Abstract
The ventral tegmental area (VTA) is the source of dopaminergic projections innervating cortical structures and ventral forebrain. Dysfunction of this mesocorticolimbic system is critically involved in psychiatric disorders such as addiction and schizophrenia. Changes in VTA dopamine (DA) neuronal activity can alter neurotransmitter release at target regions which modify information processing in the reward circuit. Here we studied the effect of α-2 noradrenergic receptor activation on the hyperpolarization-activated cation current (Ih ) in DA neurons of the rat VTA. Brain slice preparations using whole-cell current and voltage-clamp techniques were employed. Clonidine and UK14304 (α-2 receptor selective agonists) were found to decrease Ih amplitude and to slow its rate of activation indicating a negative shift in the current’s voltage dependence. Two non-subtype-selective α-2 receptor antagonists, yohimbine and RS79948, prevented the effects of α-2 receptor activation. RX821002, a noradrenergic antagonist specific for α-2A and α-2D did not prevent Ih inhibition. This result suggests that clonidine might be acting via an α-2C subtype since this receptor is the most abundant variant in the VTA. Analysis of a second messenger system associated with the α-2 receptor revealed that Ih inhibition is independent of cyclic adenosine monophosphate (cAMP) and resulted from the activation of protein kinase C. It is suggested that the α-2 mediated hyperpolarizing shift in Ih voltage dependence can facilitate the transition from pacemaker firing to afferent-driven burst activity. This transition may play a key role on the changes in synaptic plasticity that occurs in the mesocorticolimbic system under pathological conditions.
Keywords: clonidine, VTA, Ih, noradrenergic receptors, addiction, dopamine
Introduction
Dopamine (DA) released from the ventral tegmental area (VTA) is necessary for the processing of information related to rewarding properties (Grace et al. 2007; Schultz 2002). The two main patterns of activity of VTA DA neurons, i.e. pacemaker or bursting activity, are thought to modulate the neurophysiology of the mesocorticolimbic system (Cooper, 2002; Kitai et al., 1999; Overton and Clark, 1997; Grace and Bunney, 1983). In addiction and schizophrenia these two firing modes appear to be altered (Brodie et al. 1999; Nestler, 2004; Grace 2000; Grace 1991). For instance, withdrawal after chronic ethanol exposure significantly decreases pacemaker activity (Hopf et al. 2007; Okamoto et al. 2006). The mechanisms that underlie the changes in DA cell physiology that characterize drug abuse remain for the most part unclear. However, at least in addiction to some psychostimulants (e.g., cocaine and amphetamines) these alterations appear to involve changes in DA cell sensitivity to noradrenergic stimulation (Paladini et al., 2001; Peterson et al., 1990).
VTA DA cells express an inward rectifying non-selective cation current in response to membrane hyperpolarization, termed the hyperpolarization-activated cation current (Ih) (Neuhoff et al. 2002; Liu et al 2003; Hopf et al. 2007; Okamoto et al. 2006). The Ih is sensitive to the intracellular concentration of cyclic adenosine monophosphate (cAMP). A reduction in cAMP levels results in a hyperpolarizing shift of Ih voltage dependence while increments in cAMP levels cause the opposite (Robinson and Seigelbaum 2003; Hofmann et al. 2003). In VTA DA cells of rats and mice repeated ethanol administration leads to a significant reduction in Ih current density without any apparent effect on its voltage dependence (Hopf et al. 2007; Okamoto et al. 2006). Thus, the Ih conductance may play a critical role in the excitability changes that take place in drug addiction.
In many brain regions inhibition of Ih has been linked to the activation of α-2 noradrenergic receptors. Clonidine (2-[2,6 –dichloralanine]-2-imidazoline HC), a well known α-2 agonist, has been shown to effectively inhibit Ih in different neuronal types, including dorsal root ganglion (Yagi and Sumino 1998), hypoglossal motoneurons (Parkis and Berger 1997), trigeminal ganglion (Takeda et al. 2002), and pyramidal neurons of the prefrontal cortex (PFC) (Carr et al. 2007). Although activation of α-2 receptors is most commonly known to decrease intracellular levels of cAMP (Jansson et al. 1994), there is evidence showing that activation of α-2 receptors may inhibit Ih in a cAMP-independent manner. In PFC pyramidal cells, activation of α-2 receptors stimulate a protein kinase C (PKC) which causes Ih inhibition (Carr et.al. 2007). In midbrain DA neurons, activation of PKC by serotonin or neurotensin also inhibits Ih (Liu et al 2003; Cathala and Paupardin-Tritsch 1997). Consequently, we asked if activation of α-2 receptors in putative VTA DA cells from rat midbrain slices could produce similar actions as the ones induced in the PFC. Here, we show that activation of α-2 noradrenergic receptors results in Ih inhibition of putative VTA DA cells.
Materials and Methods
Animals and Slice preparation
All experimental procedures were performed accordingly to the US Public Health Service publication “Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Care and Use Committee at the Universidad Central del Caribe. Sprague-Dawley rats of either sex between 15 and 35 days postnatal were anaesthetized by intraperitoneal injection of chloral hydrate (90 mg/kg) and decapitated. After rapid removal of the brain, the cerebral hemispheres and a portion of the dorsal mesencephalon were removed. Horizontal slices (thickness: 220 μm) containing the ventral tegmental area were prepared from the remaining ventral face using a vibratome (VT1000S, Leica, Germany). Slices were cut in ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 127 NaCl; 2.5 KCl; 1.25 NaH2PO4 ; 25 NaHCO3 ; 2 CaCl2 ; 1 MgCl2 ; 25 D-glucose. The solution was previously saturated with a 95% O2 and 5% CO2 gas mixture to pH = 7.4. Slices were transferred to an intermediate chamber and incubated at 35° C in the same solution for approximately one hour before transferring them to the recording chamber.
Electrophysiological recordings
Whole-cell voltage or current clamp recordings were obtained from visually identified neurons in the VTA area (Paxinos and Watson 1998) using infrared microscopy with DIC (BX51WI Olympus, Japan) and water-immersion objectives. Putative VTA DA cells were identified by the presence of the Ih current. According to Sarti et al. (2007) Ih is present in about 84% VTA DA neurons and VTA GABA cells do not express this conductance (Margolis et al. 2006). Consequently, the contribution of non-DA cells to our data is likely to be not significant. The slice was totally submerged in a 500 μl recording chamber, which was connected to a superfusion system (1-2 mL per minute). The bath solution was the same used for slice preparation, with the chamber temperature maintained at 32°C. Borosilicate glass patch pipettes (O.D. 1.5 mm, I.D. 1,0 mm; WPI, Sarasota, FL) were pulled to a final resistance of 3-6 MΩ when filled with (in mM): 115 KCH3SO4 (potassium methylsulfate); 20 KCl ; 1.5 MgCl2; 5 (K)HEPES; 1 EGTA; 2 (Mg)ATP; 0.2 (Na)GTP; 10 (Na2)creatine phosphate (CP); pH = 7.25, 290 mOsm. (Na)GTP, (Mg)ATP and (Na2)CP were added fresh daily. Data were collected through an Axopatch1-D amplifier (Axon Instruments, Foster City, CA), filtered at 1 kHz through a Bessel filter, digitized at 5 kHz using Digidata 1322A (Axon Instruments, Foster City, CA), stored in Macintosh G4 computer using AxoGraph 4.6 (Axon Instruments, Foster City, CA), and analyzed off line using GraphPad Prism 4.5 (GraphPad Software, Inc) software. The seal quality used was typically 4-6 GΩ. Series resistance was not compensated and was monitored during the entire experiment (values ranged from 8 to 30 MΩ). Data were discarded if changes of more than 10% occurred.
To activate Ih in whole-cell voltage-clamp mode, cells were clamped at a holding potential of −55 mV, and then stepped to successively hyperpolarizing test potentials from −45 mV to −145 mV in 10 mV increments (pulse duration: 1sec, frequency: 1/9 Hz). This voltage protocol assured that steady-state current could be fully activated at most test potentials, and that upon return to −55 mV the current would deactivate completely. Furthermore, current amplitude could be measured frequently enough to monitor the temporal development of current modulation. Ih current at each voltage step was measured as the difference between the current that appears instantaneously in the beginning of the step (with 50 ms lag) (the instantaneous current) and the current developed at the end of the step reaching steady-state level (50 ms before the end of the step: the steady-state current). For current-clamp recordings, a similar protocol was used, in which successively decreasing current pulses of 100 pA amplitude were applied.
To determine the reversal potential of the current affected by α-2 noradrenergic receptor agonists, a protocol similar to the one used by others was applied (Liu et al 2003; Yagi and Sumino 1998). Briefly, membrane current was clamped at −120 mV for 1 second, and “tail currents” were then elicited by returning to different depolarized test potentials (from −10 to −60 in 10 mV steps) in normal solution (containing 1μM TTX and 200 μM Ba2+) and in the presence of α-2 agonists. Tail currents amplitudes were determined at 50 ms after the end of the hyperpolarization step to avoid contamination by capacitative current transients.
To build the current activation curve, tail current (Itail) amplitudes were measured for different voltage steps, applying the same voltage protocol used for Ih, and then normalized in relation to the maximal tail current (Itail max). The data were fitted with the following Boltzmann equation to estimate the potential of half-activation (V50) and the slope factor (k):
To reduce the contamination of Ih tail currents with other conductances, 1μM TTX and 200 μM Ba2+ were added to the external solution.
As the fast exponential component accounts for the great majority of Ih amplitude (Chen et al. 2001b; Solomon and Nerbonne 1993) we used a single exponential fit of the data as acceptable for our purpose of determining the changes in the time scale of Ih activation under different pharmacological conditions. Monoexponential time constants were analyzed by fitting an exponential function to the current trace records between 100 and 500 ms after the Ih current was elicited. This approach gave more consistent values per recorded cell than fitting the entire duration of the 1 sec. hyperpolarizing step with a double-exponential function (See Okamoto et al. 2006). Normalization of current amplitudes was always done with respect to the maximal current observed under control conditions. To compare the effect of the drug, current amplitudes were always measured at −125 mV, unless otherwise noted. Data are presented as mean ± SEM. Student’s T-test was used for statistical analysis and a value of P < 0.05 was considered statistically significant. GraphPad Prism 4.5 software was used for the statistical calculations.
Drugs
Pharmacological agents used in this study
Yohimbine hydrochloride(17-Hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride), Clonidine hydrochloride (2-(2,6-Dichloroanilino)-2-imidazoline hydrochloride), Moxonidine hydrochloride (4-Chloro-6-methoxy-2-methyl-5-(2-imidazolin-2-yl) aminopyrimidine hydrochloride), PDA (Phorbol 12,13-diacetate), UK 14,304 (5-Bromo-N-(2-imidazolin-2-yl)-6-quinoxalinamine), BAPTA (1,2-Bis(2-Aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid), RS79948 (8aR,12aS,13aS) 5,8,8a,9,10,11,12,12a,13,13 a-Decahydro-3-methoxy-12-(ethylsulfonyl)-6H-isoquino[2,1-g][1,6]naphthyridine hydrochloride), RX821002 hydrochloride (2-(2,3-Dihydro-2-methoxy-1,4-benzodioxin-2-yl)-4,5-dihy dro-1H-imidazole hydrochloride), Chelerythrine chloride (1,2-Dimethoxy-12-methyl[1,3]benzodioxolo[5,6-c]phenanth ridinium chloride) were purchased from Sigma (St Louis, MO), except for potassium methylsulfate (Pfaltz & Bauer Inc, Waterbury, CT). All substances were diluted in fresh ACSF until completely mixed, then transferred to separate graduated reservoirs connected to the chamber (1-2 ml/min). The effects on current amplitude were measured within 5 min after the beginning of the flow.
Results
Ih was studied in 137 VTA neurons using whole cell patch recordings in slice preparations. Current and voltage clamp configurations were employed. We found Ih current in cells with broad (>2 ms) overshooting action potentials (APs), slow spontaneous activity and relatively regular interspike intervals. In current-clamp mode, hyperpolarizing current pulses elicited voltage responses with pronounced voltage and time-dependent depolarizing “sag” at potentials negative to −70 mV. As Ih deactivates slowly after hyperpolarization, this current contributes to the formation of long-lasting rebound excitation. All VTA cells that were used in our experiments had the characteristics described above, which are commonly accepted electrophysiological features of DA neurons in this area (Aghajanian and Bunney 1973; Grace and Bunney 1983; Ungless et al. 2004).
Simultaneous actions of clonidine in VTA DA cells: Ih inhibition and reduction of the spontaneous firing rate
Clonidine (40 μM) application produced a small depolarizing shift of 1.7 ± 0.4 mV (n = 10) in the threshold level (i.e., the beginning of the spike upstroke) of the pacemaker potential of putative DA neurons. However, this shift was not statistically significant (from −53 ± 3 mV to −54 ± 3 mV, n = 10, P > 0.05). Interestingly, this slight depolarization was accompanied by a reduction in spontaneous firing of about 25% (interspike interval had changed from 140 ± 5 ms to 189 ± 4 ms, n = 10, P < 0.05) (Fig. 1A). Clonidine also reduced action potential (AP) amplitude in current-clamp mode (83.2 ± 0.21mV versus 73.1 ± 0.18 mV, n = 10, P < 0.05) and significantly reduced after-spike hyperpolarization by ~20% (9.57 ± 0.11 mV versus 7.07 ± 0.04 mV, n = 10, P < 0.05) (Fig. 1A). After hyperpolarization was defined as the vertical distance between the threshold potential and the most negative voltage reached at the end of the AP. Despite the fact that APs had less profound afterhyperpolarizations, under 40 μM of clonidine the membrane potential (MP) returned to the spike activation threshold level more slowly (the slope of the linear fit to the subthreshold MP without clonidine was 68 ± 2.8 mV/sec versus 49 ± 1.9 mV/sec with clonidine, n = 10, P < 0.05). All the above mentioned effects of Clonidine where not further investigated in the present study.
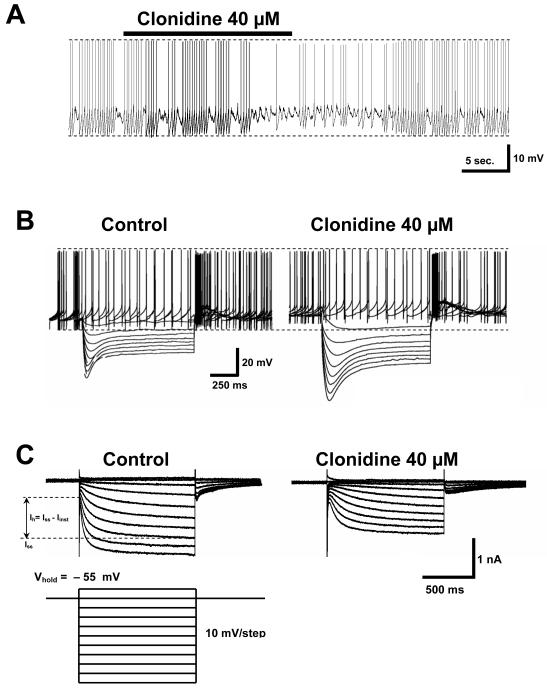
Fig. 1. Clonidine’s effect on VTA DA cells.
A. A 20 seconds bath application of the α-2 noradrenergic agonist clonidine (40 μM) evokes a slight depolarization of the membrane potential in VTA DA cells (1.7 ± 0.4 mV, n = 10). Concomitant with this event there is a significant decrease in the following measurements: action potential amplitude, afterhyperpolarization amplitude and, rate of subthreshold depolarization. There is a significant increase in the interspike interval.
B. Current-clamp response of a VTA DA cell to a family of 8 hyperpolarizing dc current pulses of 100 pA each (duration: 1 sec, frequency: 1/9 Hz) in the absence and presence of clonidine. The cell was held at 0 pA. Note that all the effects seen above (part A of the figure) are also seen here. The amplitude of voltage responses under clonidine is increased indicating an augmentation in the cell’s input resistance. Voltage “sag” is also more profound and visibly slowed indicating a shift in the voltage dependence of the underlying Ih.
C. In voltage clamp mode, bath application of clonidine reduces the difference between the steady state current (Iss) and the instantaneous current (Iinst) at all command potentials below the holding level (−55 mV), i.e., clonidine inhibits Ih = Iss − Iinst. In the presence of clonidine the membrane current reaches its steady state value at a much slower rate. As indicated by part B of the figure, clonidine’s effects on Ih, membrane potential and firing frequency follow a similar time course.
Clonidine application rapidly affected neuronal activity: for example, Fig. 1A shows a reduction in VTA cell activity within 20 sec after clonidine application. This effect could be washed out completely for clonidine concentrations up to 80 μM. Figure 1B shows the reduction of firing frequency caused by 40 μM clonidine administration, along with the effect of the drug on voltage responses to hyperpolarizing current pulses. The amplitude of voltage responses to a family of hyperpolarizing current pulses increased under clonidine application (Fig. 1B, right). This indicates an enhancement in cell membrane resistance proportional to the increase in voltage response. Voltage “sag” also became more profound and visibly slowed, suggesting slowing of Ih activation. Furthermore, the “sag” response moved to more negative potentials, which may indicate a shift in the voltage dependence of the underlying Ih. This data also suggests that Ih channels are the principal channels regulating a substantial proportion of the membrane conductance of VTA DA cells during hyperpolarization.
Under voltage-clamp, clonidine-induced Ih reduction could be seen directly as a decrease in the current response to a family of hyperpolarizing voltage steps (Fig. 1C). After every hyperpolarizing pulse below −65mV (following the fast capacitance transient), a membrane current appeared that slowly increased to a steady-state level over the course of the 1 second hyperpolarization (Fig. 1C, left). In the presence of clonidine (40 μM) current activation was slowed and the amplitude of both instantaneous and steady-state current decreased (Fig. 1C, right). Ih amplitude, i.e., the difference between instantaneous and steady-state level current, decreased by 34.9 ± 5.7% at −125mV, n = 12. Single exponential fits of the current response of VTA DA neurons with (n = 6) and without (n = 10) 40 μM of clonidine showed a ~2–fold increase in the voltage-dependent time constant of Ih activation (i.e., a decrease in the rate of activation) in the presence of clonidine (Fig. 2A).
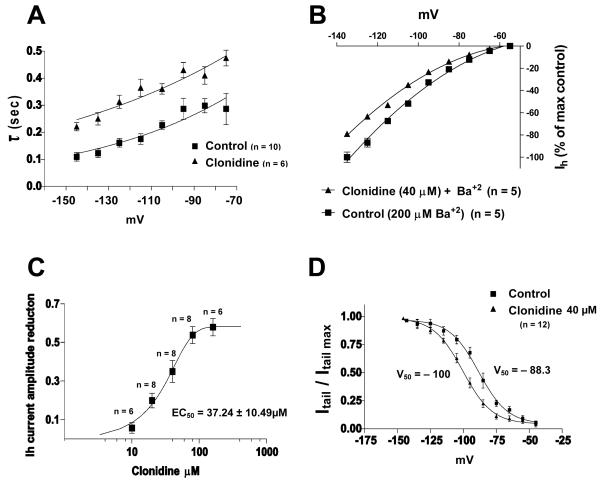
Fig. 2. Clonidine causes significant negative shift in Ih voltage dependence and Kir channels are not involved in this effect.
A. Monoexponential time constants (τ) vs membrane potential plot indicates that clonidine (40μM) slows down Ih activation upon membrane hyperpolarization, i.e., greater τ.
B. Normalized current-voltage curve showing that barium (Ba+2) does not alter clonidine’s effect on Ih, implying that Kir channels are not involved.
C. Dose-response curve of clonidine’s reduction of Ih current amplitude at −125 mV, illustrating that there is an increase inhibition at higher concentrations.
D. Activation curve showing that clonidine (40μM) produces a negative shift (−11.7 mV) in Ih voltage dependence (n = 12 is the same in both groups).
As clonidine affected both instantaneous and steady-state current amplitude, some of the effects might be attributable to inward rectifying potassium channels (Kir channels). To address this possibility, we added Ba++ (200 μM) to the external solution, which is a relatively selective blocker of Kir channels (Alagem et al. 2001; Standen and Stanfield 1978). Ba++ reduced instantaneous current (reduction of 18.2 ± 6.1% at −125mV, n = 5), but had no significant effect on the difference between instantaneous and steady-state current (i.e., Ih current). Clonidine in the presence of Ba++ still had the same pronounced inhibitory action on Ih (Fig 2B), changing not only the difference between instantaneous and steady-state currents, but also slowing Ih activation (time constant for −125 mV was 161 ± 24 ms vs. 313 ± 54 ms in the presence of clonidine, n = 5, P < 0.05).
We also determined the dose-dependence of clonidine reduction of Ih current amplitude at −125 mV in VTA DA cells (Fig. 2C), using the following concentrations: 10 μM (n = 6), 20 μM (n = 8), 40 μM (n = 8), 80 μM (n = 8), 160 μM (n = 6), and fitting the data to the sigmoidal dose-response curve using the algorithm present in GrafPad Prism. The EC50 was found to be 37.24 ± 10.49 μM (determined at −125 mV), which is about seventeen times greater than the value determined for the clonidine induced Ih blockade in freshly isolated rat dorsal root ganglion neurons (2.2 μM) (Yagi and Sumino 1998).
ZD7288 (20 μM), a well known blocker of Ih, was used to test if some other clonidine sensitive current was present in VTA DA cells. After ZD7288 blocked Ih, cells responded linearly to hyperpolarization current (in voltage clamp mode) and showed no activation of Ih (data not shown). In the presence of ZD7288 (20 μM), clonidine (40 μM) reduced this linear component only by 8.3 ± 5.1% (n = 6). The reversal potential of clonidine-sensitive current in the presence of Ba++ was calculated as −38.8 ± 3.6 mV (n = 6), using the protocol described in Liu et al. (2003) (data not shown). This is very close to the value determined by others for Ih current (Liu et al. 2003; Magee 1998; Yagi and Sumino 1998), thus confirming that the main current affected by clonidine was Ih.
We studied the voltage dependence activation of Ih in 12 putative VTA DA cells by analyzing tail current amplitudes in standard solution (with 1 μM TTX and 200 μM Ba++) and after administration of 40 μM of clonidine (data was fitted to the Boltzmann equation, see Fig. 2D). Clonidine shifted the V50 for Ih activation from −88.3 mV to −100.0 mV (P < 0.05) without affecting the slope factor. The maximum tail current achieved for the control solution was near −115 mV, and with addition of 40 μM clonidine the maximum reached approximately −125 mV.
Clonidine’s effects are due to the activation of the α-2 noradrenergic receptor
Clonidine is a known non-subtype-selective agonist of α-2 noradrenergic receptors (Harrison et al. 1991). These receptors have been identified in dopaminergic neurons of the midbrain and were shown to mediate an increase in depolarizing cationic conductance (Lee et.al.1998; Cathala et al. 2002). To determine if α-2 receptors were involved in the inhibition of Ih, we used another known non-subtype-selective agonist of α-2 noradrenergic receptors, UK14304. Application of 20 μM of UK14304 to VTA DA neurons in current-clamp mode reversibly inhibited spontaneous firing similar to clonidine’ action (from 9 ± 2 Hz to 5 ± 2 Hz, n = 6, data not shown). In voltage clamp mode the effect was also similar: Ih amplitude was reduced by 15.5 ± 4.2% (n = 6, Fig. 3A) and activation of Ih was substantially slowed during hyperpolarization (at −125 mV time constant was 89 ± 26 ms vs. 272 ± 41 ms with UK14304, n = 6).
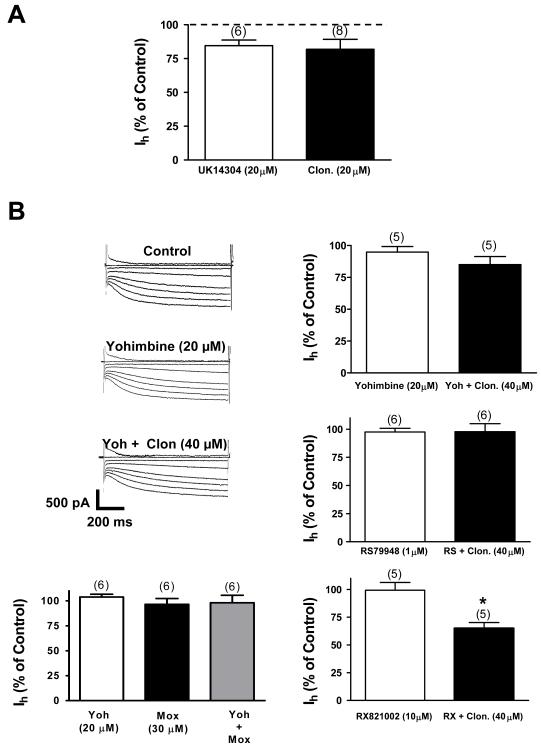
Fig. 3. Clonidine inhibits Ih by activation of the α-2 noradrenergic receptor.
A. Application of the α-2 noradrenergic agonist UK14304 (20 μM) causes Ih inhibition in VTA DA cells. This inhibition is similar to the one evoked by 20 μM of clonidine (P > 0.05). The numbers above the bars indicate the number of cells that were used for each treatment.
B. Current traces show that yohimbine, a selective non-subtype α-2 noradrenergic antagonist, blocks clonidine’s inhibitory effect. To the right, bar graphs summarize the effects of yohimbine. RS79948, another selective non-subtype- α-2 noradrenergic antagonist, also blocks clonidine’s action. Moxonidine (Mox) in the presence of yohimbine activates preferentially imidazoline receptors. The Ih was not decreased under this condition or under moxonidine alone. It seems that clonidine’s action is mediated by activation of α-2 receptors and do not involve an imidazoline receptor component. RX821002 (10 μM), an antagonist for α-2A and α-2D subtypes did not abolish clonidine’s evoked Ih inhibition. This inhibition was statistically different from that of RX 821002 alone (P < 0.05). Consequently, it appears that clonidine’s action is mediated by a specific α-2C receptor subtype. In all graphs the numbers above the bars indicate the number of cells that were used for each treatment.
Yohimbine is a non-subtype-selective α-2 noradrenergic receptor antagonist (Sakuta and Okamoto 1994). Yohimbine (20 μM) administration was able to block clonidine’s (40 μM) reduction of Ih (n=5, P > 0.05) (Fig. 3B). In addition, RS79948 (1 μM; n = 6, P > 0.05), another non-subtype-selective α-2 noradrenergic receptor antagonist, also blocked clonidine’s action on Ih (Fig. 3B). Clonidine effects on the pacemaker potential were not seen in the presence of these antagonists (data not shown). RX821002 (10 μM), a potent α-2A and α-2D specific antagonist (Trendelenburg et al., 1996), did not prevent clonidine-evoked Ih inhibition (Fig. 3B). This inhibition was not statistically different from that of clonidine alone (RX + clonidine: 35 ± 5.1% reduction at −125mV, n = 5 vs. clonidine: 34.9 ± 5.7% reduction at −125mV, n = 12, P> 0.05). As a result, it appears that clonidine’s action is mediated by an specific alpha-2 receptor subtype. Such possibility was not further investigated.
Clonidine is an imidazole derivative and thus in brain slices clonidine may activate the cell membrane imidazoline receptor I1 (Ernsberger and Shen 1997; Piletz and Sletten 1993). To rigorously confirm that effects seen on Ih are solely due to the activation of α-2 receptors and not to the I1 receptor we applied moxonidine (I1 agonist with some affinity for α-2 receptors) plus yohimbine. In the presence of yohimbie, moxonidine should only be acting through the I1 receptor (Ferry et al. 1988; Ernsberger et al. 1993). We found that moxonidine (30μM; n = 6, P > 0.05) in the presence of yohimbine or alone was unable to inhibit Ih (Fig. 3B). Thus, it appears that clonidine’s effects are mediated by α-2 noradrenergic receptors (Fig. 3B).
Clonidine induced Ih inhibition is not mediated by changes in cAMP levels
It is known that α-2 noradrenergic receptors can modulate the cAMP second messenger pathway by a G-protein coupled mechanism (Docherty 1998; Wurch et al. 2001). Alpha-2 noradrenergic receptors can differentially inhibit or stimulate adenylyl cyclases by the activation of distinct G(i/o) and G(s) protein families (Wurch et al. 2001). Most often α-2 receptors are negatively coupled to cAMP level (Jansson et al. 1994). HCN channel activation and Ih amplitude are known to be positively coupled to cAMP concentration (Hofmann et al. 2003). Thus, if clonidine affects α-2 noradrenergic receptors, it should bring down cAMP level and decrease Ih activation. We could prevent this effect by delivering large quantities of cAMP inside the cell via the patch pipette.
In order to investigate this possibility we used a recording pipette containing 3 mM of cAMP (Fig. 4A). Five minutes after the cell was exposed to cAMP Ih increased 17.7 ± 9.2% (n = 5) (P < 0.05). While this increase was small, it was consistent with previous findings that cAMP mainly augments the speed of activation of HCN channels in midbrain neurons and had little effect on steady-state current level (Pan, 2003; Liu et al, 2003). The time constant for activation of Ih at −125 mV decreased from 126 ± 27 ms in control condition to 72 ± 26 ms (P > 0.05) with added cAMP (n = 5). After clonidine (40 μM) was externally applied to the cells with 3 mM cAMP in the recording pipette, the current amplitude was reduced 29.25 ± 4.47% (n = 5, P< 0.05) (Fig. 4A). The time constant was also increased from 72 ± 26.5 ms to 283 ± 26.8 ms (n = 5, P < 0.05) under clonidine. The magnitude of changes was very similar to the changes clonidine evoked in control conditions, in spite of constant internal perfusion with cAMP (Fig. 4A).
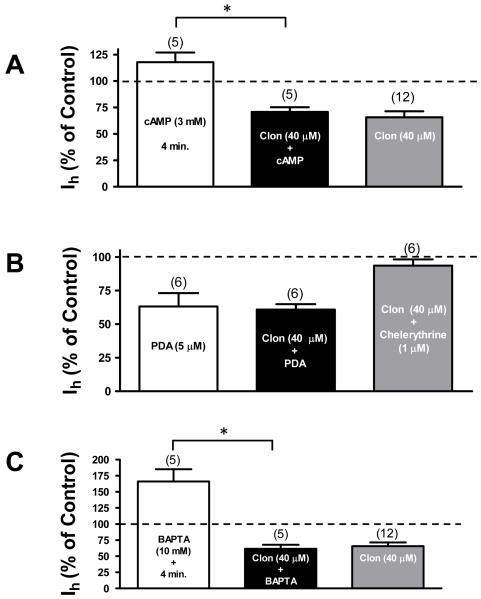
Fig. 4. Activation of α-2 noradrenergic receptor stimulates PKC in a calcium insensitive manner.
A. The bar graph shows that loading the patch pipette with 3 mM of cAMP does not block clonidine’s effect on Ih current. Minutes within the bar indicate the exposition time to cAMP. Note that there is not a significant difference between clonidine alone and clonidine plus cAMP (P > 0.05). The numbers above the bars indicate the number of cells that were used for each treatment. Asterisk denotes P < 0.05.
B. The bar graph shows that bath application of 5 μM of the PKC activator phorbol 12,13 diacetate (PDA) inhibits Ih and occludes clonidine’s effect. Additionally, bath application of 1 μM of the PKC antagonist chelerythrine blocks clonidine’s action indicating that PKC is necessary for clonidine-induced Ih inhibition in VTA DA cells. Note that there is not a significant difference between clonidine alone and clonidine plus PDA (P > 0.05). The numbers above the bars indicate the number of cells that were used for each treatment.
C. The bar graph shows that loading the patch pipette with 10 mM of the Ca2+ chelator BAPTA increases Ih amplitude. Minutes within the bar indicate the exposition time to BAPTA. This treatment does not block clonidine’s effect on Ih. Note that there is not a significant difference between clonidine alone and clonidine plus BAPTA (P > 0.05). The numbers above the bars indicate the number of cells that were used for each treatment. Asterisk denotes P < 0.05.
Clonidine-evoked Ih inhibition is mediated by PKC
Alpha-2 noradrenergic receptors can be coupled not only to the cAMP pathway, but also, in some cells simultaneously to the inositol phosphate pathway (Dochetry 1998; Wurch et al. 2001). This may partly coincide with data on Ca2+ dependence of Ih, as it is known that Ca2+ release due to activation of α-2 noradrenergic receptors is related to activation of phospholipase C (Dorn et al. 1997). There is also evidence, particularly in VTA DA neurons, that Ih is negatively coupled to the activation of PKC (Liu et al. 2003). To test the possibility that clonidine’s effect is via this pathway we used a specific activator of PKC, phorbol 12,13 diacetate (PDA). In our experiments bath application of 5 μM of PDA reduced Ih amplitude in putative VTA DA neurons by 36.84 ± 9.95% (n = 6) (Fig. 4B). Addition of 40 μM clonidine after PDA application only reduced Ih by 2.35 ± 4.09% (P > 0.05, n = 6). Under 5 μM PDA, the time constant changed, from 90 ± 20 ms to 200 ± 30 ms (n = 6, P < 0.05, at −125 mV) to 260 ± 20 ms (P > 0.05, n = 6) when clonidine (40 μM) was added. Also, we tested clonidine’s effect in the presence of 1 μM of the selective PKC inhibitor chelerythrine (Herbert et al., 1990). Bath application of this antagonist prevented clonidine’s action (P > 0.05; n = 6) (Fig. 4B). Taken together, these results suggest that clonidine’s inhibitory effect on Ih in VTA DA cells is mediated via PKC activation.
Clonidine’s effect on Ih is insensitive to Ca2+-buffering
As already mentioned, the α-2 noradrenergic receptor can be coupled to the inositol phosphate pathway (Dochetry 1998; Wurch et al. 2001) and thus its activation may increase the intracellular Ca2+ levels (Kukkonen et al. 1998; Jansson et al. 1998). Therefore, it may be possible that the α-2 evoked Ih inhibition is sensitive to Ca2+–buffering. To explore this possibility we measured clonidine’s effect on Ih in the presence of 10 mM of BAPTA (Ca2+ chelator) in the internal pipette solution (Fig. 4C). Interestingly, addition of BAPTA substantially increased Ih, the effect developing over time, reaching its peak at 3 to 4 minutes. In 4 min. Ih amplitude increased to 166 ± 19% (n = 5, P < 0.05, Fig. 4C) of its initial value. The activation time constant for Ih did not change significantly (at −125 mV, 87 ± 12 ms without BAPTA and 84 ± 5 ms with BAPTA in the pipette, P > 0.05, n = 5). After 5 min of BAPTA exposure, addition of clonidine (40 μM) to the external solution reduced Ih amplitude by 38.5 ± 6.2% (n = 5), which is not significantly different (P > 0.05) from clonidine’s effect on DA neurons with no BAPTA in the pipette (34.9 ± 5.7%, n = 12) (Fig. 4C). Activation of Ih in the presence of clonidine also was slowed down as usual (activation time constant changed from 84 ± 15 ms to 126 ± 11 ms at −125 mV, P < 0.05, that is smaller, but not statistically different than normal, P > 0.05). Hence, since clonidine’s effect on Ih did not change with 10 mM of BAPTA inside the neuron, it follows that clonidine’s action on Ih is insensitive to Ca2+-buffering.
Discussion
Using brain slice preparations and whole-cell patch clamp techniques we showed that α-2 receptor activation inhibits the Ih in putative rat VTA DA neurons.Yohimbine and RS79948, two non-subtype-selective α-2 receptor antagonists, prevented clonidine’s inhibitory action confirming that its effect is mediated by an α-2 receptor. Furthermore, RX821002, a noradrenergic specific antagonist for α-2A and α-2D did not prevent Ih inhibition. This finding suggests that clonidine is acting via an α-2C receptor moiety since this subtype is the most predominant in the VTA with other variants having a non-significant contribution (Lee et al., 1998; Rosin et al., 1996). We also found that the addition of 3 mM cAMP to the pipette’s internal solution failed to prevent clonidine’s inhibitory effect. Thus, cAMP level does not appear to be relevant in transducing clonidine’s action. PDA, a PKC specific activator, had a profound inhibitory effect on Ih. After PDA, clonidine failed to change Ih amplitude suggesting it occluded clonidine’s effect. Furthermore, bath application of chelerythrine, a PKC antagonist, prevented clonidine’s action. These results are in accordance to published data showing that PKC activation leads to an inhibition of Ih in VTA DA neurons (Liu et al. 2003). Interestingly, intracellular application of 10 mM of BAPTA was unable to stop clonidine’s effect, suggesting that Ca2+ may play a minor role in the signal transduction cascade that leads to Ih inhibition.
Here we showed that activation of α-2 noradrenergic receptors causes a depolarization of around 2 mV in the pacemaker potential of putative VTA DA cells (Fig 1A). Concomitant to this depolarization and showing a similar time course, Ih displays a negative shift in its voltage dependence (Fig 1A, B and Fig 2C). This shift should lead to membrane hyperpolarization if Ih is active during the pacemaker potential. However, our results suggest that Ih is not active during the pacemaker potential since the opposite effect was observed. This small depolarization could be the result of the activation of a small inward current. In effect, it has been reported that stimulation of α-2 receptors in midbrain DA cells of the substantia nigra activates a small depolarizing current of about 20 pA when cells are voltage-clamp at − 60 mV (Cathala et al., 2002). An identical inward current had also been recorded in the VTA DA cells in the presence of neurotensin (Chien et al. 1996). It is interesting to note that the activation of this conductance does not involve the actions of cAMP, cGMP, Ca+2, PKA or PKC (Farkas et al. 1996; Cathala and Paupardin-Tritsch 1997) but is probably the result of the direct interaction of a G protein with the ion channel similar to what happens with the muscarinic K+ channel (Chien et al. 1996). Therefore, the α-2 mediated reduction in the frequency of the pacemaker firing of VTA DA cells may be due to the direct actions of a G protein on the conductances that determine the pacemaker rhythm.
In this investigation the activation of α-2 noradrenergic receptor in putative VTA DA cells leads to Ih inhibition. This discovery may come to no surprise since in different regions of the nervous system such effect has been documented. These neural structure include the dorsal root ganglion (Yagi and Sumino 1998), hypoglossal motoneurons (Parkis and Berger 1997), trigeminal ganglion (Takeda et al. 2002), and pyramidal neurons of the PFC (Carr et al. 2007). Our results add to this group suggesting that the α-2-mediated Ih inhibition is a rule rather than exception in nerve cells that express this current. Given that the VTA is a brain structure central to reward processing and addiction (Grace et al. 2007; Schultz 2002; Brodie et al. 1999; Nestler, 2004; Grace 2000; Grace 1991) and that the Ih conductance has a profound impact in cell excitability (Wahl-Schott and Biel 2009; Chen et al., 2002; Berger et al., 2001; Berger et al., 2003, van Welie et al., 2006) the α-2 mediated Ih inhibition in VTA DA cell may be highly relevant to reward and drug abuse.
Clonidine has been shown to be an imidazole derivative thus some of clonidine’s effects can be attributed to the agonist binding to imidazoline receptors (Dardonville and Rozas 2004; Hudson 2000). However, our results with yohimbine and RS79948 suggest that this outcome is unlikely. In the central nervous system two imidazoline receptors are widely recognized: I1 and I2 (Tanabe et al. 2006). The first is located at the cell membrane (Ernsberger and Shen 1997; Piletz and Sletten 1993), while the second is localized at the outer membrane of the mitochondria and it is known to inhibit monoamine oxidase activity (Carpéné et al. 1995; Ozaita et al. 1997; Lalies et al. 1999). Hence, in brain slices clonidine may activate the I1 receptor. Moxonidine is known to be 40-fold more selective to I1 receptors than to α-2 receptors (Ferry et al. 1988; Ernsberger et al. 1993). As a result, in the presence of yohimbine, moxonidine should only be acting through the I1 receptor. Indeed, our results show that moxonidine was unable to inhibit Ih in the presence of yohimbine or alone further confirming that clonidine’s effects are mediated by α-2 noradrenergic receptors.
The present work shows that activation of PKC in putative VTA DA cells leads to a negative shift in Ih voltage dependence. Such finding is in accordance with previous reports where the activity of this kinase is clearly linked to Ih attenuation (Carr et.al. 2007; Liu et al 2003; Cathala and Paupardin-Tritsch 1997). However, in only one of these investigations the inhibition was associated with a hyperpolarizing shift in the current’s voltage dependence (Liu et al 2003). This discrepancy could be the result of the activation of PKC isoforms that are functionally distinct (Reyland, 2009). For instance, Wanat et al. (2008) have reported that in VTA DA cells corticotropin-releasing factor (CRF) activates the CRF-R1 receptor resulting in a PKC-evoked enhancement of Ih that did not alter the current’s voltage dependence. Therefore, it appears that the final outcome on Ih PKC-evoked modulation is isoform-specific.
The present investigation shows for the first time to our knowledge that the fast Ca2+ chelator BAPTA significantly augments the Ih current in putative VTA DA cells. This finding is consistent with previous reports in other brain regions. For example, in rat hippocampal pyramidal neurons BAPTA enhances Ih (Velumian et al. 1997). Additionally, in the trigeminal mesencephalic nucleus of the rat, BAPTA prevented the reduction of Ih caused by Ca2+ elevation (Khakh and Henderson 1998) and in neurons of the rat raphe nucleus BAPTA augmented the Ih (Pan 2003). Consequently, neuromodulators that control Ca2+ levels in VTA DA cells should have a significant impact on Ih modulation.
Ih conductance presents a high channel density at the membrane of the distal dendritic tree in comparison with the membrane at perisomatic regions (Wahl-Schott and Biel 2009; Chen et al., 2002; Berger et al., 2001; Berger et al., 2003, van Welie et al., 2006). Thus, Ih inhibition results in an improved temporal summation of synaptic inputs (Carr et al. 2007). In pyramidal neurons of the cerebral cortex, dendritic Ih prevents temporal summation of synaptic inputs making the neuron less likely to fire a burst of action potentials (Berger et al., 2001; Berger et al., 2003, van Welie et al., 2006). In midbrain DA cells, Ih appears to have mostly a dendritic location as indirectly predicted from the space-clamp manipulations used when recording this conductance (Arencibia-Albite et al., 2007; Cathala and Paupardin-Tritsch, 1999; Watts et al., 1996). Consequently, the Ih of midbrain DA cells may also reduce the likelihood of burst firing since this spiking mode is afferent-driven (Cooper, 2002; Kitai et al., 1999; Overton and Clark, 1997; Grace and Bunney, 1983) and therefore dependent on temporal summation. It has been proposed that neuromodulators that inhibit Ih may facilitate the transition from pacemaker activity to burst firing in VTA DA cells (Aerencibia-Albite, et al. 2007). Hence, it is possible that Ih inhibition by α-2 receptors in midbrain DA cells results in an improved temporal summation making the transition from pacemaker activity to burst firing more probable. However, Hopf et al. (2007) have shown that inhibition of Ih with ZD7288 did not facilitate the transition to burst firing. In the latter study this firing mode was evoked by bath application of NMDA plus apamin. This treatment resulted in the development of rhythmic burst firing which may be different from the afferent-driven bursting activity that takes place in the whole animal. In thalamic relay neurons of the lateral geniculate nucleus, rhythmic bursting is generated by interaction among intrinsic conductances of the cell and not by activity of excitatory afferents, i.e., it is not dependent on temporal summation of synaptic inputs (Wahl-Schott and Biel 2009; Luthi and McCormick 1998; McCormick and Pape 1990; Pape 1996). Thus, although Ih inhibition in VTA DA cells may not facilitate the transition to rhythmic burst firing (which in brain slices is pharmacologically-driven), it is possible that this inhibition can facilitate afferent-driven bursting since a reduction of this dendritic conductance leads to a better temporal summation (Carr et al. 2007; Berger et al., 2001; Berger et al., 2003, van Welie et al., 2006).
In conclusion, the present results show that activation of α-2 noradrenergic receptors in putative VTA DA cells causes Ih inhibition. A functional implication of the latter finding is that this inhibition may facilitate the transition from pacemaker firing to afferent-driven bursting. This transition may play a central role in the synaptic plasticity changes that are known to occur in the mesocorticolimbic system after drug exposure.
Acknowledgements
This work was supported by grants from NIGMS, GM-08224, 1SC1GM084854-01 and MBRS-RISE R25-GM061838 to CAJR. The authors thank Dr. Wade Pearson for proofreading an initial version of the manuscript and Maria C. Velazquez for her technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian G, Bunney BC. In: Usdin E, Snyder SH, editors. Central dopaminergic neurons: Neurophysiologic identification and responses to drugs; Frontiers in catecholamine research; proceedings; Elmsford, N.Y., Pergamon Press. 1973.pp. 643–648. [Google Scholar]
- Alagem N, Dvir M, Reuveny E. Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arencibia-Albite F, Paladini C, Williams JT, Jiménez-Rivera CA. Noradrenergic modulation of the hyperpolarization-activated cation current (Ih) in dopamine neurons of the ventral tegmental area. Neuroscience. 2007;149:303–314. doi: 10.1016/j.neuroscience.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Kalivas P. Brain norepinephrine rediscovered in addiction research. Biol Psychiatry. 2008;63:1005–1006. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Senn W, Luscher RL. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J. Neurophysiol. 2003;90:2428–2437. doi: 10.1152/jn.00377.2003. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum M, Luscher RL. High Ih channels density in distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J. Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Brodie MS, McElvain MA, Bunney EB, Appel SB. Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 1999;290:325–333. [PubMed] [Google Scholar]
- Carpéné C, Collon P, Remaury A, Cordi A, Hudson A, Nutt D, Lafontan M. Inhibition of amine oxidase activity by derivatives that recognize imidazoline I2 sites. J Phramacol Exp Ther. 1995;272:681–688. [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. α2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN current. J Physiol. 2007;584.2:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Guyon A, Eugene D, Paupardin-Tritsch D. Alpha2-adrenoceptor activation increases a cationic conductance and spontaneous GABAergic synaptic activity in dopaminergic neurones of the rat substantia nigra. Neuroscience. 2002;115:1059–1065. doi: 10.1016/s0306-4522(02)00542-0. [DOI] [PubMed] [Google Scholar]
- Cathala L, Paupardin-Tritsch D. Effect of catecholamines on the hyperpolarization-activated Ih and the inward rectifying potassium Ikir currents in rat’s substantia nigra pars compacta. Eur. J. Neurosci. 1999;11:398–406. doi: 10.1046/j.1460-9568.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- Cathala L, Paupardin-Tritsch D. Neurotensin inhibition of the hyeprpolarization-cation current (Ih) in rat substantia nigra pars compacta implicates the protein kinase C pathway. J. Physiol. 1997;503:87–97. doi: 10.1111/j.1469-7793.1997.087bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Santhakumar V, Soltesz I. H-channels in epilepsy: new targets for seizure control? Trends Pharmacol. Sci. 2002;23:552–557. doi: 10.1016/s0165-6147(02)02110-7. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001b;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien PE, Farkas RH, Nakajima S, Nakajima Y. Single-channel properties of the non-selective cation conductance induced by neurotensin in dopaminergic neurones. Proc. Natl. Acad. Sci. USA. 1996;93:14917–14921. doi: 10.1073/pnas.93.25.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DC. The significance of the action potential bursting in the brain reward circuit. Neurochem. Int. 2002;41:333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Dardonville C, Rozas I. Imidazoline binding sites and their ligands: an overview of the different chemical structures. Med Res Rev. 2004;24:639–661. doi: 10.1002/med.20007. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Oswald KJ, McCluskey TS, Kuhel DG, Liggett SB. Alpha 2A-adrenergic receptor stimulated calcium release is transduced by Gi-associated G(beta gamma)-mediated activation of phospholipase C. Biochemistry. 1997;36:6415–6423. doi: 10.1021/bi970080s. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Friedman JE, Koletsky RJ. The I1-imidazoline receptor: from binding site to therapeutic target in cardiovascular disease. J Hypertens - Suppl. 1997;15:S9–23. doi: 10.1097/00004872-199715011-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger P, Damon TH, Graff LM, Schafer SG, Christen MO. Moxonidine, a centrally acting antihypertensive agent, is a selective ligand for I1-imidazoline sites. J. Pharmacol. Exp. Ther. 1993;264:172–182. [PubMed] [Google Scholar]
- Farkas RH, Chien PY, Nakajima S, Nakajima Y. Properties of a slow nonselective cation conductance modulated by neurotensin and others neurotransmitters in midbrain dopaminergic neurons. J. Neurophysiol. 1996;76:1968–1981. doi: 10.1152/jn.1996.76.3.1968. [DOI] [PubMed] [Google Scholar]
- Ferry D, Armah BI, Goll A, Glossmann H. Characteristics of the binding of antihypertensive agent moxonidine to α2-adrenoceptors in rat brain membranes. Arzneimittelforschung. 1988;38:1442–1445. [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implication for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl. 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Harrison JK, D’Angelo DD, Zeng DW, Lynch KR. Pharmacological characterization of rat alpha 2-adrenergic receptors. Mol Pharmacol. 1991;40:407–412. [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172(3):993–9. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Kaupp UB, International Union of P International Union of Pharmacology. XLII. Compendium of voltage-gated ion channels: cyclic nucleotide-modulated channels. Pharmacol Rev. 2003;55:587–589. doi: 10.1124/pr.55.4.10. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Hudson A. Tocris Reviews. 2000. Imidazoline Receptors; pp. 1–4. [Google Scholar]
- Jansson CC, Kukkonen JP, Nasman J, Huifang G, Wurster S, Virtanen R, Savola JM, Cockcroft V, Akerman KE. Protean agonism at alpha2A-adrenoceptors. Mol Pharmacol. 1998;53:963–968. [PubMed] [Google Scholar]
- Jansson CC, Marjamaki A, Luomala K, Savola JM, Scheinin M, Akerman KE. Coupling of human alpha 2-adrenoceptor subtypes to regulation of cAMP production in transfected S115 cells. Eur J Pharmacol. 1994;266:165–174. doi: 10.1016/0922-4106(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Hyperpolarization-activated cationic currents (Ih) in neurones of the trigeminal mesencephalic nucleus of the rat. J Physiol. 1998;510:695–704. doi: 10.1111/j.1469-7793.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr. Opin. Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Renvaktar A, Shariatmadari R, Akerman KE. Ligand- and subtype-selective coupling of human alpha-2 adrenoceptors to Ca++ elevation in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1998;287:667–671. [PubMed] [Google Scholar]
- Lalies MD, Hibell A, Hudson AL, Nutt DJ. Inhibition of central monoamine oxidase by imidazoline2 site-selective ligands. Ann NY Acad Sci. 1999;881:114–117. doi: 10.1111/j.1749-6632.1999.tb09350.x. [DOI] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of α-2c-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–1096. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. H-current: properties of neuronal and network pace maker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegemental area revisited: is there an electrophysiological marker for dopaminergic neurons. J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in the thalamic relay neurons. J Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J. Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res. Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Olmos G, Boronat MA, Lizcano JM, Unzeta M, Gracia-Sevilla JA. Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver. Br J Pharmacol. 1997;121:901–912. doi: 10.1038/sj.bjp.0701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. Kappa-opioid receptor-mediated enhancement of the hyperpolarization-activated current (I(h)) through mobilization of intracellular calcium in rat nucleus raphe magnus. J Physiol. 2003;548:765–775. doi: 10.1113/jphysiol.2002.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Berger AJ. Clonidine reduces hyperpolarization-activated inward current (Ih) in rat hypoglossal motoneurons. Brain Res. 1997;769:108–118. doi: 10.1016/s0006-8993(97)00677-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peterson SL, Olsta SA, Matthews RT. Cocaine enhances medial prefrontal cortex neuron response to ventral tegmental area activation. Brain Res. 1990;24:267–273. doi: 10.1016/0361-9230(90)90214-k. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Sletten K. Nonadrenergic imidazoline binding sites on human platelets. J. Pharmacol. Exp. Ther. 1993;267:1493–1502. [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front. Biosci. 2009;14:2386–99. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG. Distribution of alpha 2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372(1):135–65. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Okamoto K. Inhibition by imidazoline and imidazolidine derivatives of glibenclamide-sensitive K+ currents in Xenopus oocytes. Eur J Pharmacol. 1994;259:223–231. doi: 10.1016/0014-2999(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute exposure alerts spine density and long-term potentiation in the ventral tegmental area. Eur. J. Neurosci. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Solomon JS, Nerbonne JM. Two kinetically distinct components of hyperpolarization-activated current in rat superior colliculus-projecting neurons. J Physiol. 1993;469:291–313. doi: 10.1113/jphysiol.1993.sp019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Ikeda M, Tanimoto T, Lipski J, Matsumoto S. Changes of the excitability of rat trigeminal root ganglion neurons evoked by alpha(2)-adrenoreceptors. Neuroscience. 2002;115:731–741. doi: 10.1016/s0306-4522(02)00481-5. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Kino Y, Honda M, Ono H. Presynaptic I1-imidazoline receptors reduce gabaergic synaptic trasnmission in striatal medium spiny neurons. J Neurosci. 2006;26(6):1795–1802. doi: 10.1523/JNEUROSCI.4642-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Wahl CA, Starke K. Antagonists that differentiate between alpha 2A-and alpha 2D-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1996;353(3):245–9. doi: 10.1007/BF00168625. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- van Welie I, Remme MW, van Hooft JA, Wadman WJ. Different levels of Ih determine distinct temporal integration in bursting and regular spiking neurons in rat subiculum. J Physiol. 2006;576(Pt 1):203–214. doi: 10.1113/jphysiol.2006.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velumian AA, Zhang L, Pennefather P, Carlen PL. Reversible inhibition of IK, IAHP, Ih and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflugers Archiv - Eur J Physiol. 1997;433:343–350. doi: 10.1007/s004240050286. [DOI] [PubMed] [Google Scholar]
- Wahl-Schott C, Biel M. HCN channels: Structure, cellular regulation and physiological function Cell. Mol. Life Sci. 2009;66:470–494. doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AE, Williams JT, Hederson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J. Neurophysiol. 1996;76:2–10. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- Wurch T, Okuda J, Pauwels PJ. Reciprocal modulation of alpha (2A)-adrenoceptor and G(alpha o) protein states as determined by carboxy-terminal mutagenesis of a G(alpha o) protein. Mol Pharmacol. 2001;60:666–673. [PubMed] [Google Scholar]
- Yagi J, Sumino R. Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol. 1998;80:1094–1104. doi: 10.1152/jn.1998.80.3.1094. [DOI] [PubMed] [Google Scholar]