Abstract
Rationale
Many of the biochemical, physiological, and behavioral effects of ethanol are known to be mediated by ionotropic glutamate receptors. Emerging evidence implicates metabotropic glutamate receptors (mGluRs) in the biobehavioral effects of ethanol and other drugs of abuse, but there is little information regarding the role of mGluRs in the reinforcing effects of ethanol.
Materials and methods
Male C57BL/6J mice were trained to lever-press on a concurrent fixed ratio 1 schedule of ethanol (10% v/v) vs water reinforcement during 16-h sessions. Effects of mGluR1, mGluR2/3, and mGluR5 antagonists were then tested on parameters of ethanol self-administration behavior.
Results
The mGluR5 antagonist MPEP (1–10 mg/kg, i.p.) dose-dependently reduced ethanol-reinforced responding but had no effect on concurrent water-reinforced responding. Analysis of the temporal pattern of responding showed that MPEP reduced ethanol-reinforced responding during peak periods of behavior occurring during the early hours of the dark cycle. Further analysis showed that MPEP reduced the number of ethanol response bouts and bout-response rate. MPEP also produced a 13-fold delay in ethanol response onset (i.e., latency to the first response) with no corresponding effect on water response latency or locomotor activity. The mGluR1 antagonist CPCCOEt (1–10 mg/kg, i.p.) or the mGluR2/3 antagonist LY 341495 (1–30 mg/kg, i.p.) failed to alter ethanol- or water-reinforced responding.
Conclusions
These data indicate that mGlu5 receptors selectively regulate the onset and maintenance of ethanol self-administration in a manner that is consistent with reduction in ethanol’s reinforcement function.
Keywords: mGluR5, mGluR1, mGluR2/3, MPEP, Ethanol self-administration, Reinforcement, Alcohol drinking, Mice
Introduction
Glutamate is the primary excitatory amino acid neurotransmitter in the mammalian central nervous system. Fast excitatory actions of glutamate are mediated by ionotropic (iGluR) N-methyl-D-aspartate (NMDA), α-amino-3-hydroxi-5-methyl-ioxyzole-4-propionic acid (AMPA), and kainate (KA) receptors. Metabotropic glutamate receptors (mGluRs) mediate slower glutamate responses through G-protein coupling to various intracellular signaling cascades that can modulate, or fine-tune, iGluR function (Benquet et al. 2002). The eight known mGluR subtypes have been classified into three groups based on amino acid sequence similarity, agonist pharmacology, and the signal transduction pathways to which they couple. Group I mGluRs (mGluR1 and 5) up-regulate Ca2+ cascades, whereas Group II (mGluR2 and 3) and Group III (mGluR4 and 6–8) receptors are negatively coupled to the cyclic adenosine monophosphate (cAMP) cascade but are distinguished functionally by their differing agonist–antagonist profiles and sequence homologies (Gereau and Conn 1995; Pin and Duvoisin 1995).
A variety of the physiological, biochemical, and behavioral effects of ethanol are known to involve iGluR function (Aschner et al. 2001; Costa et al. 2000; Dodd et al. 2000; Littleton et al. 2001; Mihic 1999; Tabakoff and Hoffman 1993; Weight et al. 1993; Woodward 1999). Noncompetitive NMDA receptor antagonists substitute for the discriminative stimulus (i.e., subjective or cue) effects of ethanol when they are administered systemically (Colombo and Grant 1992; Grant and Colombo 1993) or in specific limbic brain regions (Hodge and Cox 1998). Accordingly, NMDA receptor antagonists decrease ethanol intake by rats selectively bred for high ethanol preference (McMillen et al. 2004) and inhibit reinstatement of ethanol-seeking behavior following extinction (Backstrom and Hyytia 2004). Similarly, the NMDA2B antagonist ifenprodil reduces relapse-like behavior in rats that have been deprived of alcohol (Vengeliene et al. 2005).
Emerging evidence implicates mGluR function in ethanol’s neurobehavioral effects. Ethanol has been shown to reduce both basal and stimulated phosphoinositide hydrolysis (Gonzales et al. 1986), which indicates modulation of general metabotropic receptor activity by ethanol. Ethanol alters neuronal firing rates (Netzeband et al. 1997) and Ca2+ levels (Gruol et al. 1997) mediated by mGluRs in vitro. Chronic exposure to ethanol reduces mGluR1 mRNA levels in cerebellar Purkinje neurons of mice (Simonyi et al. 1996), and early withdrawal from ethanol leads to alterations in mGluR-evoked Ca2+ signaling in cerebellar neurons (Netzeband et al. 2002). Physiologically relevant concentrations of ethanol inhibit glutamate-induced Ca2+-dependent Cl− currents in Xenopus oocytes expressing mGluR5 but have no effect on currents in oocytes expressing mGluR1 (Minami et al. 1998), which suggests that ethanol may selectively alter mGluR5 function. In rats, chronic exposure to an ethanol-containing liquid diet decreased mRNA levels for mGluR3 and mGluR5 in the dentate gyrus, whereas mGluR1, mGluR5, and mGluR7 mRNA was decreased in the CA3 regions of the hippocampus (Simonyi et al. 2004). In addition, recent evidence indicates that the mGluR5 antagonist MPEP decreases relapse to alcohol self-administration in outbred Long–Evans rats (Backstrom et al. 2004) and in selectively bred alcohol-preferring P rats (Schroeder et al. 2005) and blocks the discriminative stimulus effects of ethanol (Besheer and Hodge 2005).
The goal of the present study was to characterize involvement of mGluRs in the reinforcing effects of ethanol. To accomplish this goal, we trained inbred C57BL/6J mice to self-administer ethanol on a concurrent fixed ratio 1 (CONC FR1) schedule of ethanol (10% v/v) vs water reinforcement during 16-h sessions. The effects of mGluR1, mGluR2/3, and mGluR5 antagonists were then assessed on various parameters of self-administration behavior. Results suggest that full expression of the reinforcing effects of ethanol requires mGlu5 receptor activity. Preliminary results of this study were presented at the annual meeting of the Research Society on Alcoholism (Sharko et al. 2002).
Materials and methods
Mice
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, N=32) were housed in standard Plexiglas cages (17.8×29.2×12.7 cm; W×L×H) with food and water always available. Mice were 20 weeks of age (body weight, 28–35 g) at the start of testing. Animal housing rooms were maintained on a 12:12 light–dark cycle (lights on at 0600 hours) at 22°C. All procedures were approved by the Institutional Animal Care and Use Committee and followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Ethanol self-administration
Apparatus
Ethanol self-administration sessions were conducted in eight Plexiglas operant-conditioning chambers (Med Associates, Georgia, VT) measuring 15.9×14×12.7 cm with stainless steel grid floors. Each chamber was housed in a sound-attenuating cubicle equipped with a house fan that provided ventilation and helped mask external noise. The left and right wall of each operant chamber was equipped with one ultrasensitive stainless steel response lever and a liquid delivery system. Liquid solutions (ethanol or water) were maintained in 60-ml syringes mounted on a programmable pump (PHM-100, Med Associates), which delivered 0.01 ml per activation into a stainless steel cup located to the left of the associated response lever. Each chamber also contained a houselight (illuminated between 1600–1800 hours and 0600–0800 hours) and a stimulus light located above each lever (which was activated each time the lever was pressed). The operant-conditioning chambers were interfaced (Med Associates) to an IBM-compatible PC, which was programmed to record all lever presses and liquid deliveries.
Self-administration procedure
Mice (n=24; 8 per antagonist) were trained to lever-press using reinforcement (10% sucrose w/v) of successive approximations. After initial response-shaping sessions, mice were run during 16-h overnight (1600–0800 hours) training sessions. During these training sessions, both response levers were active on a concurrent fixed ratio 1 (CONC FR1) schedule, with 10% sucrose vs water presented as the reinforcers. The position of each solution (left or right) was fixed for each animal but counterbalanced between animals to control for side preference. After 4 days, mice were trained to orally self-administer ethanol (10% v/v) vs water using a sucrose substitution procedure (Hodge et al. 1993a; Samson 1986), which we have adapted for use in the mouse (Besheer et al. 2004). Briefly, ethanol (2, 4, 8, or 10% v/v) was incrementally added to the sucrose (10% w/v) solution for 4 days at each increasing concentration. Afterwards, sucrose (10, 5, or 2% w/v) was incrementally faded out of the ethanol-containing solution with 4 days at each decreasing concentration. After sucrose-substitution training, all mice reliably responded on the CONC FR1 schedule of reinforcement with ethanol (10% v/v) vs water presented as the reinforcers.
After sucrose substitution, baseline behavior was established over a 6-week period, during which, mice were tested in overnight (16-h) sessions running from 1600–0800 hours as previously described (Schroeder et al. 2003). Mice were weighed immediately before being placed in the chambers to quantify and express the ethanol consumed (grams per kilogram) after each operant session. Sessions were conducted 4 days/week (i.e., Monday–Thursday evenings). Following each test session, mice were returned to the home cages. The 12-h light–dark cycle was maintained in the operant-conditioning chambers by regulation of the houselight (lights off at 1800 hours and lights on at 0600 hours).
Testing drug effects on mouse operant ethanol self-administration
After the 6-week baseline period, the effects of pre-session administration of mGluR antagonists were tested on parameters of operant ethanol self-administration. Mice were removed from home cages at approximately 1545 hours, weighed, administered a test drug, and placed immediately in the operant-conditioning chambers. Houselights were illuminated immediately, and operant sessions began. All drugs and doses were administered on Tuesday or Thursday in a randomized Latin-square manner to control for dose order effects. Control data from noninjection and vehicle sessions were obtained similarly as part of the randomized sequence. Each mGluR antagonist was examined in separate groups of mice (n=8 per group).
Locomotor activity
Potential locomotor effects of MPEP were examined in Plexiglas chambers (43 cm2) (Med Associates). Two sets of 16 pulse-modulated infrared photo beams were placed on opposite walls at 1-in. centers to record x–y ambulatory movements. Activity chambers were computer-interfaced (Med Associates) for data sampling at 100-ms resolution. Mice (n=8) were handled and weighed daily for 1 week before activity testing. Prior to each session, the activity chamber was wiped clean with 2.5% glacial acetic acid to limit any confounding odors. Saline or MPEP (10 mg/kg, i.p.) was administered, and mice were placed in the center of the chamber. Horizontal distance traveled (in centimeters) was recorded as an index of motor function.
Drugs
Ethanol solutions for self-administration were prepared by mixing appropriate volumes of ethanol (95% v/v) and distilled water. Sucrose (w/v) solutions consisted of granulated cane sugar dissolved in tap water. Ethanol, sucrose, and water solutions were presented at room temperature. The mGluR1 selective antagonist 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), mGluR5 selective antagonist 2-methyl-6-(phenylethynyl) pyridine (MPEP), and group II selective (mGluR2 and mGluR3) antagonist (2S)-2-amino-2-[(1S,2S)-2-carboxy-cycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY 341495) were obtained from Tocris (Ellisville, MO, USA). MPEP was dissolved in physiological saline (0.9%). CPCCOEt and LY 341495 were suspended in (2-hydroxypropyl)-β-cyclodextrin (20% w/v, Sigma, St. Louis, MO, USA) in distilled water. Drug and vehicle solutions were administered to mice in a volume of 0.01 ml/10 g body weight.
Data analyses
Measures of self-administration were response latency (i.e., time at which the first response occurred relative to the beginning of the session), the total number of ethanol- and water-reinforced lever presses, volume of ethanol and water consumed (milliliters), self-administration bouts, number of lever presses per bout, and rate of lever pressing during each bout (i.e., number of lever presses per bout divided by length of bout). Response bouts were defined as previously described by our group (Olive et al. 2000). Briefly, a bout was defined as self-administration of at least 0.2 g/kg of ethanol, with no two responses occurring more than 6 min apart. Ethanol intake (grams per kilogram) was estimated using each animal’s daily body weight and the volume of ethanol consumed. Drug dose effects were analyzed statistically by repeated-measures analysis of variance (ANOVA) followed by a Tukey’s post hoc multiple comparisons test using SigmaStat v. 3.0 (SPSS, Chicago, IL, USA). Planned comparisons were conducted with paired t test where indicated.
Results
Total ethanol-reinforced responding
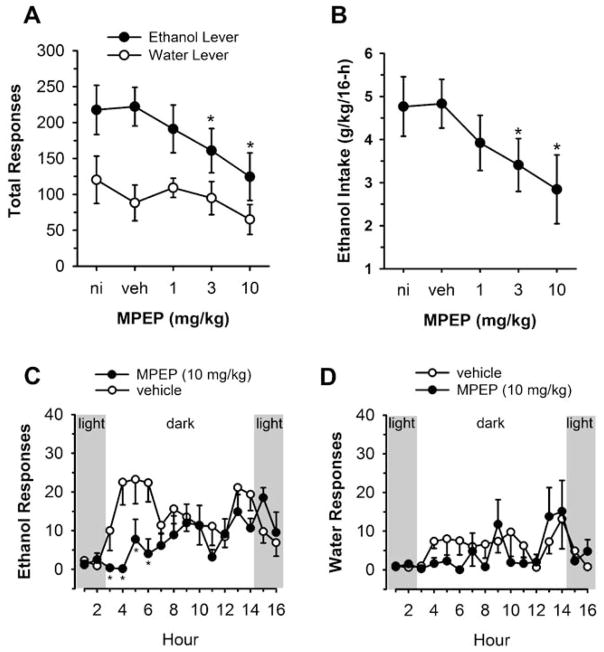
Systemic administration of the mGluR5 antagonist MPEP produced dose-dependent decreases in total operant ethanol self-administration by C57Bl/6J mice during the 16-h sessions (Fig. 1a). Two-way repeated-measures ANOVA showed a significant effect of reinforcer condition [F(1,7)= 5.8, p<0.05], which indicated increased self-administration of ethanol vs water. There was also an overall significant effect of MPEP dose [F(4,28)=13.7, p<0.001] on total responses. Planned comparisons (paired t test) showed that MPEP (3 or 10 mg/kg) produced dose-dependent reductions in responding as compared to vehicle control but had no effect on water-reinforced responses (MPEP 3 mg/kg, t=4.1, p=0.004; MPEP 10 mg/kg, t=4.2, p=0.002; Fig. 1a). Accordingly, analysis by one-way ANOVA confirmed that MPEP decreased [F(4,28)=7.6, p<0.001] the dose of self-administered ethanol (grams per kilogram) achieved during the total 16-h session (Fig. 1b). The mGluR1 antagonist CPCCOEt and the mGluR2/3 antagonist LY 341495 were both without effect on total ethanol- or water-reinforced responding or dosage of ethanol (grams per kilogram) that was self-administered (Table 1).
Fig. 1.
The mGluR5 antagonist MPEP significantly decreased the reinforcing function of ethanol. a Total number of ethanol- and water-reinforced responses plotted as a function of MPEP dosage. Asterisk indicates significantly different from vehicle (veh) control, paired t test planned comparison (p<0.05). b Ethanol intake (grams per kilogram per 16 h) plotted as a function of MPEP dosage. Asterisk significantly different from veh control (Tukey test, p<0.05). c Number of ethanol- or d water-reinforced responses following vehicle or MPEP (10 mg/kg) injection plotted as a function of time (hours). Shaded areas of the graphs indicate responding during the light portion of the diurnal cycle, and the unshaded area indicates responding during the 12-h dark phase. Asterisk significantly different from veh at the same time point (Tukey test, p<0.05). All data are plotted as mean±SEM
Table 1.
The mGluR1 antagonist CPCCOEt or the mGluR2/3 antagonist LY 341495 produced no significant differences in measures of ethanol self-administration at the doses tested
| Drug | Dosage (mg/kg, i.p.) |
|||||
|---|---|---|---|---|---|---|
| Ni | Veh | 1 | 3 | 10 | 30 | |
| CPCCOEt | ||||||
| EtOH responses | 246 (31) | 212 (26) | 261 (32) | 200 (49) | 258 (44) | – |
| H2O responses | 112 (33) | 115 (49) | 71 (22) | 128 (48) | 88 (39) | – |
| EtOH (g/kg) | 5.0 (0.6) | 4.3 (0.5) | 5.2 (0.6) | 4.0 (0.9) | 5.3 (0.9) | – |
| LY 341495 | ||||||
| EtOH responses | 208 (36) | 209 (28) | 217 (41) | 192 (29) | 222 (33) | 165 (31) |
| H2O responses | 109 (23) | 78 (18) | 164 (26) | 143 (21) | 107 (12) | 87 (17) |
| EtOH (g/kg) | 4.6 (0.8) | 4.5 (0.6) | 4.8 (0.9) | 3.9 (0.6) | 4.4 (0.5) | 3.4 (0.6) |
Data are expressed as mean (±SEM)
Temporal pattern of ethanol-reinforced responding
Analysis of the temporal distribution of alcohol self-administration showed that ethanol responses peaked once during the first half (hours 4–6) and once during the second half (hours 13–14) of the dark cycle under control conditions (Fig. 1c, open circles). Two-way repeated-measures ANOVA on ethanol response patterns found significant main effects of MPEP Dose [F(3,21)=11.1, p<0.001] and Hour [F(15,105)=4.3, p<0.001]. In addition, the MPEP Dose×Hour interaction was statistically significant [F(45,315)=3.2, p<0.001], which suggests that the effects of MPEP depended on the hour at which responding occurred. Multiple comparison procedure showed that MPEP (10 mg/kg) significantly decreased ethanol-reinforced responding during the initial period of the dark cycle (hours 2–6) of the 16-h session (Fig. 1c, closed circles). The low dose of MPEP (1 mg/kg) significantly decreased ethanol-reinforced responding only at hour 14 (data not shown). Likewise, MPEP (3 mg/kg) significantly decreased ethanol-reinforced responding only at hour 13 but produced marginal reductions at hours 4, 8, and 10, which contributed to the overall reduction in total responding (data not shown). Water-reinforced responding was not significantly altered by MPEP at any dose or time point tested (i.e., Fig. 1d), confirming the total session analysis. The mGluR1 or mGluR2/3 antagonist produced no changes in the temporal pattern of ethanol- or water-reinforced responding.
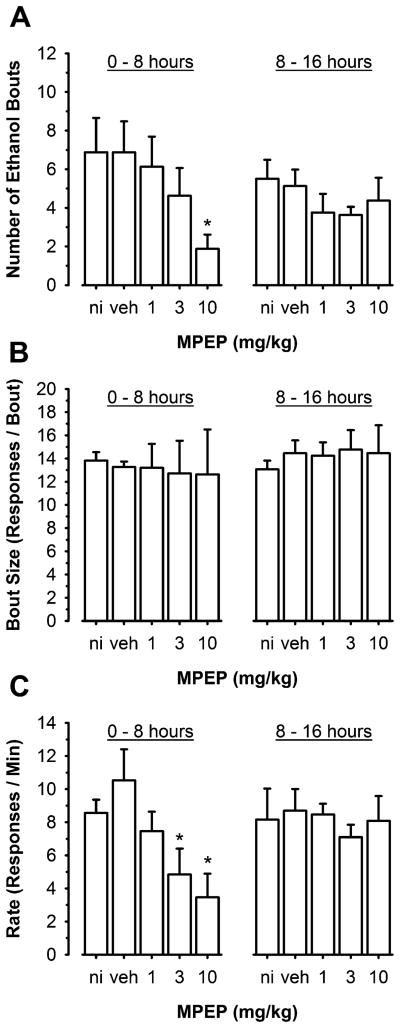
Effects of MPEP on ethanol-reinforced response bouts
Since the primary effects of the mGluR5 antagonist MPEP (10 mg/kg) were observed during the first 8 h of the session, analysis of response bouts (see “Materials and methods” for the definition of a bout) was conducted during two temporal epochs (0–8 and 8–16 h). Repeated-measures ANOVA found a significant effect of MPEP dose [F(4,28)= 6.1, p=0.001] on the number of ethanol-reinforced response bouts. Although there was no significant effect of time, the effects of MPEP depended on time, as indicated by the significant Dose × Time interaction [F(4,28)=2.8, p=0.04]. Multiple comparison procedures (Tukey test) showed that MPEP (10 mg/kg) decreased the number of bouts relative to both no-injection and saline controls only during the first 8 h of the session (p<0.05, Fig. 2a). This decrease in the number of instances of ethanol self-administration was not accompanied by a change in bout size (Fig. 2b). However, MPEP dose-dependently [F(4,28)=5.5, p=0.002] decreased response rate during bouts. The effect of MPEP depended on time, as indicated by the significant Dose × Time interaction [F(4,28)=2.7, p=0.048] Tukey tests showed that both the 3- and 10-mg/kg doses of MPEP significantly decreased response rate relative to control (Fig. 2c).
Fig. 2.
The mGluR5 antagonist MPEP significantly altered parameters of ethanol-reinforced response bouts. a Total number of ethanol response bouts plotted as a function of MPEP dosage. b Total number of responses per bout plotted as a function of MPEP dosage. c Response rate (responses/minute) per bout plotted as a function of MPEP dosage. All data are plotted as mean±SEM during the first and second 8-h periods of the sessions. Asterisk significantly different from no-injection (ni) and vehicle (veh) control (Tukey test, p<0.05)
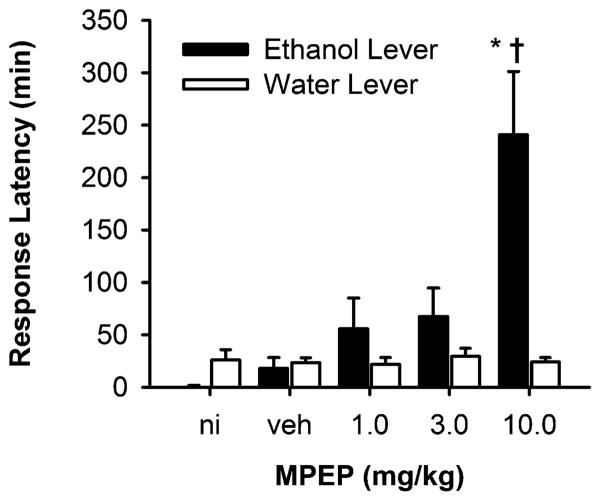
Onset of ethanol-reinforced responding
The onset of ethanol-reinforced responding (i.e., the latency to the first response) was selectively (as compared to water) delayed by the mGluR5 antagonist MPEP (Fig. 3). Statistically significant main effects were observed for MPEP Dose [F(4,28)=8.3, p<0.001] and Reinforcer (ethanol vs water) [F(1,7)=12.1, p=0.01]. In addition, the Dose × Reinforcer interaction was significant [F(4,28)= 7.5, p<0.001], indicating that the effects of MPEP Dose depended on the reinforcement condition. Multiple comparison procedures (Tukey test) indicated that MPEP (10 mg/kg) significantly increased the latency to the first ethanol-reinforced response in the absence of any effect on the onset of water-reinforced responding (Fig. 3). The range of latencies observed after MPEP (10 mg/kg) was 29–489 min, with five of eight mice showing values greater than the mean of 240.8 min. The mGluR1 antagonist CPCCOEt and the mGluR2/3 antagonist LY 341495 were both without effect on the latency to the first ethanol- or water-reinforced response.
Fig. 3.
The mGluR5 antagonist MPEP selectively increased the latency to the first ethanol-reinforced response. Response latency (minutes) is plotted as a function of MPEP dosage. Data represent mean±SEM. Asterisk significantly different from no-injection (ni) and vehicle (veh) control; dagger significantly different from water at the same dose of MPEP (Tukey p<0.05)
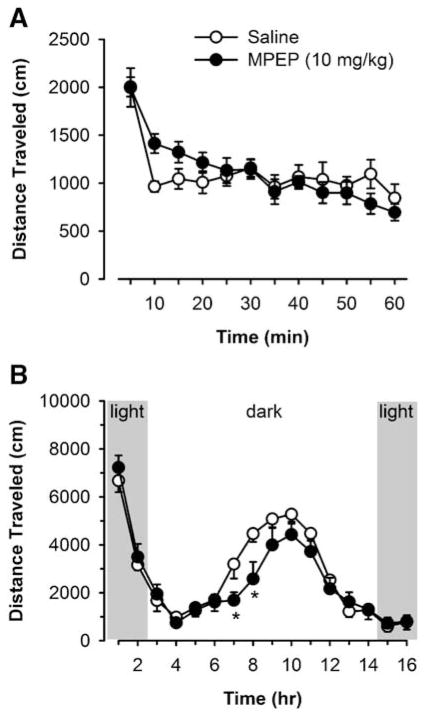
Locomotor activity
To determine if reductions in ethanol-reinforced responding were associated with the inhibition of motor ability, the effects of MPEP (10 mg/kg) were tested on locomotor activity in two separate experiments. First, mice were administered MPEP (10 mg/kg) and placed in locomotor activity chambers after a 30-min delay. Distance traveled (in centimeters) during a 1-h test period decreased as a function of time [F(11,156)= 13.2, p<0.001], indicating normal spontaneous activity and habituation to the environment. However, MPEP produced no statistically significant effect on motor activity at any time point (Fig. 4a). Second, mice were administered MPEP (10 mg/kg) 2 h prior to the onset of the dark cycle and were immediately placed in locomotor-activity chambers for a 16-h test session, which corresponds with the full self-administration test sessions. Distance traveled varied as a function of time [F(15,195)=45.5, p<0.001], again indicating normal spontaneous activity and habituation to the environment. Although there was no significant effect of MPEP alone, MPEP did produce time-dependent decreases in locomotor activity, as indicated by a significant Drug × Time interaction [F(15,195)=1.8, p=0.04]. Post hoc comparisons within each hour showed that MPEP significantly decreased motor activity during hours 7 and 8 of the 16-h session (Tukey, p<0.05; Fig. 4a), which did not correspond with the time course of effects on self-administration behavior.
Fig. 4.
Effects of the mGluR5 antagonist MPEP on spontaneous locomotor activity and habituation. a Effects of MPEP (10 mg/kg)-administered 3 h prior to a 1-h locomotor test session. b Effects of MPEP (10 mg/kg) administered immediately prior to a 16-h locomotor test session. Data represent mean±SEM distance traveled (cm). Asterisk indicates significantly different from saline at the corresponding time point
Discussion
The main finding of the present study is that the mGluR5 antagonist MPEP decreased the reinforcing effects of ethanol in alcohol-preferring inbred C57BL6/J mice. The mGluR1 antagonist CPCCOEt or the mGluR2/3 antagonist LY 341495 were without effect on ethanol-reinforced responding. These data are consistent with emerging evidence implicating mGluR5 in the general regulation of the reinforcing effects of drugs of abuse. For example, mice lacking the mGluR5 gene do not self-administer cocaine and show no cocaine-induced increase in locomotor activity (Chiamulera et al. 2001), which indicates a significant role of mGluR5 in the behavioral effects of psychomotor stimulants. MPEP dose-dependently reduced nicotine self-administration in rats (Paterson et al. 2003). Recent evidence also indicates that MPEP decreases ethanol self-administration and blocks relapse to ethanol self-administration in rats (Backstrom et al. 2004; Schroeder et al. 2005). Together, these data suggest that mGlu5 receptor activity is required for the full expression of cocaine, nicotine, and ethanol reinforcement in both rats and mice, which demonstrates interspecies generality of mGluR5 involvement in addiction.
Results from the present study also suggest that mGluR5 modulation of ethanol reinforcement is specific. That is, MPEP decreased ethanol self-administration in the absence of effect on concurrent water-reinforced responding. This is consistent with MPEP reductions in nicotine self-administration, with no concomitant effect on food-maintained responding (Markou et al. 2004; Paterson et al. 2003; Tessari et al. 2004) and evidence that mGluR5 null mice respond for food reinforcement (Chiamulera et al. 2001). This interpretation warrants caution, however, because ethanol- and water-reinforced response rates were significantly different, which may reflect differential reinforcement function and sensitivity to mGluR5 modulation. In addition, mGluR5 antagonists decrease food-reinforced responding (Paterson and Markou 2005; Varty et al. 2005), and caloric content influences ethanol self-administration by C57BL/6NHsd (McMillen and Williams 1998) and C57BL/6ByJ (Bachmanov et al. 1996a; Bachmanov et al. 1996b) mice, which suggests that observed effects of MPEP on ethanol self-administration may have been influenced by the caloric properties of ethanol. That said, MPEP also decreases ethanol- but not water-reinforced responding in selectively bred alcohol-preferring P rats (Schroeder et al. 2005), which are considered a valid animal model of alcoholism because their ethanol intake does not appear to be influenced by taste or caloric factors (Lankford et al. 1991; McMillen 1997). Together, these data suggest that mGluR5 activity selectively regulates drug and ethanol reinforcement when alternative reinforcers are available but may also play a more general role in motivation.
In addition to effects on the total number of ethanol reinforced responses, the mGluR5 antagonist MPEP produced specific changes in the temporal distribution of responding that suggest reduced motivation to self-administer ethanol. First, MPEP (10 mg/kg) produced a 13-fold delay in the onset of the first ethanol-reinforced response, which is consistent with reduced motivation to start drinking (Schroeder et al. 2003). Second, ethanol-reinforced responding is well known to occur in temporally contiguous units called “bouts.” This is observed in primates (Henningfield and Meisch 1979), rats (Samson et al. 1988), and mice (Olive et al. 2000). In the present study, MPEP (10 mg/kg) decreased the total number of ethanol-reinforced bouts and the rate of responding during each bout. Thus, mice engaged in fewer temporally clustered units of self-administration behavior, and responding during each cluster was slower following MPEP injection. When taken together with the significant delay in response onset, these data are consistent with reduced motivation to self-administer ethanol.
However, a number of interpretations other than altered motivation are also plausible. First, MPEP may have altered ethanol-reinforced responding via nonspecific effects on motor ability. For the most part, however, locomotor data from the present study argue against this interpretation because the mGluR5 antagonist did not alter motor activity during the first 6 h of a 16-h locomotor session, which is when the compound reduced ethanol self-administration. A second alternative interpretation is that MPEP may have altered the rewarding effects of ethanol or produced independent rewarding or aversive effects that decreased ethanol self-administration. This possibility also appears unlikely because MPEP does not alter ethanol-induced conditioned place preference (CPP) or produce a CPP or conditioned place aversion when administered alone (McGeehan and Olive 2003; Popik and Wrobel 2002). Third, a plausible alternative explanation to altered motivation is that MPEP may have altered the pharmacological effects of ethanol that underlie its reinforcing function. Research from our laboratory has shown that MPEP blocks the discriminative (i.e., subjective) stimulus effects of ethanol (Besheer and Hodge 2005), which are fundamental to addiction liability (Stolerman 1992). Thus, MPEP may have blocked the discriminative stimulus effects of self-administered ethanol, which could functionally shift the concentration response curve for ethanol reinforcement to the left and occasion less responding. It would be of significant interest to determine in future studies if MPEP can alter the discriminative stimulus effects of self-administered ethanol (e.g., Hodge et al. 2001), which would suggest a highly specific behavioral mechanism of action.
Data from the present experiment show that mGluR1 or mGluR2/3 antagonists were without effect on ethanol- or water-reinforced responding. This result is consistent with recent evidence showing that glutamate activity at mGluR5, but not mGluR1 or mGluR2/3, regulates the reinforcing effects of ethanol in selectively bred alcohol-preferring P rats (Schroeder et al. 2005), which raises the possibility that mGluR regulation of ethanol reinforcement is specific to the mGluR5 subtype. However, reduced potency of the mGluR1 antagonist at its receptor should be considered as an alternative explanation for lack of effect of CPCCOEt. Future studies employing different antagonists, doses, or agonist compounds may find functional involvement of mGluR1 or mGluR2/3 receptors in ethanol reinforcement. At the present time, however, the results of this study suggest both reinforcer and receptor subtype specificity in the modulation of ethanol reinforcement by mGluR5.
It is of significant interest to consider why the mGluR antagonists showed differential involvement in ethanol reinforcement. Although group I mGluRs (mGluR1 and mGluR5) are highly related, these receptors have a distinct pattern of expression in the brain that may determine functional involvement in ethanol self-administration. MGluR1 receptors show low expression in most limbic brain regions but are highly expressed in the cerebellum where it regulates motor coordination (Ichise et al. 2000). MGluR5 (and mGluR2/3) receptors are almost completely absent in the cerebellum, but are highly expressed in limbic brain regions such as the nucleus accumbens, cortex, and hippocampus, where mGluR1 receptors are less abundant (Bordi and Ugolini 1999; Spooren et al. 2001; Tamaru et al. 2001). This differential mesocorticolimbic distribution pattern suggests differential involvement in ethanol self-administration. For example, evidence from site-specific infusion studies shows ethanol self-administration is modulated by the activity of specific brain regions, including the ventral tegmental area, nucleus accumbens, and frontal cortex (Hodge et al. 1995, 1996a,b, 1997, 1993b, 1994; Rassnick et al. 1992; Samson et al. 1993) that express high levels of mGluR5 and mGluR2/3. Thus, based on expression patterns, it is plausible that mGlu5 receptors might specifically regulate ethanol reinforcement, but mGluR1 might not.
Given the similar expression patterns of mGlu2/3 and mGlu5 receptors, it does not seem likely that the differential effect of antagonists at these two receptors can be attributed to brain regional influence. However, evidence suggests that these receptors may be predominantly expressed at different cellular locations. For example, mGluR5 immunoreactivity is predominantly postsynaptic (Paquet and Smith 2003), whereas mGlu2/3 receptors are mostly presynaptic (Tamaru et al. 2001). Thus, the mGluR5 antagonist may have inhibited primarily the postsynaptic effects of glutamate (e.g., O’Leary et al. 2000), which appeared to interfere with ethanol reinforcement. By contrast, the pharmacological blockade of presynaptic mGlu2/3 receptors increases extracellular glutamate levels (Baker et al. 2002), which did not alter ethanol reinforcement. Therefore, the actions of glutamate at postsynaptic mGlu5 receptors appear to be necessary for full expression of ethanol reinforcement. Moreover, it appears plausible that decreasing synaptic glutamate levels via administration of an mGluR2/3 agonist might also decrease ethanol reinforcement, which has been shown to decrease reinstatement of cocaine self-administration (Baptista et al. 2004).
The data from this study suggest that mGluR5 activity is required for the full expression of ethanol’s reinforcing effects. However, the long time course of MPEP effect on ethanol self-administration (i.e., up to 6 h postadministration) indicates that the observed behavioral effects are not correlated with direct pharmacological blockade of the receptor. That is, in vivo occupancy of mGluR5 by MPEP (10 mg/kg) decreases linearly to control levels after 2 h, with an approximate half-life of 1 h in C57BL/6J mice (Anderson et al. 2003). This suggests that MPEP administration may have altered the activity of signaling pathway (s) downstream of mGluR5 that inhibited ethanol self-administration during the effective period of 3–6 h postadministration. Indeed, activation of postsynaptic mGluR5 up-regulates phospholipase C (PLC) activity, which in turn activates the second messenger diacylglycerol (DAG) (Linn 2000; Pellegrini-Giampietro et al. 1996). DAG activates protein kinase C (PKC), which can increase Ca2+ influx via phosphorylation of ionotropic NMDA receptors (MacDonald et al. 1998), which influence ethanol intake (McMillen et al. 2004). Accordingly, we have shown that gene deletion of PKCε, which is DAG-sensitive, decreases ethanol self-administration (Hodge et al. 1999), and that the effects of MPEP on two-bottle ethanol intake are dependent on PKCε (Olive et al. 2005). Together, these findings suggest that MPEP administration may produce long-term reductions in alcohol self-administration by changing the function of mGluR5 coupled signaling pathways.
In conclusion, many studies have focused on iGluR involvement in ethanol’s neurobiological effects, but the clinical utility of manipulating iGluRs remains illusive due to numerous side effects associated with their widespread central nervous system (CNS) expression (Kemp and McKernan 2002). MGluRs are rapidly emerging as important therapeutic targets in numerous areas of neuropathology (Bordi and Ugolini 1999; Spooren et al. 2001), but few studies have addressed their involvement in ethanol’s reinforcing effects. The results of this preclinical study indicate that the mGluR5 antagonist MPEP decreases the reinforcing effects of ethanol and may therefore have utility in the medical management of alcohol abuse and alcoholism, insomuch as reduced drinking is a viable therapeutic outcome.
Acknowledgments
This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism (AA014983 and AA011605) to C.W.H.
Contributor Information
Clyde W. Hodge, Email: chodge@med.unc.edu, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA, Tel.: +1-919-8434823, Fax: +1-919-9665679
Michael F. Miles, Departments of Pharmacology/Toxicology and Neurology, Virginia Commonwealth University, P.O. Box 980599, Rm. 630, 1217 E. Marshall St., Richmond, VA 23298, USA
Amanda C. Sharko, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA
Rebekah A. Stevenson, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA
Jennie R. Hillmann, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA
Veronique Lepoutre, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA.
Joyce Besheer, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA.
Jason P. Schroeder, Department of Psychiatry, Bowles Center for Alcohol Studies School of Medicine, CB#7178, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178, USA
References
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Mutkus L, Allen JW. Aspartate and glutamate transport in acutely and chronically ethanol exposed neonatal rat primary astrocyte cultures. Neurotoxicology. 2001;22:601–605. doi: 10.1016/s0161-813x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996a;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996b;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl) 2004;174:358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Colombo G, Grant KA. NMDA receptor complex antagonists have ethanol-like discriminative stimulus effects. Ann N Y Acad Sci. 1992;654:421–423. doi: 10.1111/j.1749-6632.1992.tb25986.x. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Gereau RWt, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Theiss C, Crews FT. Effects of ethanol on stimulated inositol phospholipid hydrolysis in rat brain. J Pharmacol Exp Ther. 1986;237:92–98. [PubMed] [Google Scholar]
- Grant KA, Colombo G. Pharmacological analysis of the mixed discriminative stimulus effects of ethanol. Alcohol Alcohol Suppl. 1993;2:445–449. [PubMed] [Google Scholar]
- Gruol DL, Parsons KL, DiJulio N. Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res. 1997;773:82–89. doi: 10.1016/s0006-8993(97)00912-8. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Meisch RA. Ethanol drinking by rhesus monkeys with concurrent access to water. Pharmacol Biochem Behav. 1979;10:777–782. doi: 10.1016/0091-3057(79)90332-0. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993a;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS, Erickson HL. Specific decreases in ethanol- but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205-930. Alcohol. 1993b;10:191–196. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Tolliver GA, Haraguchi M. Effects of intraaccumbens injections of dopamine agonists and antagonists on sucrose and sucrose–ethanol reinforced responding. Pharmacol Biochem Behav. 1994;48:141–150. doi: 10.1016/0091-3057(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996a;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Chappelle AM, Samson HH. Effects of ventral tegmental microinjections of the GABAA agonist muscimol on self-administration of ethanol and sucrose. Pharmacol Biochem Behav. 1996b;53:971–977. doi: 10.1016/0091-3057(95)02146-9. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacology (Berl) 2001;154:13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;(Suppl 5):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Linn CL. Second messenger pathways involved in up-regulation of an L-type calcium channel. Vis Neurosci. 2000;17:473–482. doi: 10.1017/s0952523800173134. [DOI] [PubMed] [Google Scholar]
- Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S. Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol Clin Exp Res. 2001;25:132S–136S. doi: 10.1097/00000374-200105051-00023. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Xiong XG, Lu WY, Raouf R, Orser BA. Modulation of NMDA receptors. Prog Brain Res. 1998;116:191–208. doi: 10.1016/s0079-6123(08)60438-0. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci. 2004;1025:491–503. doi: 10.1196/annals.1316.061. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- McMillen BA. Toward a definition of a valid model of alcoholism: multiple animal models for multiple diseases. Alcohol. 1997;14:409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Williams HL. Role of taste and calories in the selection of ethanol by C57BL/6NHsd and Hsd:ICR mice. Alcohol. 1998;15:193–198. doi: 10.1016/s0741-8329(97)00111-0. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Joyner PW, Parmar CA, Tyer WE, Williams HL. Effects of NMDA glutamate receptor antagonist drugs on the volitional consumption of ethanol by a genetic drinking rat. Brain Res Bull. 2004;64:279–284. doi: 10.1016/j.brainresbull.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW, 4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Parsons KL, Sweeney DD, Gruol DL. Metabotropic glutamate receptor agonists alter neuronal excitability and Ca2+ levels via the phospholipase C transduction pathway in cultured Purkinje neurons. J Neurophysiol. 1997;78:63–75. doi: 10.1152/jn.1997.78.1.63. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL. Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcohol Clin Exp Res. 2002;26:386–393. [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol. 1996;118:1035–1043. doi: 10.1111/j.1476-5381.1996.tb15503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi K, Haraguchi M. Relation of ethanol self-administration to feeding and drinking in a nonrestricted access situation in rats initiated to self-administer ethanol using the sucrose-fading technique. Alcohol. 1988;5:375–385. doi: 10.1016/0741-8329(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Iller KA, Hodge CW. Neuropeptide-Y Y5 receptors modulate the onset and maintenance of operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1912–1920. doi: 10.1097/01.ALC.0000098873.80433.BA. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Iller K, Koenig H, Lau K, Ou CJ, Camarini R, Hodge CW. Involvement of metabotropic glutamate receptor subtype 5 (mglu5) in alcohol self-administration. Alcohol Clin Exp Res. 2002;26 (Suppl 5):112A. [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport. 1996;7:2115–2118. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13:170–176. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Ethanol, sedative hypnotics, and glutamate receptor function in brain and cultured cells. Behav Genet. 1993;23:231–236. doi: 10.1007/BF01067428. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Weight FF, Peoples RW, Wright JM, Lovinger DM, White G. Ethanol action on excitatory amino acid activated ion channels. Alcohol Alcohol Suppl. 1993;2:353–358. [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int. 1999;35:107–113. [PubMed] [Google Scholar]