Abstract
The role of platelets in hemostasis may be influenced by alteration of the platelet redox state—the presence of antioxidants and the formation of reactive oxygen and nitrogen species. We investigated the effects of two antioxidants, resveratrol and trolox, on platelet activation. Trolox and resveratrol inhibited aggregation of washed platelets and platelet-rich plasma activated by ADP, collagen, and thrombin receptor-activating peptide. Resveratrol was a more effective agent in reducing platelet static and dynamic adhesion in comparison with trolox. The antioxidant capacity of resveratrol was, however, the same as that of trolox. After incubation of platelets with antioxidants, the resveratrol intraplatelet concentration was about five times lower than the intracellular concentration of trolox. Although both antioxidants comparably lowered hydroxyl radical and malondialdehyde production in platelets stimulated with collagen, TxB2 levels were decreased by resveratrol much more effectively than by trolox. Cyclooxygenase 1 was inhibited by resveratrol and not by trolox. Our data indicate that antioxidants, apart from nonspecific redox or radical-quenching mechanisms, inhibit platelet activation also by specific interaction with target proteins. The results also show the importance of studying platelet activation under conditions of real blood flow in contact with reactive surfaces, e.g., using dynamic adhesion experiments.
Keywords: Platelet, Adhesion, Aggregation, Resveratrol, Trolox, Free radicals
Platelets exert a crucial function in hemostasis, wound repair, and the formation of vascular plugs, underlying thrombotic diseases such as stroke and myocardial infarction. Exposure to the subendothelial matrix induces rapid platelet activation giving rise to formation of a vascular plug and release of stimulatory molecules that initiate the repair process [1,2]. The activation of platelets is regulated and modulated by numerous relatively well-characterized factors, including ADP, serotonin, and thromboxane A2 (TxA2)1, which are released from activated platelets and further potentiate platelet aggregation [3]. Because platelets perform their functions mainly at high shear, the availability of the cone and plate(let) analyzer makes it possible to measure platelet adhesion in whole blood, under physiologic blood flow conditions [4–6].
Intracellular signaling, which is necessary for platelet cytoskeletal reorganization and granule secretion, includes the phosphoinositide hydrolysis pathway [7,8], the eicosanoid synthesis pathway [9], etc. The formation of eicosanoids from arachidonic acid catalyzes cyclooxygenase 1 (COX-1) and COX-2. COX-1 is involved in platelet functions; COX-2 is primarily present at sites of inflammation [9]. Several publications have suggested that reactive oxygen species (ROS) represent a new modulator of platelet activity. It has been shown that either exogenous or platelet-derived ROS have influences on platelet function [10]. The release of several ROS, including O2·−, HO·, and H2O2, from platelets was reported [11], both from resting platelets and after platelet stimulation with agonists such as collagen [12,13] or thrombin [14].
There is mounting evidence that antioxidants modulate platelet activation [15]. The effects of antioxidant supplementation on platelet function in vivo are still controversial. Nevertheless in vitro-added antioxidants, such as vitamin C, vitamin E, resveratrol, flavonoids, and others, generally attenuate platelet activation either by ROS quenching or by substance-specific mechanisms [16–18].
In this study, we concentrated our experimental effort on two antioxidants: trolox, a vitamin E analogue, and resveratrol. Resveratrol (trans-3,4′,5-trihydroxystilbene), a naturally occurring hydroxystilbene, is contained in red wine and possesses chemopreventive properties and also cytostatic activities. It has been published that resveratrol inhibits platelet activation [19–21]. The inhibitory effects of resveratrol possibly involve inhibition of the p38 MAP kinase, cytosolic phospholipase A2, arachidonic acid, Ca2+ cascade and activation of NO/cyclic GMP, inhibition of phospholipase C, and protein kinase C activation [22].
There are a lot of papers describing the influence of vitamin E on blood platelets [23–25] but information that trolox (stable analogue of vitamin E) is able to inhibit platelet aggregation is meager [26,27].
The aim of this study was to determine the effects of either resveratrol or trolox on normal washed platelet functions assessed by platelet aggregation induced by collagen and platelet adhesion. In particular, the relationship between the antioxidant capacity of the antioxidants, their bioavailability in platelets, the concentration of hydroxyl radicals produced during platelet activation, malondialdehyde (MDA) and thromboxane B2 (TxB2) formation, and the tendency for platelets to aggregate and to adhere under static or dynamic conditions were investigated.
Methods
Materials
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), 3,4′,5-trihydroxystilbene (resveratrol), adenosine 5′-diphosphate (ADP), thrombin receptor-activating peptide SFLLRNP (TRAP), prostaglandin E1, Triton X-100, p-nitrophenyl phosphate, human fibrinogen, acetonitrile, ethanol and methanol (HPLC grade), myoglobin, 2,2′-azinobis(3-ethylbezothiazoline-6-sulfonic acid) (ABTS), sodium salicylate, 2,3-dihydroxybenzoic acid (2,3-DHB), and 2,5-dihydroxybenzoic acid (2,5-DHB) were from Sigma–Aldrich Prague (Czech Republic); collagen was from Bio/Data (USA); Sephadex G-10 was from Pharmacia (Switzerland); and bovine serum albumin (BSA) was from Imuna (Slovakia). Other chemicals were purchased from Lachema (Czech Republic) and were of analytical grade.
Preparation of blood platelets and their incubation with antioxidants
Blood was drawn from healthy volunteers who had not ingested an acetylsalicylic acid-containing drug for at least 2 weeks. All individuals tested agreed to this study at the time of blood collection. The study was approved by the Institute of Hematology and Blood Transfusion Ethics Committee and all samples were obtained in accordance with the regulations of the ethical commission of the institute.
Platelet-rich plasma (PRP) was prepared from blood (9 ml) that was drawn by venipuncture into 1 ml of 3.8% trisodium citrate (w/v). PRP was obtained by centrifugation of blood at 250 g at 37°C for 15 min and the platelet count in the PRP was estimated by a blood counter (Coulter Counter Onyx, Coultronix, France). Platelet-poor plasma was obtained by centrifugation of blood or PRP at 1400 g at 25°C for 10 min. The platelet count of the PRP was adjusted with Platelet poor plasma (PPP) to 200,000 platelets/μl.
Washed blood platelets were isolated by differential centrifugation of blood collected into citric acid/citrate/dextrose solution at 8.1:1.9 (v/v). Briefly, PRP was obtained by centrifugation of blood at 250 g at 37°C for 15 min. The PRP, after the addition of prostaglandin E1 (1 μM, final concentration), was incubated in a water bath at 37°C for 10 min and centrifuged at 1000 g at 37°C for 10 min. The platelet pellet was resuspended in modified (Ca2+ free) Tyrode’s buffer, pH 6.2, in the presence of 1 μM prostaglandin E1 and centrifuged at 600 g at 37°C for 10 min. The platelets were finally resuspended in Tyrode’s buffer, pH 7.4, with fibrinogen (final concentration 0.1% (w/v)) to a concentration of 200,000 platelets/μl, equilibrated for 30 min at 37°C, and used for experiments within 1 h.
Platelet suspensions were incubated at 37°C with various final concentrations of either resveratrol (0.300, 0.150, 0.075, 0.038, 0.019, 0.009, 0.005, and 0.002 mM) or trolox (4.20, 3.15, 2.10, 1.05, 0.53, 0.26, and 0.13 mM) for 30 min. Antioxidants were diluted in ethanol. The control samples were treated under the same conditions with a final concentration of 0.6% ethanol (v/v) without any antioxidant [23].
Measurement of platelet aggregation
Platelet aggregation was studied in platelet suspensions turbidimetrically [28] using a Chrono-Log aggregometer (Chrono-Log, Havertown, PA, USA). Tests were performed at 37°C in cuvettes stirred at 1000 rpm. Two hundred fifty microliters of sample was stimulated with ADP (0.01 mM final concentration), TRAP (0.01 mM final concentration), or collagen (18.8 μg/ml final concentration). The concentrations of agonists were the lowest ones sufficient to induce a full platelet aggregation response (from five independent experiments). The final percentage of aggregation was recorded at 6 min. Percentage inhibition was calculated using the aggregation response of a sample containing 0.6% ethanol (v/v, final concentration) as a control.
Trolox equivalent antioxidant capacity assay—measurement of resveratrol and trolox antioxidant capacity
The antioxidant activity of vitamins was measured by the trolox equivalent antioxidant capacity assay and the results were expressed in trolox equivalents (TEAC; the millimolar concentration of a trolox solution having the antioxidant capacity equivalent to a 1.0 mM solution of the substance under investigation) [29,30]. The TEAC value is based on the ability of the antioxidant to scavenge the blue-green ABTS·+ radical cation relative to the ABTS·+ radical cation scavenging ability of the water-soluble vitamin E analogue, trolox. The assay was slightly modified and performed in microplates. Briefly, in a separate Eppendorf tube, 8.4 μl of either tested antioxidant (1 mM antioxidant solution in PBS buffer) or trolox solution was thoroughly mixed with 36 μl of 70 μM metmyoglobin and 300 μl of 500 μM ABTS. The solution was transferred in three 70-μl aliquots to microplate wells and brought to 35°C in an ELISA reader for 5 min. The production of ABTS·+ was started by the addition of 130 μl of freshly prepared and prewarmed (5 min, 35°C) solution of 100 μM hydrogen peroxide. All substances were dissolved in PBS buffer (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 · 12 H2O, 1.5 mM KH2PO4, pH 7.4). The microplate was read at 15-s intervals with a microplate reader at 750 nm for 8 min. A calibration curve based either on absorbance values or on lag phase was constructed.
Measurement of resveratrol concentration in platelets
The platelet suspension incubated with resveratrol (0.3 mM, final concentration) was briefly washed several times in Tyrode’s buffer. Fifty microliters of washed platelet suspension was vortexed with 10 μl of methanol, 25 μl of Na2HPO4 (0.25 mM), and 600 μl of ethyl acetate. The organic phase was separated using a centrifuge, dried out, and dissolved in 200 μl of mobile phase (acetonitrile, 25 mM Na2HPO4, pH 4.2, 30:70, v/v). Forty microliters was injected onto an SGX C18 column, 150×3 mm, 5 μm (Tessek, Prague, Czech Republic). The chromatography was performed isocratically with a flow rate of 0.5 ml/min at room temperature using UV detection at 310 nm.
Measurement of trolox concentration in platelets
The platelet suspension incubated with trolox (4.2 mM, final concentration) was briefly washed several times in Tyrode’s buffer. Two hundred microliters of washed platelet suspension was vortexed with 200 μl of acetonitrile and centrifuged. Five microliters of supernatant was injected onto an SGX C18 column, 150×3 mm, 5μm (Tessek). The mobile phase consisted of methanol and 30 mM H3PO4 adjusted to pH 3 with NaOH (58:42, v/v). The chromatography was performed isocratically with a flow rate of 0.5 ml/min at room temperature using UV detection at 290 nm.
Measurement of OH· production in collagen-activated platelets
The production of OH· by platelets was determined by incubation of washed platelets with 5 mM salicylic acid and measurement of its hydroxylated products 2,3-DHB and 2,5-DHB as previously described [31,32]. Briefly, a platelet suspension (250 μl) in the presence of either resveratrol or trolox was incubated for 3 min with the sodium salt of salicylic acid (5 mM final concentration) and subsequently 10 min with collagen (37°C, stirred at 1000 rpm). The concentration of collagen was 18.8 μg/ml. The sample was transferred into an Eppendorf tube containing 250 μl of acetonitrile and 12.5 μl of HCl (1 M) and vortexed. One hundred microliters of the centrifuged supernatant was added to 400 μl of 50 mM CH3COONa, 50 mM citric acid, pH 2.5. Two hundred microliters of the sample was injected onto a Nucleosil C18 column, 125×3 mm, 5 μm (Macherey-Nagel, Düren, Germany). The mobile phase consisted of methanol and 50 mM CH3COONa, 50 mM citric acid, pH 2.5 (25:75, v/v). The chromatography was performed isocratically with a flow rate of 0.4 ml/min at room temperature using a Coulochem II electrochemical detector (ESA, Chelmsford, MA, USA; guard cell U=800 mV, electrode U=250 mV, 20 nA).
Measurement of thromboxane B2 concentration in collagen-activated platelets
Thromboxane A2 is spontaneously and rapidly transformed into thromboxane B2 [33]. Platelet suspensions with either resveratrol or trolox (250 μl) were incubated for 10 min with collagen (37°C, stirred at 1000 rpm). The concentration of collagen was 18.8 μg/ml. TxA2 production was determined using a thromboxane B2 ELISA kit (Cayman Chemicals, Ann Arbor, MI, USA).
Measurement of malondialdehyde concentration in collagen-activated platelets
A platelet suspension with either resveratrol or trolox (250 μl) was incubated for 10 min with collagen (37°C, stirred at 1000 rpm). The concentration of collagen was 18.8 μg/ml. The sample was transferred into an Eppendorf tube, mixed with 30 μl of 100 mM EDTA in 2% NaOH (w/v), 30 μl of H2O or MDA standards, and 315 μl of acetonitrile. The sample was incubated at 60°C for 30 min and centrifuged (40,000 g, 10 min). Seventy-five microliters of supernatant was added to 300 μl of 25 mM 2-Thiobarbituric acid (TBA) in 2 M CH3COOH, pH 3, and incubated at 100°C for 60 min. Twenty-five microliters of centrifuged supernatant was injected onto a Nucleosil C18 column, 125×3 mm, 5 μm (Macherey-Nagel). The mobile phase was a mixture of methanol and 100 mM H3PO4, adjusted to pH 6.5 with NaOH (40:60, v/v). Chromatography was performed with a flow rate of 0.5 ml/min at room temperature, using UV–Vis detection at 532 nm [34].
Measurement of cyclooxygenase 1 inhibition by resveratrol or trolox
Inhibition of COX-1 by resveratrol or trolox was determined by a COX inhibitor screening assay ELISA kit (Cayman Chemicals). This assay directly measures prostaglandin F2α by SnCl2 reduction of COX-derived prostaglandin H2 produced in the COX reaction. The prostanoid product is quantified via enzyme immunoassay using a broadly specific antiserum that binds to all the major prostaglandin compounds. Various concentrations of either resveratrol (0.300, 0.150, 0.075 mM) or trolox (4.20, 2.10, 1.05 mM) were used.
Measurement of static adhesion
Platelet adhesion under static conditions was estimated by the modified method of Bellavite et al. [35]. A platelet suspension was treated with various concentrations of either resveratrol or trolox. Microplate wells were coated with 100 μl of fibrinogen solution (20 μg/ml) in PBS buffer for 60 min and blocked with 200 μl of BSA solution (1% dissolved in PBS buffer). A platelet suspension (100 μl) was added to the PBS-washed wells coated with fibrinogen and incubated at room temperature for 1 h. Nonadherent platelets were removed by aspiration followed by washing the wells 10 times with PBS. The amount of platelets adherent to protein-coated wells was estimated by measurement of the platelet acid phosphatase activity using a calibration curve estimated in standard suspensions of platelets (50 μl, 0–2000×103 platelets per well). The wells were rapidly filled with 100 μl of 0.1 M citrate buffer, pH 5.4, containing 5 mM p-nitrophenyl phosphate and 1% Triton X-100 (v/v). After 60 min incubation at room temperature, the reaction was stopped by the addition of 70 μl of 2 M NaOH. The p-nitrophenol produced was measured with a Synergy HT microplate reader (Biotek Instruments, Winooski, VT, USA) at 405 nm against a platelet-free blank [36]. A linear relationship exists between optical density at 405 nm and cell number in the calibration range used. The influence of antioxidants on platelet adhesion was expressed as a percentage of inhibition: platelet count per square centimeter in the presence of antioxidant versus platelet count per square centimeter of control.
Measurement of dynamic adhesion by cone and plate(let) analyzer
A suspension of washed platelets with various concentrations of either resveratrol or trolox (200 μl) was mixed with washed red blood cells in a ratio of 1:1 (final count of platelets 200,000/μl), and fibrinogen was added (final concentration 0.1%, w/v). Red blood cells were prepared from the same person’s blood. Briefly, the whole blood was first centrifuged (250 g, 37°C, 15 min) and the PRP was removed. The remaining blood sample was diluted in PBS (final volume of the whole blood sample) and centrifuged (220 g, 25°C, 10 min) and the supernatant was consequently removed. This step was repeated three times. The conditions of the last centrifugation were altered (2000 g, 25°C, 10 min) and red blood cells were resuspended in PBS (final concentration 8,000,000/μl). High shear was applied with the Impact-R cone and plate(let) analyzer (DiaMed, Cressier, Switzerland). One hundred thirty microliters of the sample (washed platelets with antioxidant/red blood cells/fibrinogen) was placed onto a polystyrene plate onto which a Teflon cone was perfectly fitted and shear was immediately applied (shear rate 1800 s−1) for 2 min. Adhered plates were then washed with deionized water and stained with May–Grünwald solution according to the manufacturer’s manual. Samples were analyzed with an image analyzing system. Platelet adhesion and aggregation were recorded by examination of the percentage of total area covered with platelets, expressed as surface coverage (%) and the average size (μm2) of surface-bound objects. Seven images were collected from each run and the medians of the respective values were calculated by the analyzing system. The influence of antioxidant on platelet adhesion was expressed as the percentage of inhibition, surface coverage in the presence of antioxidant versus surface coverage of control.
Statistics
All results were statistically analyzed using the Student t test and p<0.05 was considered statistically significant. In a single experiment platelets from one donor were used. Data in the paper are means ± SD from at least four independent experiments.
Results
Platelet aggregation
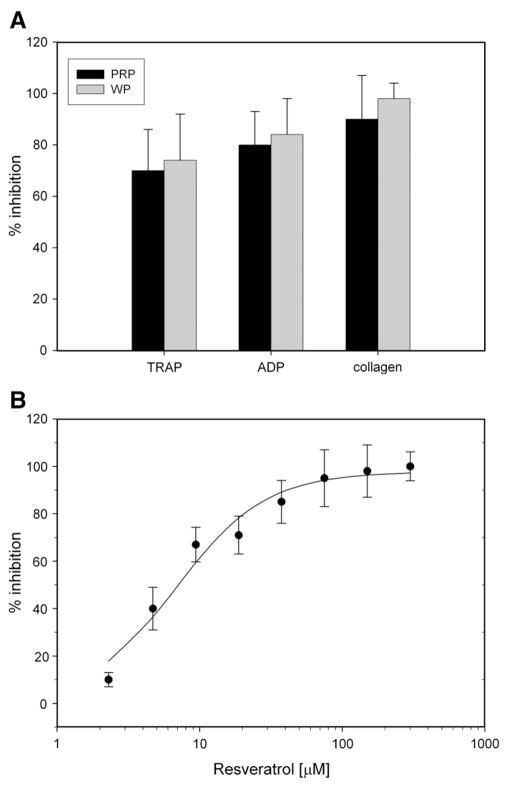
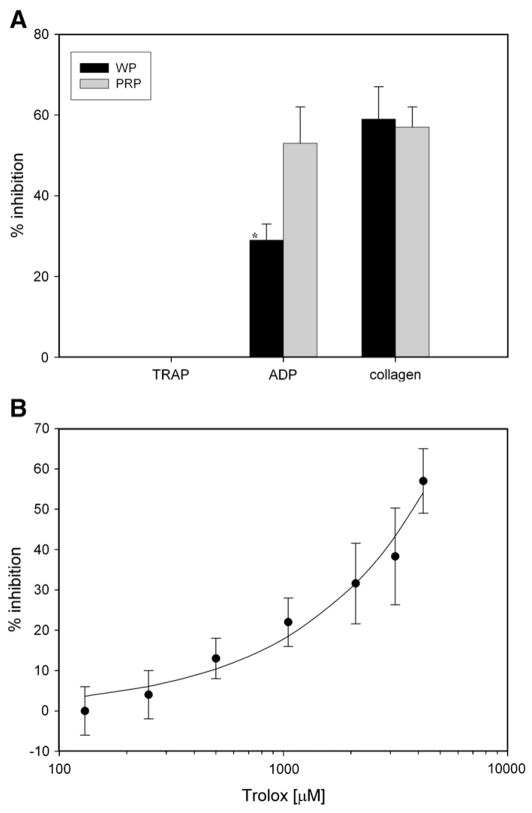
Resveratrol (Fig. 1A) inhibited aggregation of both PRP and washed platelets induced by either collagen (90±17% inhibition in PRP, 98±6% inhibition in washed platelets) or ADP (80±13% in PRP, 84±14% in washed platelets). Trolox (Fig. 2A) inhibited aggregation of both PRP and washed platelets induced by either collagen (59±8% in PRP, 57±5% in washed platelets) or ADP (29±4% in PRP, 53±9% in washed platelets). Resveratrol inhibited aggregation induced by a potent activator, TRAP (70±16% in PRP, 74±18% in washed platelets). Trolox did not inhibit aggregation induced by the potent activator TRAP. Collagen activation of platelets was mostly suppressed with these antioxidants (Fig. 3), compared with other agonists.
Fig. 1.
(A) Inhibition of PRP and washed platelet aggregation in the presence of resveratrol. Platelet-rich plasma or washed platelets were incubated with resveratrol (0.3 mM) and activated by ADP (0.01 mM), TRAP (0.01 mM), or collagen (18.8 μg/ml). (B) The influence of resveratrol concentration on inhibition of aggregation response. The resveratrol concentration range was 0–300 μM. The suspensions of washed platelets were activated by collagen (18.8 μg/ml). Data are means±SD of % inhibition of aggregation response from seven independent experiments done in duplicate for each data point.
Fig. 2.
(A) Inhibition of PRP and washed platelet aggregation in the presence of trolox. Platelet-rich plasma or washed platelets were incubated with trolox (4.2 mM) and activated by ADP (0.01 mM), TRAP (0.01 mM), or collagen (18.8 μg/ml). (B) The influence of trolox concentration on inhibition of aggregation response. The trolox concentration range was 0–4200 μM. The suspensions of washed platelets were activated by collagen (18.8 μg/ml). Data are means±SD of % inhibition of aggregation response from seven independent experiments done in duplicate for each data point. *Statistical significance of difference between washed platelets and PRP incubated with trolox and ADP (Student’s t test, p<0.05).
Fig. 3.
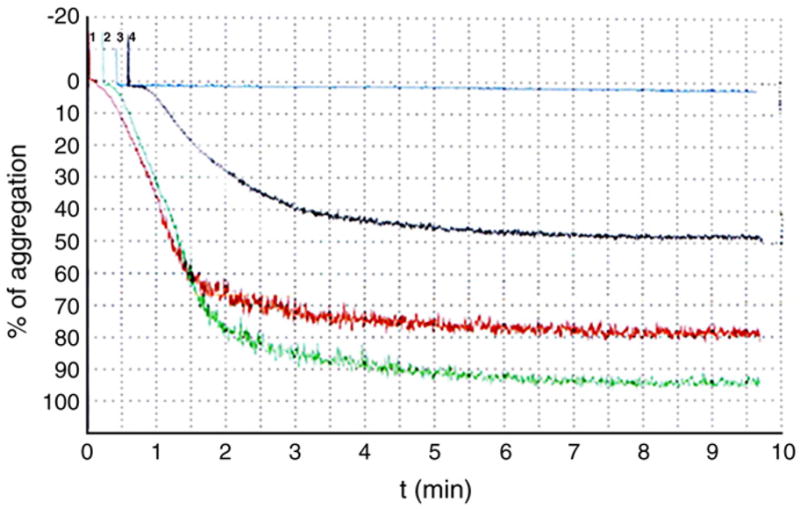
Aggregation curves of washed platelets activated with collagen. Washed platelets were incubated with 0.3 mM resveratrol (blue) or 4.2 mM trolox (black) or 0.6% ethanol as a control (green) or Tyrode’s buffer, pH 7.4 (red), and activated by collagen (18.8 μg/ml).
The dependence of the washed platelet aggregation response to collagen addition with increasing concentrations of either resveratrol (0.300, 0.150, 0.075, 0.038, 0.019, 0.009, 0.005, and 0.002 mM) (Fig. 1B) or trolox (4.20, 3.15, 2.10, 1.05, 0.53, 0.26, and 0.13 mM) (Fig. 2B) was estimated. Resveratrol inhibited collagen-induced aggregation with an IC50 value of about 7 μM, whereas the trolox inhibition constant IC50 could not be estimated using the data obtained, but its value is probably in the millimolar range. Therefore, resveratrol is a much stronger inhibitor of collagen-induced aggregation in comparison with trolox. The maximum concentration of trolox (4.2 mM) in platelet suspensions was limited by the trolox solubility.
Antioxidants
The antioxidant capacity of resveratrol measured by the TEAC assay was 0.97±0.05 TEAC, which means that it has the same antioxidant capacity as trolox (1 TEAC by definition). Data are means±SD from five independent experiments done in triplicate for each data point.
The ability of both antioxidants to quench hydroxyl radicals produced during platelet aggregation was estimated using the concentration measurement of the hydroxylated products of salicylic acid (DHB) (Fig. 4). The concentration of DHB in collagen-activated platelets was significantly lower in the presence of either resveratrol (0.236±0.097 μM, p=0.0166, n=6) or trolox (0.280±0.0.070 μM, p=0.0384, n=6), compared to the control (0.377±0.071 μM). DHB concentrations in collagen-activated platelets in the presence of resveratrol were not significantly different from DHB concentrations produced in the presence of trolox (p=0.3888, n=6). The level of DHB in resting platelets was 0.010±0.006 μM. The presence of the antioxidants did not affect the level of DHB in resting platelets. Both antioxidants have comparable power to quench hydroxyl radicals produced in collagen-activated platelets.
Fig. 4.
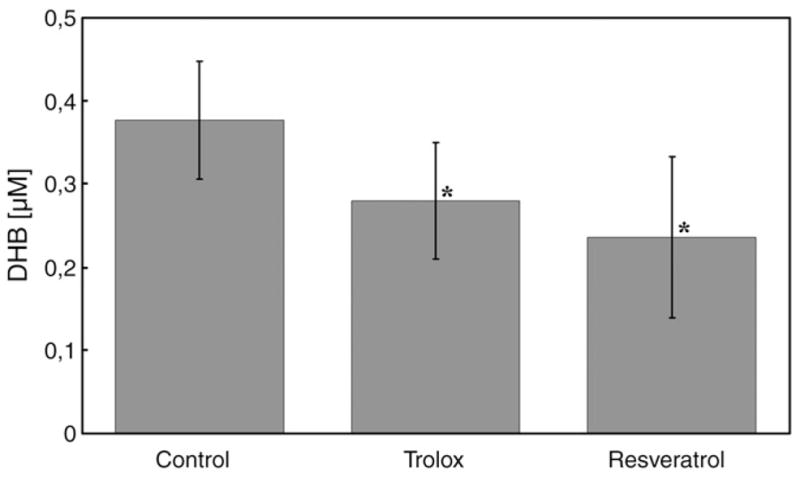
Concentrations of hydroxyl radical produced in washed platelets activated with collagen. Concentrations of dihydroxybenzoic acids agree with the hydroxyl radical concentrations. Dihydroxybenzoic acids in washed platelets incubated with salicylic acid and with either trolox (4.2 mM) or resveratrol (0.3 mM) activated by collagen (18.8 mg/ml) were measured. Data are means±SD of results from six independent experiments. *Statistical significance of difference between antioxidant and control (Student’s t test, p<0.05).
The concentrations of both antioxidants resorbed in platelets were analyzed by HPLC using UV detection. The resveratrol concentration in platelets (6.5±1.3 nmol/109 platelets) was about five times lower compared with trolox (33.5±6.2 nmol/109 platelets). Data are means±SD from five independent experiments done in duplicate for each data point.
Thromboxane B2 in collagen-activated platelets
The concentration of thromboxane B2 generated in collagen-activated platelets was 1048.5±173.9 pmol/109 platelets. Collagen activation of platelets in the presence of resveratrol produced significantly lower amounts of TxB2 (13.9±11.1 pmol/109 platelets) compared with production of TxB2 in the presence of trolox (293.3± 73.9 pmol/109 platelets). This difference was statistically significant (p<0.001) (Fig. 5).
Fig. 5.
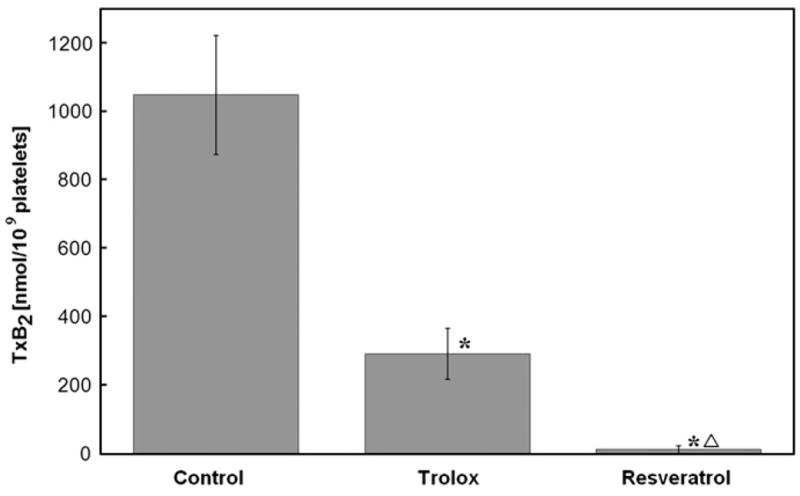
Production of thromboxane B2 in washed platelets incubated with antioxidants. Washed platelets were incubated with either trolox (4.2 mM) or resveratrol (0.3 mM) and activated with collagen (18.8 mg/ml). Data are means±SD of results from four independent ELISAs done in triplicate for each data point. *Statistical significance of difference between antioxidant and control; Δstatistical significance of difference between resveratrol and trolox (Student’s t test, p<0.05).
Malondialdehyde concentration in collagen-activated platelets
The concentration of malondialdehyde produced in control samples of collagen-activated platelets was 3.09±0.71 μM. The concentration of MDA produced in collagen-induced platelets in the presence of resveratrol was 2.40±0.20 μM, whereas in the presence of trolox was it was 2.21±0.48 μM. The difference between these concentrations and that of the control was statistically significant (trolox, p=0.031; resveratrol, p=0.045). The MDA concentrations in collagen-activated platelet samples in the presence of either trolox or resveratrol were not significantly different (p=0.392). Data are means±SD from six independent experiments done in duplicate for each data point.
Inhibition of cyclooxygenase 1
We did not find any inhibition of COX-1 by incubation with either 0.6% ethanol (control) or trolox (4.20, 2.10, 1.05 mM). Resveratrol (0.300, 0.150, 0.075 mM) strongly inhibited COX-1 but inhibition was not concentration-dependent (Fig. 6).
Fig. 6.
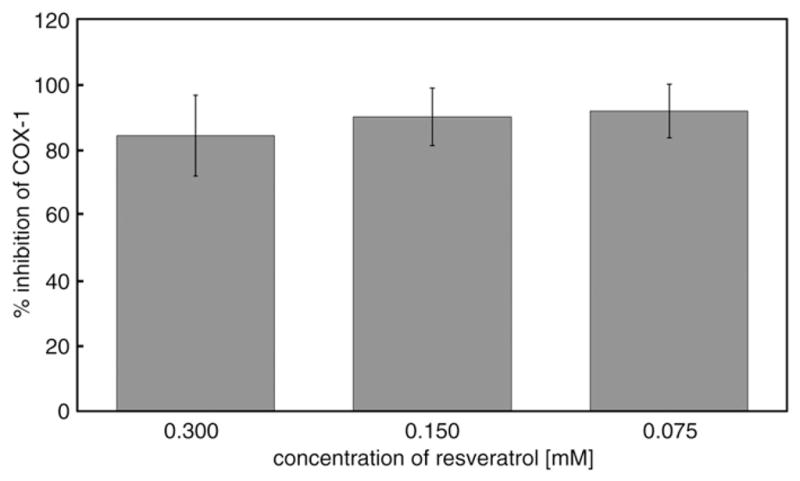
Inhibition of cyclooxygenase 1 by resveratrol. Resveratrol (0.300, 0.150, 0.075 mM) was incubated with COX-1. Data are means±SD of % inhibition of COX-1 from six independent ELISAs done in duplicate for each data point.
Static and dynamic adhesion
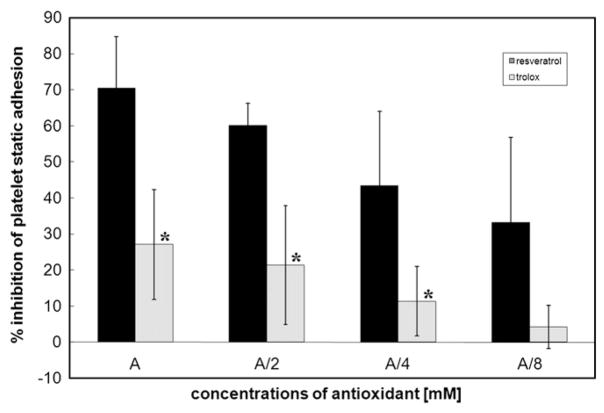
Trolox and resveratrol inhibited static adhesion (Fig. 7) and dynamic adhesion (Figs. 8 and 9). Resveratrol (0.3 mM, concentration used in the other experiments) inhibited platelet adhesion under static conditions and under dynamic conditions, 60.10±6.20 and 53.94±10.37%, respectively. Resveratrol at 0.6 mM also inhibited platelet adhesion under static conditions and under dynamic conditions, 70.52±14.23 and 68.65±18.67%, respectively. Trolox (4.2 mM, concentration used in the other experiments) inhibited platelet adhesion under static conditions and under dynamic conditions, 20.07±15.20 and 49.38±25.51%, respectively. The inhibition of platelet adhesion under either static or dynamic conditions was more pronounced in the presence of resveratrol compared with trolox.
Fig. 7.
Inhibition of platelet adhesion in the presence of antioxidants under static conditions. Washed platelets were incubated with either trolox (4.20, 2.10, 1.05, 0.53 mM) or resveratrol (0.600, 0.300, 0.150, 0.075 mM). A, antioxidant. Calculated % inhibition of platelet adhesion induced by antioxidants represents the means±SD of results from four independent experiments done in triplicate for each data point. *Statistical significance of difference between resveratrol and trolox (Student’s t test, p<0.05).
Fig. 8.
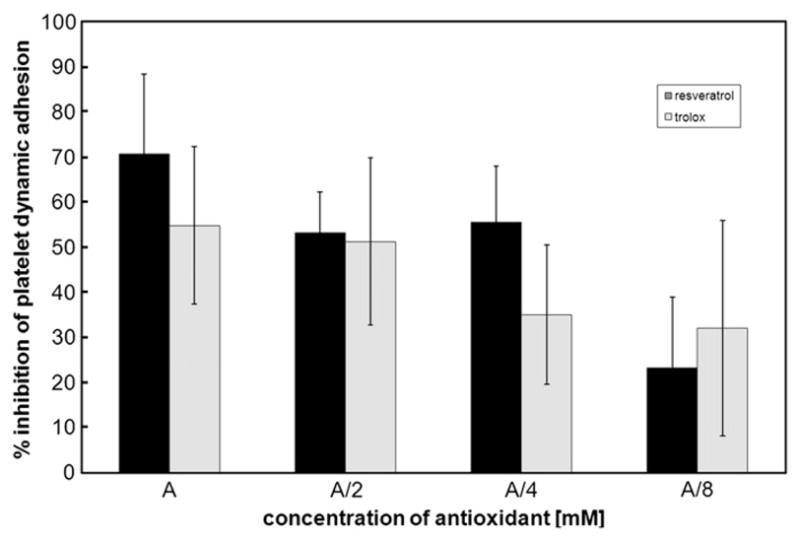
Inhibition of platelet adhesion in the presence of antioxidants under dynamic conditions. Washed platelets were incubated with either trolox (4.20, 2.10, 1.05, 0.53 mM) or resveratrol (0.600, 0.300, 0.150, 0.075 mM). A, antioxidant. Calculated % inhibition of platelet adhesion induced by antioxidants represents the means±SD of results from four independent experiments done in triplicate for each data point.
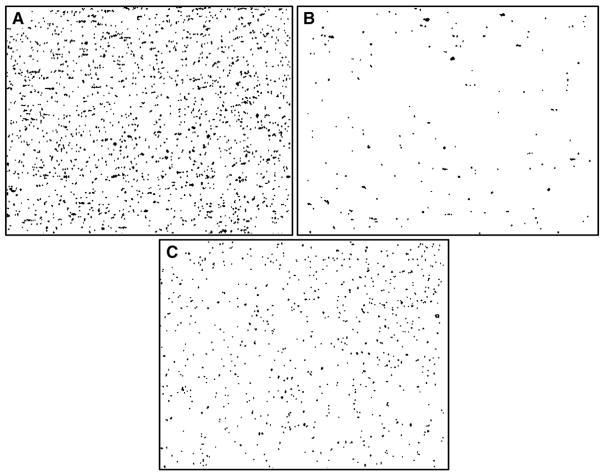
Fig. 9.
Adhered platelets under dynamic conditions analyzed by Impact-R (DiaMed). Washed platelets were incubated with antioxidants in the presence of red blood cells. Representative images of platelet adhesion: (A) control, (B) 0.6 mM resveratrol, (C) 4.2 mM trolox.
The dependence of platelet adhesion under static or dynamic conditions on increasing concentrations of either resveratrol (0.075, 0.150, 0.300, 0.600 mM) or trolox (0.53, 1.05, 2.10, 4.20 mM) was estimated (Figs. 7 and 8). Inhibition of platelet adhesion by resveratrol was concentration-dependent only under static conditions, as was inhibition of platelet adhesion by trolox (Fig. 7).
Discussion
Platelets play a crucial role in hemostasis, and their function may be influenced by alteration of the platelet redox state, the presence of endogenous or exogenous antioxidants, and the formation of reactive oxygen and nitrogen species. Recently, trolox and resveratrol were said to be potent inhibitors of platelet activation [21,26].
We have found that resveratrol inhibits platelet aggregation. Resveratrol and trolox are strong inhibitors of aggregation in washed platelets and platelet-rich plasma. The strongest inhibition of aggregation in washed platelets and PRP was observed using collagen as the activator. Because the influence of antioxidants on platelet aggregation by ADP or TRAP was not as significant, the rest of the experiments were performed using only collagen as the platelet activator. It is known that platelets activated by collagen produce reactive oxygen species, especially hydroxyl radicals and hydrogen peroxide [17,19]. The lower levels of ROS were found during activation of platelets by ADP and thrombin [11], but there are few data about the production of ROS in platelets activated by TRAP.
The antioxidant power of the antioxidants used in our experiments was estimated to assess their possible role in mechanisms of platelet activation. The antioxidant capacity of both trolox and resveratrol was almost the same and yet resveratrol inhibited platelet aggregation much more in comparison with trolox, despite the fact that the latter was used at about 10 times higher concentration. The maximum concentration of resveratrol (0.3 mM) was the concentration inducing the maximum inhibition of aggregation. The maximum concentration of trolox (4.2 mM) in platelet suspensions was limited by the trolox solubility. Determination of the concentrations of antioxidants inside the platelets showed that resveratrol penetrated into the platelets more easily than trolox. Nevertheless, the final resveratrol concentration in platelet suspensions was approximately 5 times lower than the trolox concentration. Therefore, the lower effectiveness of trolox in the inhibition of the platelet’s responses to activators in comparison with resveratrol cannot be explained by its lower bioavailability in platelets.
We estimated the ability of both antioxidants to quench the hydroxyl radicals produced during activation of platelets with collagen using the salicylic acid assay. Salicylic acid absorbed within washed platelets is converted to dihydroxybenzoates, and antioxidants present in platelets react with hydroxyl radicals in concurrent reactions. Salicylic acid itself does not affect the cyclooxygenase pathway and does not interfere with platelet aggregation [32]. We found that both antioxidants had similar reactivity with hydroxyl radicals. Our results thus support the idea that both resveratrol and trolox influence platelet agonist responses by several different mechanisms, including ROS quenching.
The possible role of the arachidonate pathway in resveratrol inhibition of platelet activation was examined by several authors [37]. Arachidonic acid is transformed into prostaglandin endoperoxides, which are then converted by thromboxane synthase into thromboxane A2, MDA, and 12(L)-hydroxyheptadeca-5,8,10-trienoic acid in the ratio 1:1:1 [33].
We estimated the MDA concentration by chromatography of the malondialdehyde–thiobarbituric acid adduct as one of the markers of cyclooxygenase activity. There were no distinct differences between the decrease in MDA concentrations caused by either resveratrol or trolox. The decrease in MDA production induced by both antioxidants (about 75%) corresponded with the lowered production of hydroxyl radicals (about 70%). It is in good agreement with data reporting alternative MDA production due to free radical-induced lipoperoxidation [38].
A much more specific marker of arachidonic acid pathway activity is the measurement of TxB2 as a TxA2 product. We estimated TxB2 concentration in platelets activated by collagen in the presence of either resveratrol or trolox using a commercial ELISA kit. Both antioxidants decreased TxB2 levels, but resveratrol was much more effective than trolox. We tested the inhibition of pure cyclooxygenase 1 with either resveratrol or trolox to elucidate the influence of the respective antioxidants on thromboxane synthesis. We did not find any inhibition of COX-1 with trolox, whereas inhibition of COX-1 activity with resveratrol, which was used in the concentration range of 0.075–0.300 mM, was about 87%. These data are in accordance with the average platelet aggregation response (about 90%) to collagen within the concentration range of resveratrol used (0.075–0.300 mM).
Our data do not exclude the possibility that there may be some influence of trolox on thromboxane synthesis in activated platelets. Trolox action on the arachidonate metabolic pathway may, however, be a result rather of some overall nonspecific interactions with platelet proteins. Our data support the theory that resveratrol, in addition to ROS-dependent mechanisms, influences platelet activation by direct inhibition of COX-1.
Traditional platelet aggregometry measurements examine platelet function in the absence of red and white blood cells and at low shear rates (<100 s−1) [5]. This is far from the physiological conditions in blood vessels. Various types of perfusion chambers for platelet adhesion measurement have been used, and recently Varon’s cone and plate(let) analyzer [39] was introduced by DiaMed [6]. We have used the cone and plate(let) analyzer for the study of platelet adhesion under physiological shear rates (1800 s−1) and in the presence of various concentrations of both antioxidants. The washed platelets were incubated with different antioxidants and the washed red blood cells were added subsequently to evoke a proper contact of platelets with the surfaces of the cone and plate chamber under defined shear rates. The presence of red blood cells is essential for this measurement [5]. An influence of both antioxidants on washed-platelet adhesion under dynamic conditions in the presence of fibrinogen was detected and we found the effect of resveratrol to be more pronounced in comparison to trolox. Actually, there was no concentration-dependent effect on dynamic platelet adhesion in the presence of resveratrol or trolox. It is known that platelet adhesion to fibrinogen depends on both cell activation and shear stress [39]. The platelet adhesion efficiency decreased with increasing shear rates up to 2000 s−1. The inhibition of dynamic platelet adhesion in the presence of fibrinogen induced by resveratrol at a shear rate 1800 s−1 was, however, especially pronounced at the highest concentrations of antioxidant. It is possible that the diminished interaction of platelets with fibrinogen under the dynamic conditions mentioned above [40] can be further suppressed only by a strong inhibitor, e.g., by resveratrol at the highest concentration. This fact could help to explain some of the controversial results of antioxidant usage in the prevention of cardiovascular disease.
A relatively easy method of estimating platelet–protein interactions is to examine the adhesion of platelets under static conditions to various proteins of interest adsorbed on the surfaces of microplates. It is often utilized by many authors as one of the important criteria of hemocompatibility [36]. Nevertheless, this method suffers from being carried out under nonphysiological conditions, as mentioned concerning platelet aggregometry. Some authors have shown that the integrin αvβ3 bears a receptor site for resveratrol with potential for inhibition of blood platelet activation [41]. Other authors have found that the inhibitory effect of resveratrol on platelet aggregation does not involve binding to the glycoprotein αIIbβ3 [16]. Nevertheless our results from static adhesion on fibrinogen surfaces revealed that both antioxidants tested efficiently modulate platelet adhesion to the adhesive protein fibrinogen. The comparison of resveratrol with trolox has shown that resveratrol is a more effective agent at reducing platelet adhesion. Inhibition of platelet adhesion was concentration-dependent for both antioxidants.
In developed countries, cardiovascular diseases, including angina pectoris, myocardial infarction, and stroke, represent the most important causes of mortality and morbidity [42]. It was shown that fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia–reperfusion in vivo [43]. Thus, the study of the dynamic adhesiveness of platelets in the presence of fibrinogen can help to assess the influence of platelet adhesion on the postischemic vessel wall.
Conclusion
We have shown that ROS play an important role in platelet activation, especially when collagen was used as an agonist. Both resveratrol and trolox inhibited platelet aggregation and both static and dynamic platelet adhesion, but resveratrol was a more effective inhibitor compared with trolox. Although the bioavailability of resveratrol in platelets incubated with the used antioxidants was lower compared with trolox, both antioxidants had similar antioxidant capacity and ability to quench hydroxyl radicals produced during platelet activation. Our data agree with the proposition that some antioxidants can inhibit platelet activation also by specific interaction with target proteins, apart from nonspecific redox or radical-quenching mechanisms. Resveratrol, in addition to hydroxyl radical quenching, influences platelet activation by direct inhibition of the arachidonate pathway via COX-1. Platelets play a key role in the development of cardiovascular diseases and therefore the study of possible inhibitors of platelet activation (e.g., antioxidants) should be performed under conditions of real blood flow in contact with reactive surfaces, e.g., using dynamic adhesion experiments.
Acknowledgments
This study was supported by Grants NS10633-3/2009 and MZ 02373601 from the Ministry of Health, Czech Republic, and by Grant KAN200670701 from the Academy of Sciences, Czech Republic.
Abbreviations
- ABTS
2,2′-azinobis(3-ethylbezothiazoline-6-sulfonic acid)
- COX
cyclooxygenase
- DHB
dihydroxybenzoic acid
- ELISA
enzyme-linked immunosorbent assay
- HO·
hydroxyl radical
- HPLC
high-performance liquid chromatography
- MDA
malondialdehyde
- O2·−
superoxide
- PBS
phosphate-buffered saline
- PRP
platelet-rich plasma
- ROS
reactive oxygen species
- TEAC
trolox equivalent antioxidant capacity
- TRAP
thrombin receptor activating peptide
- TxA2
thromboxane A2
- TxB2
thromboxane B2
- WP
washed platelets
References
- 1.Gibbins JM. Platelet adhesion signaling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 2.Ozaki Y, Asazuma N, Suzuki-Inoue K, Berndt MC. Platelet GPIb-IX-V-dependent signaling. J Thromb Haemostasis. 2005;3:1745–1751. doi: 10.1111/j.1538-7836.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 3.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 4.Stohlawetz PJ, Horvath M, Eichelberger B, Koren D, Jilma B, Panzer S. Platelet function under high-shear conditions from platelet concentrates. Transfusion. 2008;48:129–135. doi: 10.1111/j.1537-2995.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- 5.Peerschke EI, Silver RT, Weksler BB, Yin W, Bernhardt B, Varon D. Examination of platelet function in whole blood under dynamic flow conditions with cone and plate(let) analyzer. Am J Clin Pathol. 2007;127:422–428. doi: 10.1309/B29E2AHMTQA1KAKJ. [DOI] [PubMed] [Google Scholar]
- 6.Morrison A, Hornsey VS, Prowse CV, MacGregor IR. Use of the DiaMed Impact R to test platelet function in stored platelet concentrates. Vox Sang. 2007;93:166–172. doi: 10.1111/j.1423-0410.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- 7.Rittenhouse SE. Phosphoinositide 3-kinase activation and platelet function. Blood. 1996;88:4401–4414. [PubMed] [Google Scholar]
- 8.Jackson SP, Yap CL, Anderson KE. Phosphoinositide 3-kinases and the regulation of platelet function. Biochem Soc Trans. 2004;32(Pt 2):387–392. doi: 10.1042/bst0320387. [DOI] [PubMed] [Google Scholar]
- 9.Caughey GE, Cleland LG, Gamble JR, James MJ. Up-regulation of endothelial cyclooxygenase-2 and prostanoid synthesis by platelets. J Biol Chem. 2001;276:37839–37845. doi: 10.1074/jbc.M010606200. [DOI] [PubMed] [Google Scholar]
- 10.Krötz F, Sohn HY, Pohl U. Reactive oxygen species—players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 11.Principe D, Menichelli A, Matteis W, Giulio S, Giordani M, Savini I, Agro AF. Hydrogen peroxide is an intermediate in the platelet activation cascade triggered by collagen, but not by thrombin. Thromb Res. 1991;62:365–375. doi: 10.1016/0049-3848(91)90010-t. [DOI] [PubMed] [Google Scholar]
- 12.Balleissen L, Marx R, Kuhn K. Platelet–collagen interaction: the influence of native and modified collagen (type I) on the aggregation of human platelets. Haemostasis. 1976;5:155–164. doi: 10.1159/000214131. [DOI] [PubMed] [Google Scholar]
- 13.Fauvel F, Legrand YJ, Caen JP. Platelet adhesion to type I collagen and alpha 1 (I)3 trimers: involvement of the C-terminal alpha 1 (I) CB6A peptide. Thromb Res. 1978;12:273–285. doi: 10.1016/0049-3848(78)90298-0. [DOI] [PubMed] [Google Scholar]
- 14.Berndt MC, Phillips DR. Interaction of thrombin with platelets: purification of the thrombin substrate. Ann N Y Acad Sci. 1981;370:87–95. doi: 10.1111/j.1749-6632.1981.tb29724.x. [DOI] [PubMed] [Google Scholar]
- 15.Joseph M. Immunopharmacology of Platelets. Academic Press; New York: 1996. The generation of free radicals by blood platelets; pp. 209–225. [Google Scholar]
- 16.Caccese D, Pratico D, Ghiselli A, Natoli S, Pignatelli P, Sanguigni V, Iuliano L, Violi F. Superoxide anion and hydroxyl radical release by collagen-induced platelet aggregation—role of arachidonic acid metabolism. Thromb Haemostasis. 2000;83:485–490. [PubMed] [Google Scholar]
- 17.Olas B, Wachowicz B. Resveratrol and vitamin C as antioxidants in blood platelets. Thromb Res. 2002;106:143–148. doi: 10.1016/s0049-3848(02)00101-9. [DOI] [PubMed] [Google Scholar]
- 18.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood. 1998;91:484–490. [PubMed] [Google Scholar]
- 19.Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets. 2005;16:251–260. doi: 10.1080/09537100400020591. [DOI] [PubMed] [Google Scholar]
- 20.Zbikowska HM, Olas B. Antioxidants with carcinostatic activity (resveratrol, vitamin E and selenium) in modulation of blood platelet adhesion. J Physiol Pharmacol. 2000;51:513–520. [PubMed] [Google Scholar]
- 21.Olas B, Wachowicz B, Szewczuk J, Saluk-Juszczak J, Kaca W. The effect of resveratrol on the platelet secretory process induced by endotoxin and thrombin. Microbios. 2001;105:7–13. [PubMed] [Google Scholar]
- 22.Shen MY, Hsiao G, Liu ChL, Fong TH, Lin KH, Chou DS, Sheu JR. Inhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol. 2007;139:475–485. doi: 10.1111/j.1365-2141.2007.06788.x. [DOI] [PubMed] [Google Scholar]
- 23.Kakishita E, Suehiro A, Oura Y, Nagai K. Inhibitory effect of vitamin E (alpha-tocopherol) on spontaneous platelet aggregation in whole blood. Thromb Res. 1990;60:489–499. doi: 10.1016/0049-3848(90)90233-3. [DOI] [PubMed] [Google Scholar]
- 24.Polette A, Blache D. Effect of vitamin E on acute iron load-potentiated aggregation, secretion, calcium uptake and thromboxane biosynthesis in rat platelets. Atherosclerosis. 1992;96:171–179. doi: 10.1016/0021-9150(92)90063-m. [DOI] [PubMed] [Google Scholar]
- 25.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Vitamin E inhibits collagen-induced platelet activation by blunting hydrogen peroxide. Arterioscler Thromb Vasc Biol. 1999;19:2542–2547. doi: 10.1161/01.atv.19.10.2542. [DOI] [PubMed] [Google Scholar]
- 26.Jardín I, Redondo PC, Salido GM, Pariente JA, Rosado JA. Endogenously generated reactive oxygen species reduce PMCA activity in platelets from patients with non-insulin-dependent diabetes mellitus. Platelets. 2006;17:283–288. doi: 10.1080/09537100600745187. [DOI] [PubMed] [Google Scholar]
- 27.Redondo PC, Jardin I, Hernandes-Cruz JM, Pariente JA, Salido GM, Rosado JA. Hydrogen peroxide and peroxynitrite enhance Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem Biophys Res Commun. 2005;333:794–802. doi: 10.1016/j.bbrc.2005.05.178. [DOI] [PubMed] [Google Scholar]
- 28.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 29.Rice-Evans C, Miller NJ, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 30.Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 31.Ghiselli A, Laurenti O, Mattia G, Maiani G, Ferro-Luzzi A. Salicylate hydroxylation as an early marker of in vivo oxidative stress in diabetic patients. Free Radic Biol Med. 1992;13:621–626. doi: 10.1016/0891-5849(92)90036-g. [DOI] [PubMed] [Google Scholar]
- 32.Blandini F, Martignoni E, Ricotti R, Jeso F, Nappi G. Determination of hydroxyl free radical formation in human platelets using high-performance liquid chromatography with electrochemical detection. J Chromatogr B. 1999;732:213–220. doi: 10.1016/s0378-4347(99)00286-8. [DOI] [PubMed] [Google Scholar]
- 33.Panse M, Block HU, Forster W, Mest HJ. An improved malondialdehyde assay for estimation of thromboxane synthase activity in washed human blood platelets. Prostaglandins. 1985;30:1031–1040. doi: 10.1016/0090-6980(85)90174-1. [DOI] [PubMed] [Google Scholar]
- 34.Suttnar J, Masova L, Dyr JE. Influence of citrate and EDTA anticoagulants on plasma malondialdehyde concentrations estimated by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;751:193–197. doi: 10.1016/s0378-4347(00)00453-9. [DOI] [PubMed] [Google Scholar]
- 35.Bellavite P, Andrioli G, Guzzo P, Adrigliano P, Chirumbolo S, Manzato F, Santonastaso CA. colorimetric method for the measurement of platelet adhesion in microtiter platelets. Anal Biochem. 1994;216:445–450. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
- 36.Vaníčková M, Suttnar J, Dyr JE. The adhesion of blood platelets on fibrinogen surface: comparison of two biochemical microplate assays. Platelets. 2006;17:470–476. doi: 10.1080/09537100600758875. [DOI] [PubMed] [Google Scholar]
- 37.Moreno JJ. Resveratrol modulates arachidonic acid release, prostaglandin synthesis, and 3T6 fibroblast growth. J Pharmacol Exp Ther. 2000;294:333–338. [PubMed] [Google Scholar]
- 38.Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim Biophys Acta. 1988;937:205–210. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- 39.Varon D, Dardik R, Shenkman B, Kotev-Emeth S, Farzame N, Tamarin I, Savion N. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb Res. 1997;85:283–294. doi: 10.1016/s0049-3848(97)00014-5. [DOI] [PubMed] [Google Scholar]
- 40.Bonnefoy A, Liu Q, Legrand C, Frojmovic MM. Efficiency of platelet adhesion to fibrinogen depends on both cell activation and flow. Biophys J. 2000;78:2834–2843. doi: 10.1016/S0006-3495(00)76826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin HY, Lansing L, Merillon JM, Davis FB, Tang HY, Shih A, Vitrac X, Krisa S, Keating T, Cao HJ, et al. Integrin αvβ3 contains a receptor site for resveratrol. FASEB J. 2006;26:144–150. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 42.Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. 2002;1:273–288. doi: 10.1016/s1474-5151(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 43.Massberg S, Enders G, Matos FC, Tomic LID, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia–reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]