Abstract
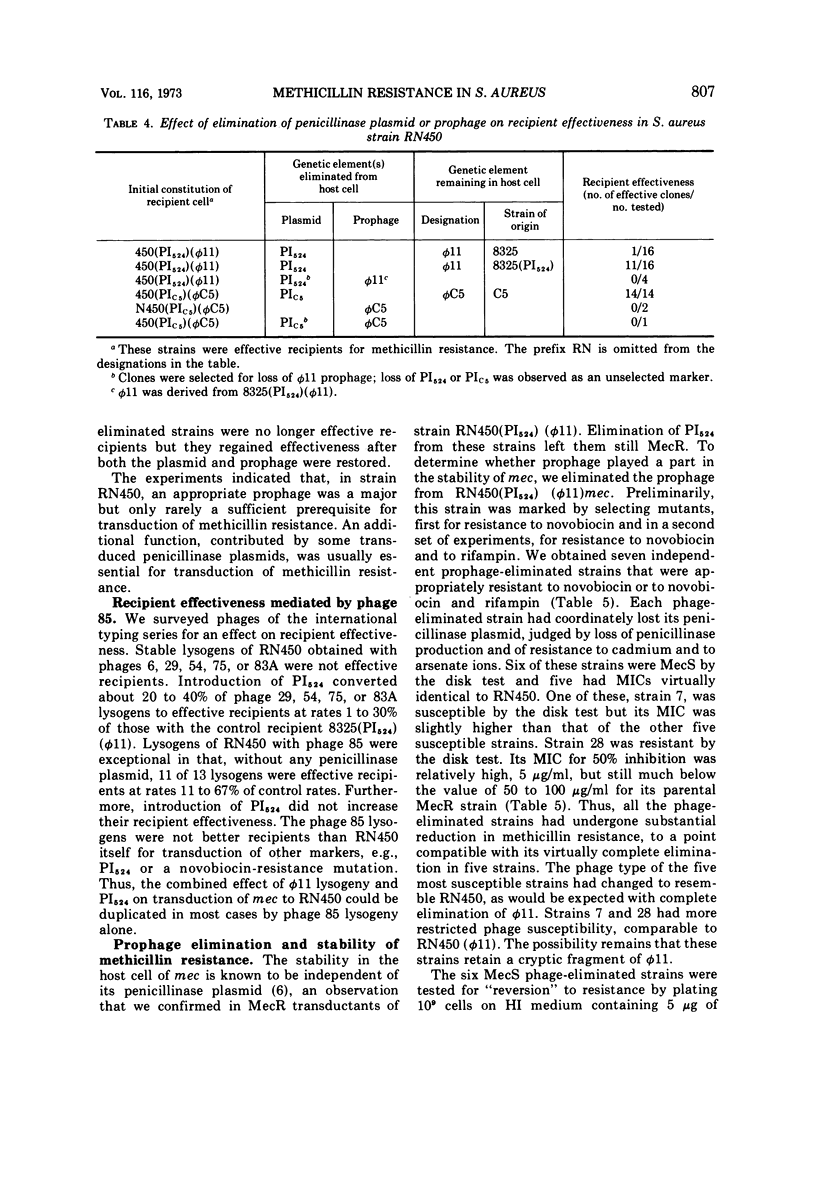
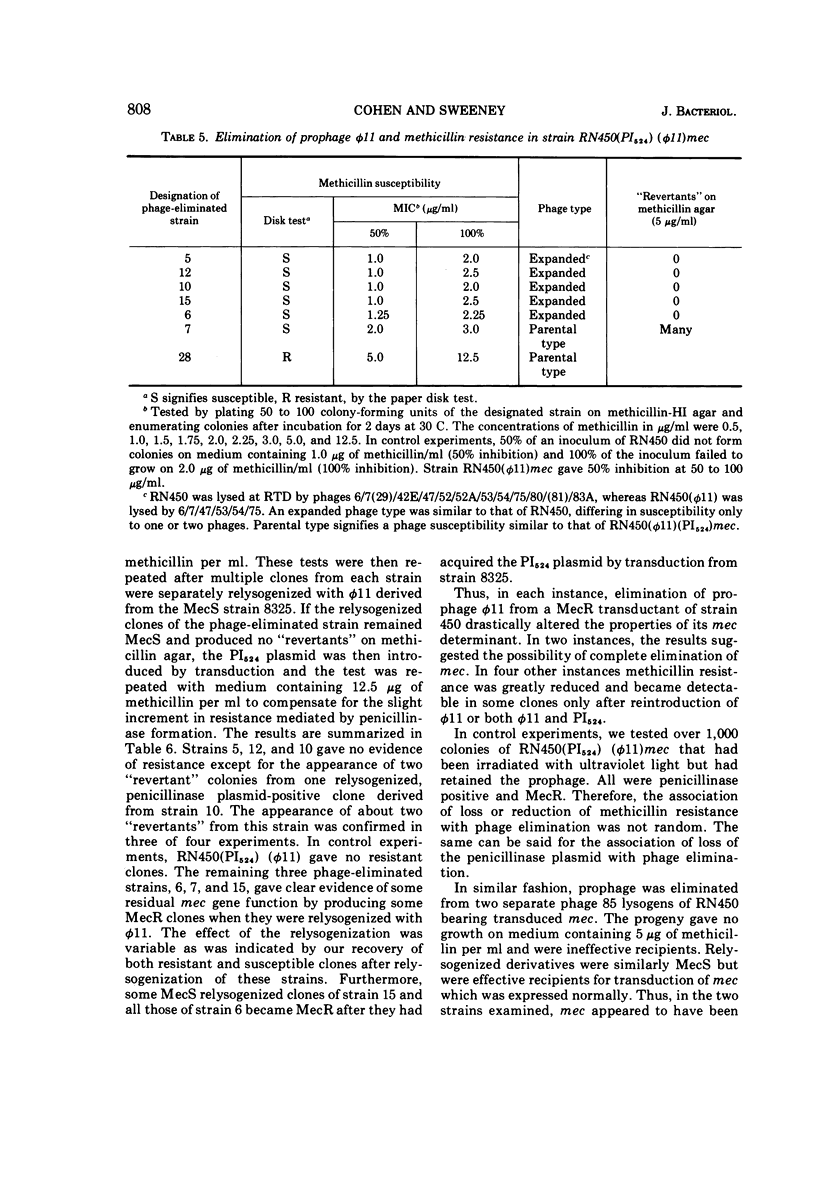
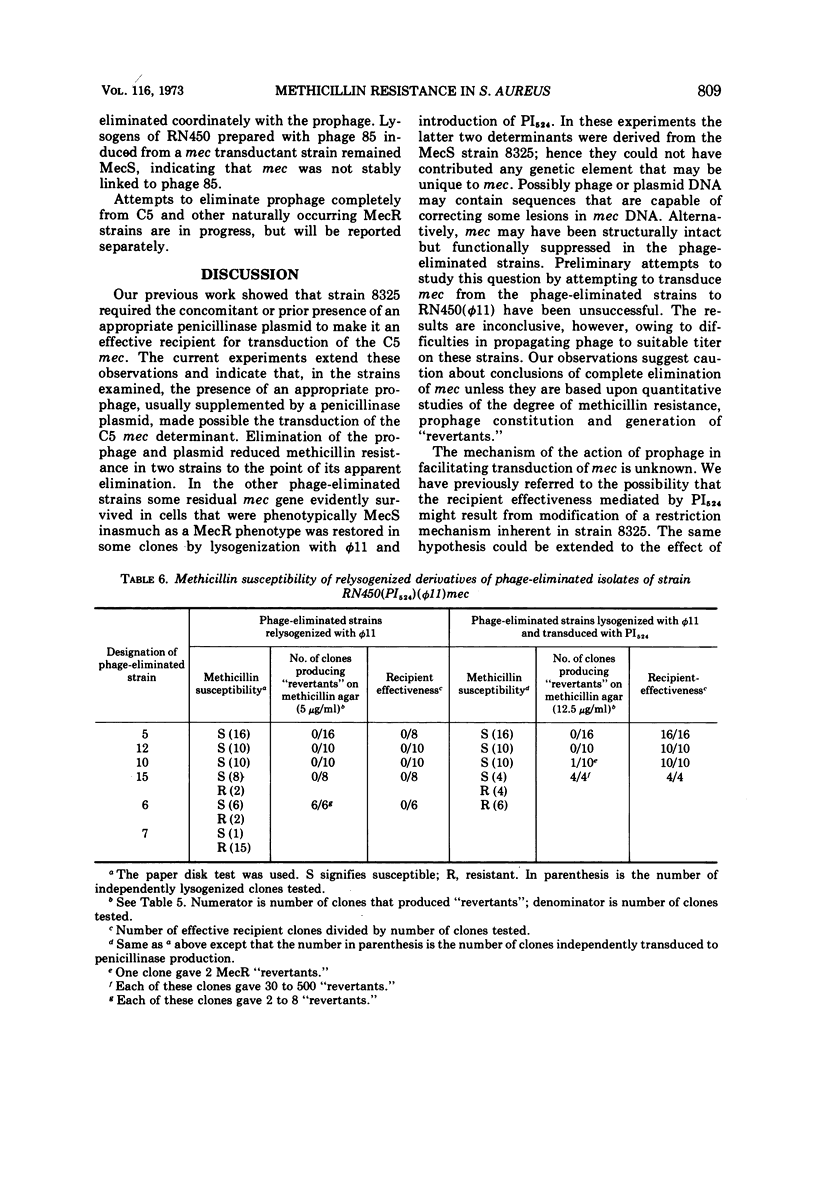
Transduction of a methicillin-resistance determinant (mec) in Staphylococcus aureus RN450 was dependent on its prior lysogenization with an appropriate temperate phage. In addition, an appropriate transduced penicillinase plasmid was usually required. Some phage 80-resistant variants of RN450 or of its parental lysogenic strain, NCTC 8325, were also effective recipients for transduction of mec. Elimination of prophage from RN450 abrogated its effectiveness as a transductional recipient of mec. Elimination of prophage from a methicillin-resistant transductant of RN450 reduced resistance to undetectable levels in six of seven phage-eliminated strains. In four of these a variable number of clones again became phenotypically resistant after lysogenization alone or lysogenization combined with reintroduction of a penicillinase plasmid. In two prophage-eliminated strains, no evidence of residual mec could be adduced. The establishment, expression, or stability of the transduced mec in strain RN450 appeared to depend on some function determined by a prophage or a prophage and a penicillinase plasmid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Annear D. I., Grubb W. B. Spontaneous loss of methicillin resistance in Staphylococcus aureus. Lancet. 1973 Jan 13;1(7794):110–110. doi: 10.1016/s0140-6736(73)90518-7. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Chabbert Y. A., Baudens J. G., Acar J. F., Gerbaud G. R. La résistance naturelle des staphylocoques à la méthicillin et l'oxacilline. Rev Fr Etud Clin Biol. 1965 May;10(5):495–506. [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAVENKEMPER C. F., BRODIE J. L., KIRBY W. M. RESISTANCE OF COAGULASE-POSITIVE STAPHYLOCOCCI TO METHICILLIN AND OXACILLIN. J Bacteriol. 1965 Apr;89:1005–1010. doi: 10.1128/jb.89.4.1005-1010.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb W. B., Annear D. I. Spontaneous loss of methicillin resistance in Staphylococcus aureus at room-temperature. Lancet. 1972 Dec 9;2(7789):1257–1257. doi: 10.1016/s0140-6736(72)92315-x. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. III. Effet du rayonnement ultraviolet sur la recombinaison génétique. Ann Inst Pasteur (Paris) 1955 Jun;88(6):724–749. [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Selective stimulation of one of the mechanisms for genetic recombination of bacteriophage S13. Science. 1968 Aug 2;161(3840):481–482. doi: 10.1126/science.161.3840.481. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Hirota Y. Impaired transduction of R213 and its recovery by a homologous resident R factor. J Bacteriol. 1971 May;106(2):523–528. doi: 10.1128/jb.106.2.523-528.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Salihy S. M., James A. M. Loss of methicillin-resistance from resistant strains of Staphylococcus aureus. Lancet. 1972 Aug 12;2(7772):331–332. doi: 10.1016/s0140-6736(72)92937-6. [DOI] [PubMed] [Google Scholar]



