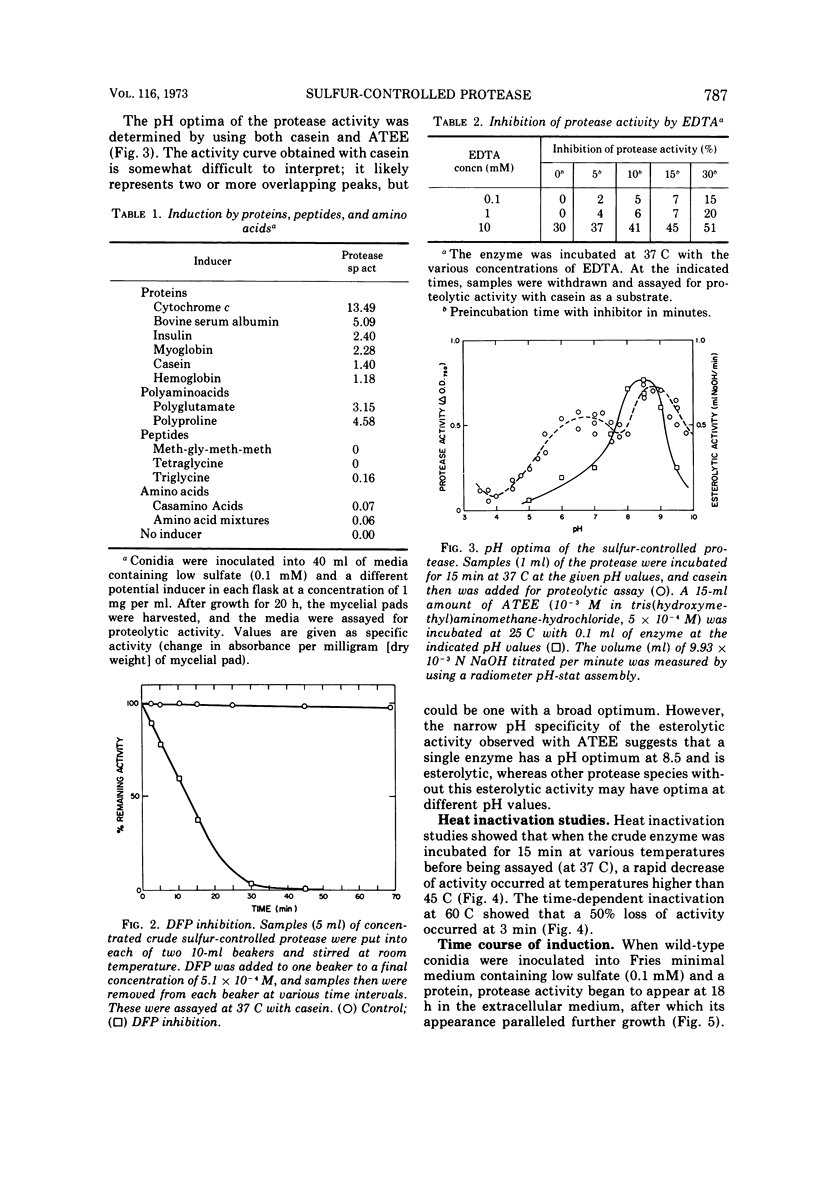
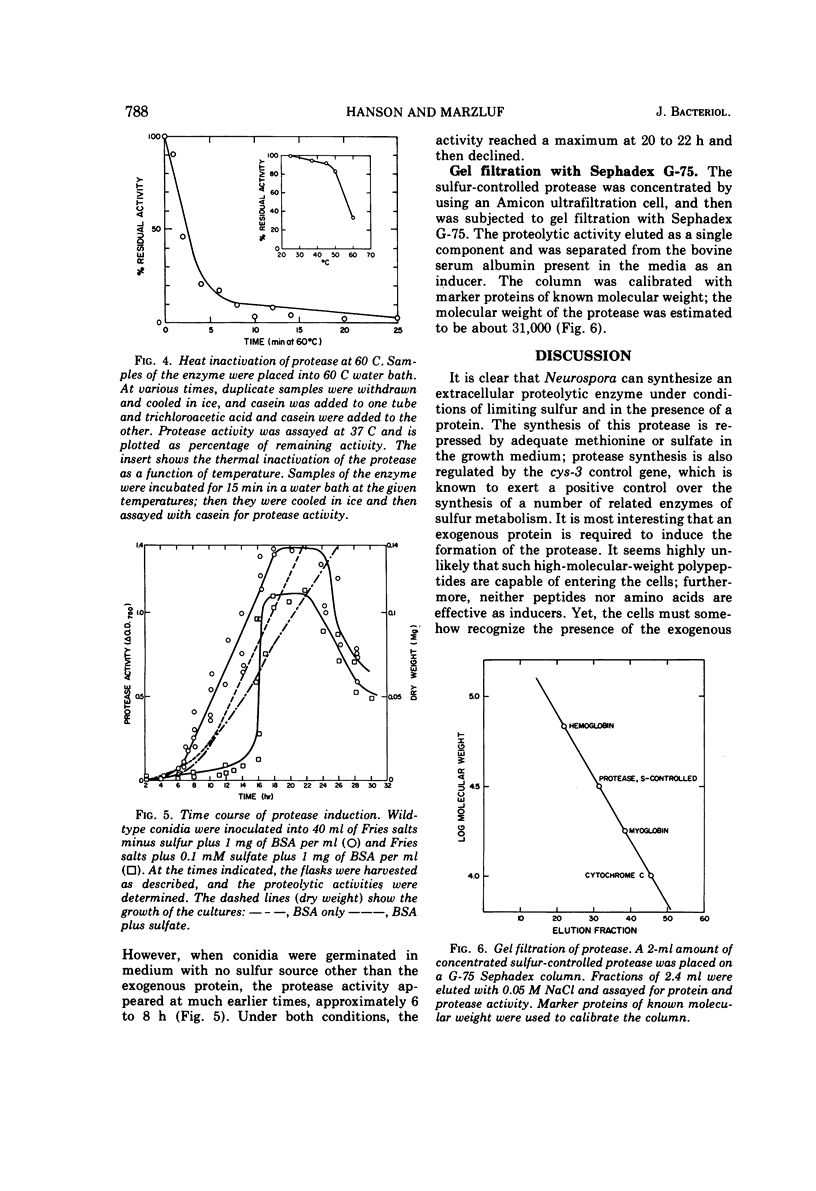
Wild-type Neurospora crassa produces and secretes extracellular protease(s) when grown on a medium containing a protein as its principle sulfur source. Readily available sulfur sources, such as sulfate or methionine, repress the synthesis of the proteolytic activity. Preliminary characterization of the proteolytic enzyme shows it to have a molecular weight of about 31,000, a pH optimum of 6 to 9 with casein as substrate, and esterolytic activity against acetyl-tyrosine ethyl ester with a pH optimum of 8.5. The enzyme activity is completely inhibited by diisopropylfluorophosphate, partially inhibited by ethylenediaminetetraacetate, but unaffected by iodoacetate. The proteolytic activity is temperature labile and is reduced by 75% within 15 min at 60 C. Synthesis of the protease activity is induced by proteins, and to a lesser extent by large-molecular-weight polyamino acids, but not at all by small peptides or amino acid mixtures. During conidial out-growth, the protease(s) first appears at about 8 h and continues to increase while the cells are in an active growth phase. When a low concentration of sulfate is present, the protease(s) is not produced until about 18 h, suggesting that the sulfate must first be used by the cells before the protease is either synthesized or released.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson S., Holme T., Lindholm B. The formation of extracellular proteolytic enzymes by Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):835–844. doi: 10.1111/j.0365-5563.1973.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol. 1972 Jun;110(3):1041–1049. doi: 10.1128/jb.110.3.1041-1049.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATILE P. INTRAZELLULAERE LOKALISATION PROTEOLYTISCHER ENZYME VON NEUROSPORA CRASSA. I. FUNKTION UND SUBZELLULAERE VERTEILUNG PROTEOLYTISCHER ENZYME. Z Zellforsch Mikrosk Anat. 1965 Mar 16;65:884–896. [PubMed] [Google Scholar]
- Marzluf G. A., Metzenberg R. L. Positive control by the cys-3 locus in regulation of sulfur metabolism in Neurospora. J Mol Biol. 1968 Apr 28;33(2):423–437. doi: 10.1016/0022-2836(68)90199-x. [DOI] [PubMed] [Google Scholar]
- Murakami M., Fukunaga K., Matsuhashi M., Ono M. Stimulative effect of proteins on protease formation by Serratia sp. Biochim Biophys Acta. 1969 Nov 18;192(2):378–380. doi: 10.1016/0304-4165(69)90385-7. [DOI] [PubMed] [Google Scholar]



