Summary
Circulating leptin and insulin convey information regarding energy stores to the central nervous system, particularly the hypothalamus. Hypothalamic pro-opiomelanocortin (POMC) neurons regulate energy balance and glucose homeostasis and express leptin and insulin receptors. However, the physiological significance of concomitant leptin and insulin action on POMC neurons remains to be established. Here we show that mice lacking both insulin and LepRs in POMC neurons (Pomc-Cre, Leprflox/flox IRflox/flox mice) display systemic insulin resistance, which is distinct from the single deletion of either receptor. In addition, Pomc-Cre, Leprflox/flox IRflox/flox female mice display elevated serum testosterone levels and ovarian abnormalities resulting in reduced fertility. We conclude that direct action of insulin and leptin on POMC neurons is required to maintain normal glucose homeostasis and reproductive function.
Introduction
Identifying mechanisms linking obesity and insulin resistance is crucial for understanding type 2 diabetes. Changing levels of circulating insulin and leptin inform the CNS regarding energy stores. Thus brain-specific disruption of the insulin receptor causes mild obesity, hyperleptinemia, and insulin resistance (Bruning et al., 2000). Hypothalamic insulin signaling also influences hepatic glucose production (HGP), and its blockade is implicated in diabetes (Gelling et al., 2006; Inoue et al., 2006; Obici et al., 2002). Likewise, leptin action in the hypothalamus is required to maintain both normal body weight and insulin sensitivity (Balthasar et al., 2004; Coppari et al., 2005; Dhillon et al., 2006; Morton et al., 2003; Morton et al., 2005; van de Wall et al., 2008). Indeed, leptin and insulin can engage similar hypothalamic intracellular signaling pathways (Carvalheira et al., 2005; Mirshamsi et al., 2004; Niswender et al., 2003; Niswender et al., 2001).
Within the hypothalamus, POMC neurons are critical regulators of energy balance and glucose homeostasis (Baskin et al., 1999; Benoit et al., 2002; Cheung et al., 1997; Elmquist et al., 1998; Porte et al., 2002). Deletion of SOCS-3, a negative regulator of the actions of leptin, insulin, and various cytokines, in POMC neurons results in modest changes in body weight but substantially improved glucose homeostasis and insulin sensitivity, as well as resistance to dietary obesity (Kievit et al., 2006). However, deletion of leptin receptors (LepRs) in POMC neurons alone causes mild obesity (Balthasar et al., 2004), and reportedly induces no (Balthasar et al., 2004) or mild effects on glucose homeostasis in males only (Shi et al., 2008). In addition, deletion of POMC insulin receptors (IRs) results in no discernable impact on body weight or glucose regulation (Konner et al., 2007).
These results call into question the physiological importance of direct leptin and insulin action on POMC neurons for modulating glucose homeostasis. Recent work from our laboratory has shown that leptin and insulin induce changes in membrane potential in disparate subgroups of ARC POMC cells (Williams et al., 2010). Thus, we hypothesized that previous studies deleting one receptor type from a subgroup of POMC neurons failed to eliminate the collective POMC neuronal regulation of glucose levels maintained by the other adiposity signal. To this end, we characterized mice lacking both insulin and LepRs specifically in POMC neurons (Pomc-Cre, Leprflox/flox IRflox/flox mice).
Results
We crossed mice lacking LepRs in POMC cells (Pomc-Cre, Leprflox/flox mice) (Balthasar et al., 2004) with mice carrying a loxP-modified IR allele (IRflox/flox) (Konner et al., 2007) to create Pomc-Cre, Leprflox/flox IRflox/flox mice. These mice lack IRs and LepRs in POMC-expressing cells in the hypothalamus and pituitary but retain them in other cell types and tissues, such as liver and ovary (Figure S1a–d). POMC expressing neurons are found in brainstem, although many (Coppari et al., 2005; Huo et al., 2006; Morton et al., 2003; Morton et al., 2005; Perello et al., 2007), but not all (Ellacott et al., 2006), reports suggest that action in the hypothalamus underlies leptin’s role in energy balance and glucose regulation. However, it should be noted that we cannot rule out a potential role of NTS neurons in these studies.
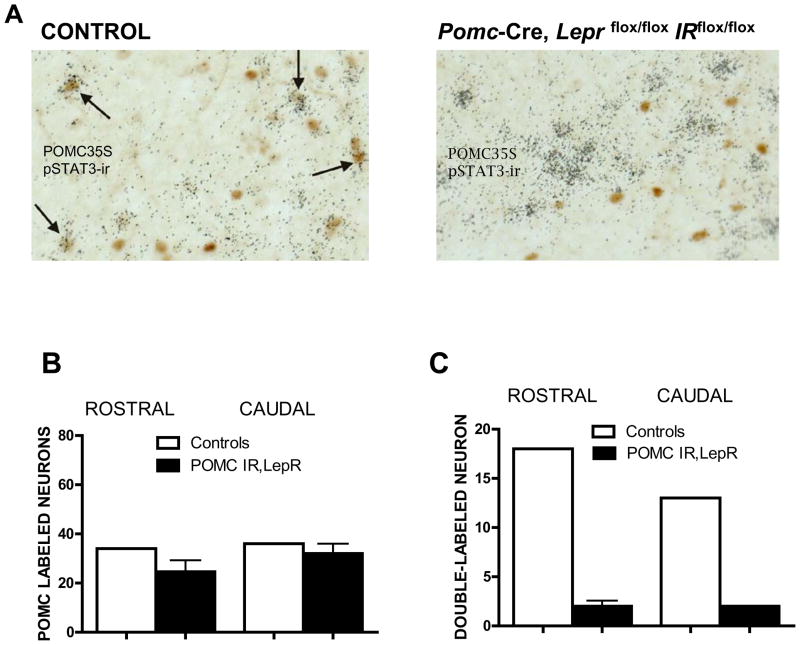
In mice lacking both leptin and IRs in POMC neurons, hypothalamic POMC neuronal distribution resembled controls (Figure 1b). As expected, leptin-induced phosphorylation of STAT3 was absent in POMC neurons of Pomc-Cre, Leprflox/flox IRflox/flox mice (Figure 1a,c). We also used our new insulin signaling reporter mouse to examine the effect of the targeted deletions on FOXO1a translocation (Fukuda et al., 2008). Despite administration of a pharmacological dose of insulin to hypothalamic slices, nuclear exclusion of FOXO1a was absent in POMC neurons of double knockout mice (Figure 2a,b). Since POMC is also expressed in the corticotropes in the anterior pituitary gland and glucocorticoids can cause glucose intolerance and insulin resistance (Jacobson et al., 2005; Zinker et al., 2007), we assessed corticosterone levels under baseline conditions and with a moderately stressful social challenge. We found that corticosterone levels of Pomc-Cre, Leprflox/flox IRflox/flox mice were similar to controls (Table 1), indicating that these mice are not hypersensitive to stress.
Figure 1. POMC neurons in Pomc-Cre, Leprflox/flox IRflox/flox mice no longer respond to leptin.
Leptin was administered ip (10microg) and animals perfused. B, POMC35S-labeled neurons and C, STAT3 phosphorylation colocalized with POMC35S-labeled neurons were quantified in the rostral and caudal ARC (Paxinos levels 43 and 47) in littermate controls (white bars) and Pomc-Cre, Leprflox/flox IRflox/flox mice (black bars). Values are presented as means +/− SE. See also figure S1.
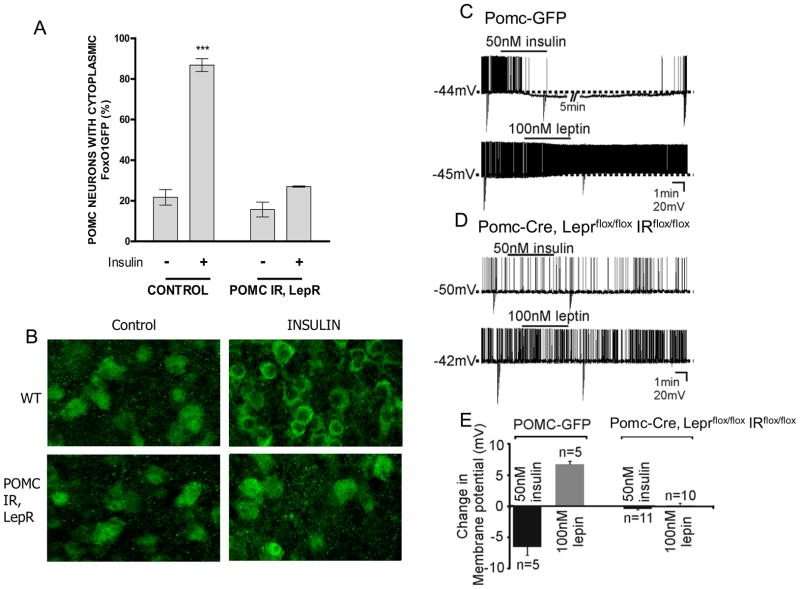
Figure 2. POMC neurons in Pomc-Cre, Leprflox/flox IRflox/flox mice do not respond to insulin.
A, Hypothalamic organotypic slices from FoxO1GFP-POMC reporter mice were treated with insulin (100 nM for 30 min) or vehicle and compared with slices from Pomc-Cre, Leprflox/flox IRflox/flox mice carrying the FoxO1GFP reporter and subjected to anti-GFP immunohistochemistry. Scale bar, 20 μm. B, The percentage of neurons with cytoplasmic FoxO1GFP. C. Current-clamp recordings at resting membrane potential depicting an insulin-induced hyperpolarization and a leptin-induced depolarization in separate POMC neurons from POMC-GFP mice. Downward deflections are responses to rectangular current steps. D. Current-clamp recordings show insulin and leptin fail to influence the membrane potential of POMC neurons in Pomc-Cre, Leprflox/flox IRflox/flox mice. E. Histogram of insulin- and leptin-induced responses in identified POMC neurons from POMC-GFP and Pomc-Cre, Leprflox/flox IRflox/flox mice. Values are means +/− SE. ***p < 0.001, compared by 2-way ANOVA followed by Bonferroni post-hoc. See also figure S2.
Table 1. Endocrine Profile of LepR/IRflox, POMC-cre mice.
Mouse trunk blood was collected from 3 month old mice and assayed for hormone levels. Serum corticosterone levels measured by EIA from mice in basal and under conditions of psychosocial stress (Hill et al., 2008b). Blood was collected from female mice on diestrus for assay of LH, FSH by RIA, and prolactin and estradiol levels by EIA kit. Groups were compared by t-test. Values are presented as means +/− SE.
| Littermate Controls | s.e. | n | LepR/IR flox, POMC-cre | s.e. | n | |
|---|---|---|---|---|---|---|
| Corticosterone (ng/ml): males, basal | 158.2 | 20.83 | 8 | 140.5 | 17.99 | 6 |
| Corticosterone (ng/ml): males, psychosocial stress | 264.2 | 31.82 | 13 | 278.8 | 16.27 | 6 |
| Corticosterone (ng/ml): females, basal | 166 | 47.09 | 6 | 150.4 | 33.95 | 6 |
| Corticosterone (ng/ml): females, psychosocial stress | 324 | 27 | 6 | 361.9 | 43.82 | 6 |
| FSH (ng/ml): females | 6.439 | 1.029 | 16 | 8.29 | 1.273 | 12 |
| LH/FSH ratio: females | 0.089 | 0.02 | 16 | 0.07274 | 0.0189 | 12 |
| Estradiol (pg/ml): females | 13.08 | 5.842 | 5 | 12.14 | 2.873 | 7 |
Previous reports suggest that leptin activates POMC neurons, while insulin inhibits POMC neuronal activity (Choudhury et al., 2005; Claret et al., 2007; Cowley et al., 2001; Hill et al., 2008b; Plum et al., 2006). Thus we examined the acute effects of leptin and insulin in POMC neurons from Pomc-Cre, Leprflox/flox IRflox/flox mice carrying a floxed Rosa-GFP allele and POMC-GFP control mice using whole-cell patch-clamp electrophysiology. Similar to previous reports, leptin (100nM) superfusion resulted in a depolarization in 5 of 8 POMC neurons from POMC-GFP mice (6.6 ± 0.4mV; resting membrane potential = −45.4 ± 1.9 mV; n=5; fig 2c,e). Likewise, some POMC neurons from POMC-GFP mice hyperpolarized in response to 50nM insulin (5 of 10 POMC neurons; −6.6 ± 1.4mV, resting membrane potential = −44.2 ± 2.8mV; n=5; fig 2c,e). However, POMC neurons from Pomc-Cre, Leprflox/flox IRflox/flox mice failed to respond to either leptin (0.1 ± 0.3mV; n=10; fig 2d,e) or insulin (−0.4 ± 0.2mV, n=11; fig 2d,e). The resting membrane potential, average input resistance, and whole cell capacitance of POMC neurons from Pomc-Cre, Leprflox/flox IRflox/flox mice were statistically similar to POMC-GFP neurons (Figure S2). These data suggest POMC neurons from Pomc-Cre, Leprflox/flox IRflox/flox mice have similar cellular properties to POMC neurons from POMC-GFP mice, but the acute effects of leptin and insulin are disrupted in POMC neurons from Pomc-Cre, Leprflox/flox IRflox/flox mice.
Assessment of Energy Homeostasis
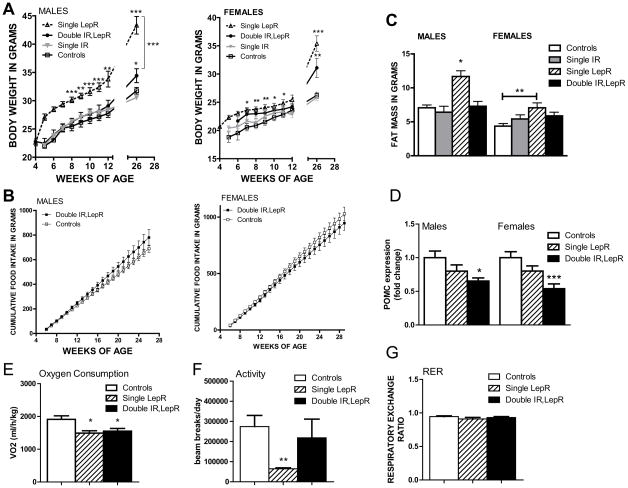
We next examined the effect of simultaneous deletion of insulin and LepRs in POMC neurons on energy balance. As previously shown (Konner et al., 2007), deletion of the IR alone in POMC neurons did not affect body weight (Figure 3a). Deletion of LepRs alone in POMC neurons produced obesity within the first 3 months of age, as we previously reported (Balthasar et al., 2004; Konner et al., 2007). Interestingly, the additional deletion of IRs in the context of POMC specific deletion of LepRs ameliorated obesity (Figure 3a). Specifically, males lacking insulin and LepRs in POMC neurons were significantly heavier by 6 months than littermate controls, but weighed significantly less than the single LepR deleted mice. No change was seen in food intake in Pomc-Cre, Leprflox/flox IRflox/flox mice (Figure 3b). To examine the underlying tissue contribution to the body weight phenotype in Pomc-Cre, Leprflox/flox IRflox/flox mice, body composition was assessed using NMR. As previously reported, the deletion of IR alone did not change adipose tissue deposition (Fig 3c). However, at 6 months of age, both males and female Pomc-Cre, Leprflox/flox IRflox/flox mice had less body fat than Pomc-Cre, Leprflox/flox mice (Fig 3c).
Figure 3. Altered metabolism and POMC expression in Pomc-Cre, Leprflox/flox IRflox/flox mice.
A, Body weight curves of male Leprflox/flox IRflox/flox mice (open squares, n = 12), Pomc-Cre, IRflox/flox (filled grey triangles, n = 16), Pomc-Cre, Leprflox/flox (open black triangles and dashed line, n = 8), and Pomc-Cre, Leprflox/flox IRflox/flox mice (filled black circles, n = 9), and body weight curves of female Leprflox/flox IRflox/flox mice (open squares, n = 10), Pomc-Cre, IRflox/flox (filled grey triangles, n = 18), Pomc-Cre, Leprflox/flox (open black triangles and dashed line, n = 8), and Pomc-Cre, Leprflox/flox IRflox/flox mice (filled black circles, n = 8) on standard chow. B, Cumulative food intake in male and female Leprflox/flox IRflox/flox mice (filled dark grey squares, n = 13,13) and Pomc-Cre, Leprflox/flox IRflox/flox mice (open light grey circles, n = 9,10) over time. C, Fat mass in a separate cohort of 6 month old Lepr flox/flox, IR flox/flox (white bars), Pomc-Cre, IR flox/flox (grey bars), Pomc-Cre, Lepr flox/flox (striped bars), and Pomc-Cre, Leprflox/flox IRflox/flox mice (black bars) as measured by NMR (n=7–15 per group). Values are means +/− SE. ANOVA: p<0.0001 males, p=0.0124 females. For entire figure, * = p< 0.05, ** = p<0.01, *** = p<0.0001, determined by Bonferroni’s Multiple Comparison Test following one-way ANOVA for each group or time point. D, Relative expression of POMC as measured by qPCR in Leprflox/flox IRflox/flox (white bars, n=14–18), Leprflox/flox,POMC-Cre (striped bars, n=7–9), and Leprflox/flox IRflox/flox POMC-Cre (black bars, n=7–8) mouse hypothalami. E, O2 consumption, F, ambulatory activity, and G respiratory exchange rate in 3 month old female mice lacking insulin and LepRs in POMC neurons (black bars, n=9), lacking only LepRs (striped bars, n=7), and littermate controls (white bars, n=12)
We next examined the source of body weight variation among the mice. As the IR only deletion had no effect on body weight or fat mass, these mice were not examined further. Deletion of the LepR only from POMC neurons had previously been reported to suppress POMC mRNA expression (Balthasar et al., 2004), and we saw a similar trend (Figure 3d). In addition, we found that the double deletion showed a significant suppression in POMC expression, apparently exacerbating the effect of the single deletion. We then examined the metabolic parameters involved in energy balance. As females mice lacking LepRs only in POMC neurons reportedly exhibit clear decreases in energy expenditure (Shi et al., 2008), we chose to examine female mice. Both Pomc-Cre, Leprflox/flox and Pomc-Cre, Leprflox/flox IRflox/flox mice showed a significant suppression of oxygen consumption with no alteration in substrate preference (Figure 3e,g). While Pomc-Cre, Leprflox/flox mice showed significant suppression of ambulatory activity, Pomc-Cre, Leprflox/flox IRflox/flox activity levels were similar to controls (Figure 3f). Thus, insulin and leptin signaling in POMC neurons may have opposing effects on activity levels, although no causal link has been established between increased activity levels and reduced weight in the double knockout.
Blood Glucose Regulation
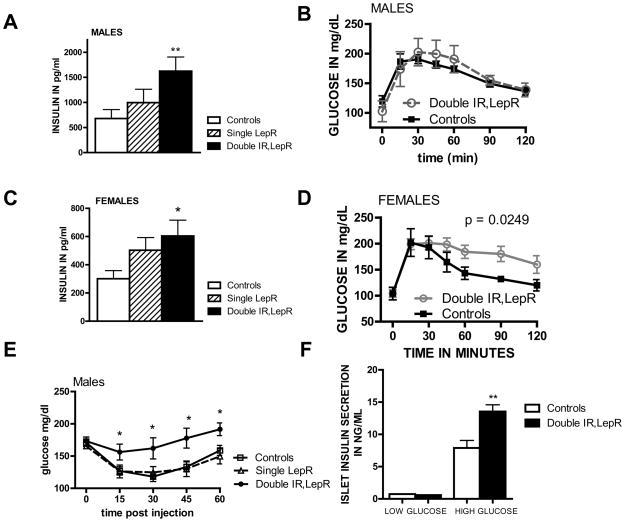
We next investigated possible alterations in glucose homeostasis. As previously shown, the single deletion of IRs from POMC neurons did not affect glucose parameters (Figure S3). Male Pomc-Cre, Leprflox/flox mice fed normal chow reportedly exhibit insulin resistance (Shi et al., 2008). We therefore examined Pomc-Cre, Leprflox/flox and Pomc-Cre, Leprflox/flox IRflox/flox mice for evidence of hyperinsulinemia. Basal insulin levels in mice lacking LepRs only in POMC neurons were not significantly above control mouse levels, although they tended to be higher (Fig 4a,c). In contrast to either single deletion, the double deletion mice showed significantly increased insulin levels in both males and females. An ITT in male mice revealed insulin resistance in Pomc-Cre, Leprflox/flox IRflox/flox mice but not Pomc-Cre, Leprflox/flox mice (Fig 4e). In response to a glucose tolerance test (GTT), the double receptor knockout females (but not males) displayed abnormally elevated glucose levels (Fig 4b,d). We next isolated islets from the pancreas of the double knockout males to examine pancreatic function. High glucose treatment induced significantly greater insulin release from islets isolated from Pomc-Cre, Leprflox/flox IRflox/flox mice (Fig 4f), as expected with pancreatic beta cell compensation for reduced insulin sensitivity. Our results suggest that deletion of insulin and LepRs in POMC neurons leads to insulin resistance despite increased pancreatic insulin secretion, independent of effects on body weight.
Figure 4. Insulin resistance in mice lacking both IRs and LepRs in POMC neurons.
A, Serum insulin levels were measured by ELISA following removal of food for 2 hours in 3 month old male (n=14–19) and female (n=7–21) Pomc-Cre, Leprflox/flox IRflox/flox and Cre negative littermate controls and analyzed by one-way ANOVA followed by Tukey’s post-hoc test. B, D, Adult male and female Pomc-Cre, Leprflox/flox IRflox/flox and littermates lacking POMC-cre (n = 7–8) were matched by weight and subjected to a GTT (1mg/kg). E, Male Pomc-Cre, Leprflox/flox IRflox/flox and Leprflox/flox IRflox/flox mice (n = 7) littermates 3 month of age were subjected to an ITT. Blood glucose levels were measured following injection of insulin (0.75U/kg). Values are presented as means +/− SE. Statistical significance as shown by p value was determined by comparison of area under the curve, and significant differences at individual time points were evaluated by t-test. F. Isolated islets (6 islets per well) from 3 month old male mice were incubated in 5mM or 17.5mM glucose for 1 hour and the secreted insulin in the media was harvested for insulin assay. Values are presented as means +/− SE. * p<0.05, ** p<0.01 See also figure S3.
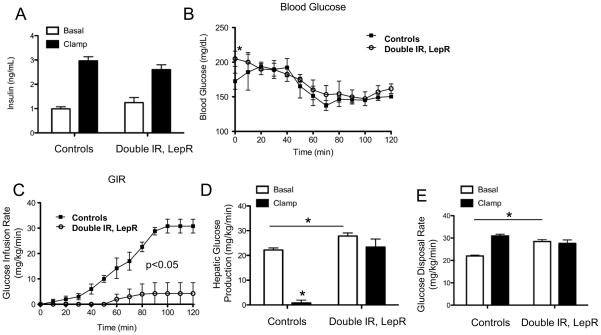
Hyperinsulinemic-euglycemic clamps were then performed in cohorts of female and male Pomc-Cre, Leprflox/flox IRflox/flox mice and Leprflox/flox IRflox/flox mice to assess insulin action. We first examined two-month-old female mice in which body weight did not differ between genotypes (data not shown). Basal plasma insulin levels were comparable and similarly elevated during the clamp steady-state period (Figure 5a). Fasted blood glucose levels were elevated in young female Pomc-Cre, Leprflox/flox IRflox/flox mice compared to control mice, but this difference normalized during the clamp (Figure 5b). The glucose infusion rate (GIR) required to clamp euglycemia in young female Pomc-Cre, Leprflox/flox IRflox/flox was markedly reduced compared to Leprflox/flox IRflox/flox controls, indicating impaired whole-body insulin action (Figure 5c). Fasting HGP and whole-body glucose disposal rates were also elevated in young female Pomc-Cre, Leprflox/flox IRflox/flox mice (Figure 5d,e) consistent with the modest increase in blood glucose. As expected, suppression of HGP in young female control Leprflox/flox IRflox/flox mice was complete (Figure 5d). HGP, in contrast, was not suppressed in young female Pomc-Cre, Leprflox/flox IRflox/flox mice indicating insulin resistance (Figure 5d). We found no differences in clamp glucose disposal (Figure 5e). In 6 month old male mice, body weight was greater in Pomc-Cre, Leprflox/flox IRflox/flox mice due to higher total fat mass. Fasted blood glucose was similar between groups (178.6 +/− 30.25 mg/dL in controls, 188 +/− 41.11 mg/dL in Pomc-Cre, Leprflox/flox IRflox/flox) and target euglycemia was achieved during the clamp stead-state (Figure S4a). The GIR required to clamp blood glucose in older male Pomc-Cre, Leprflox/flox IRflox/flox was 80% lower than in Leprflox/flox IRflox/flox controls (Figure S4b). HGP was suppressed by 43% in older male Leprflox/flox IRflox/flox mice, but this effect was absent in Pomc-Cre, Leprflox/flox IRflox/flox mice (Figure S4c). These findings are consistent with the results in young female mice and further demonstrate hepatic insulin resistance. Whole-body glucose disposal was similar between the two groups of older male mice again suggesting no differences in skeletal muscle glucose uptake (Figure S4d).
Figure 5. Failure to suppress endogenous glucose production during hyperinsulinemic-euglycemic clamps.
A, Basal and clamp insulin B, blood glucose C, Glucose infusion rate (GIR) D, HGP (EndoRa) and E, Glucose disposal rate (Rd) in conscious 2 month old female Leprflox/flox IRflox/flox (white bars, n=5), and Pomc-Cre, Leprflox/flox IRflox/flox (black bars, n=5) mice. Values are presented as means +/− SE. * p<0.05 See also figure S4.
Assessments of Reproductive Function
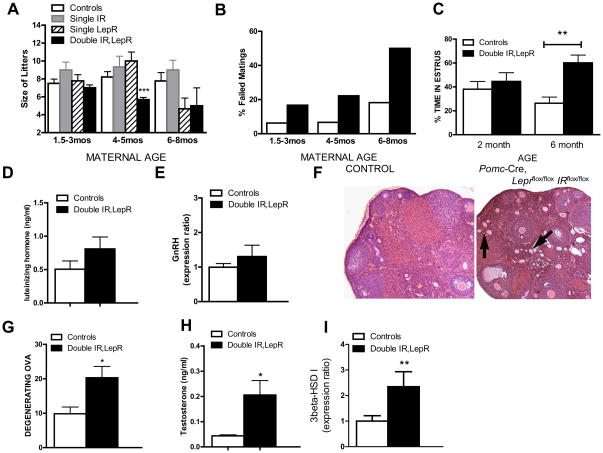
In the course of our studies, we noted that female mice lacking leptin and IRs in POMC neurons had difficulty producing offspring. Thus, we examined their fertility by mating them with control male mice that had previously sired pups. We saw a significant difference in the average number of pups born to littermate controls vs. Pomc-Cre, Leprflox/flox IRflox/flox females older than 4 months of age (Fig 6a). This phenotype was not seen in Pomc-Cre, IRflox/flox or Pomc-Cre, Leprflox/flox mice, although Pomc-Cre, Leprflox/flox mice showed a trend towards reduced litter sizes at 6–8 months of age. In addition, the percentage of matings not resulting in a litter after two months was higher for Pomc-Cre, Leprflox/flox IRflox/flox females across all maternal ages (Fig 6b). No pups born to Pomc-Cre, Leprflox/flox IRflox/flox dams died after birth (data not shown), arguing against a lactational deficiency in the knockouts. Monitoring of cycle stages by assessing vaginal cytology revealed a lengthened estrus period in older Pomc-Cre, Leprflox/flox IRflox/flox females (Fig 6c). These reproductive deficits did not result from large alterations in LH, prolactin, or estrogen levels (Figure 6d, Table 1). In addition, hypothalamic GnRH expression levels were comparable among the groups (Fig 6e). These results indicate that gonadotroph and GnRH neuronal function is not grossly impaired in these mice.
Figure 6. Reduced fertility in Pomc-Cre, Leprflox/flox IRflox/flox mice.
A, Number of pups born to Lepr flox/flox, IR flox/flox (white bars), Pomc-Cre, IR flox/flox (grey bars), Pomc-Cre, Lepr flox/flox (striped bars), and Pomc-Cre, Leprflox/flox IRflox/flox dams (black bars) that were 1.5–3 months of age (n=7–30), 4–5 months of age (n=7–14), or 6–8 months of age (n = 7–9). B, Percentage of Lepr flox/flox, IR flox/flox (white bars) and Pomc-Cre, Leprflox/flox IRflox/flox dams (black bars) caged with control males that failed to produce a litter in two months. C, Pomc-Cre, Leprflox/flox IRflox/flox mice (black bars, n=7) and littermate controls (Lepr flox/flox, IR flox/flox; white bars, n=7) were examined daily for 3 weeks for estrus length via vaginal cytology. Smears showing predominantly cornified cells were considered estrus-like. Data was analyzed by t-test. D, Blood was collected from female mice on diestrus as determined by vaginal cytology for assay of luteinizing hormone levels by RIA. Groups were compared by t-test. (n=16, 20) E, relative expression of GnRH as measured by qPCR in Leprflox/flox IRflox/flox (white bars, n=7), Leprflox/flox,POMC-Cre (striped bars, n=7), and Leprflox/flox IRflox/flox POMC-Cre (black bars, n=7) female mouse hypothalami. Values are means +/− SE. E,F, Sliced and H&E stained paraffin-embedded ovarian tissue from Pomc-Cre, Leprflox/flox IRflox/flox mice (black bars, n=10) and littermate controls (Lepr flox/flox, IR flox/flox; white bars, n=10, 6) was examined for number of degenerating ova. G, Blood was collected from female mice on diestrus for assay of testosterone levels by RIA. (n=7) H, relative expression of murine 3β-HSD type I as measured by qPCR in Leprflox/flox IRflox/flox (white bars, n=10), and Leprflox/flox IRflox/flox POMC-Cre (black bars, n=11) female mouse ovaries by qPCR. Values are presented as means +/− SE. Groups were compared by t-test.
Histological examination of their ovaries showed that double knockout females exhibited more degenerating follicles (Figure 6f,g), as well as occasional cysts (not shown). In addition, we observed significantly elevated serum testosterone levels in Pomc-Cre, Leprflox/flox IRflox/flox females (Figure 6h). This rise was accompanied by a significant elevation in the expression of ovarian enzyme 3beta-HSD I (Fig 6i), which produces androstenedione, the precursor of testosterone. Interestingly, 3β-HSD I has been found to be upregulated in models of polycystic ovarian syndrome (PCOS) (Zurvarra et al., 2009), and its human ortholog 3β-HSD II is upregulated in theca cells from patients with PCOS (Nelson et al., 2001). The gene encoding the enzyme upstream of 3beta-HSD in the testosterone synthesis pathway, CYP17, showed a trend toward increased expression as well (controls: 1.000 ± 0.3606 N=10 vs. double knockout: 2.412 ± 0.5784 N=10, p = 0.0530). Notably, insulin drives transcriptional activity of the CYP17 gene in primary cultures of theca cells (Zhang and Veldhuis, 2004), and increases 3β-HSD expression in human granulosa cells (McGee et al., 1995). Thus, the reproductive deficits seen in Pomc-Cre, Leprflox/flox IRflox/flox mice may reflect inappropriate regulation of fertility secondary to peripheral insulin resistance and hyperandrogenemia.
Discussion
POMC neurons are critical regulators of energy balance and glucose homeostasis that sense circulating adiposity signals such as insulin and leptin (Baskin et al., 1999; Benoit et al., 2002; Cheung et al., 1997; Elmquist et al., 1998). Our understanding of insulin and leptin sensing by these neurons is evolving rapidly. Functional LepRs have recently been found on approximately 25–40% of POMC/CART neurons in the mediobasal hypothalamus using electrophysiology and immunohistochemistry (Williams et al., 2009). Similar percentages of POMC neurons display immunoreactive pStat3 following leptin treatment (~40%) (Xu et al., 2007). While leptin-induced excitation is seen throughout the retrochiasmatic area (RCA) and ARC, a higher percentage (40–70%) of leptin-excited POMC cells exist in the lateral RCA and medial ARC (Hill, 2010; Williams et al., 2009). In contrast, insulin-inhibited POMC cells are largely found in the medial RCA and rostromedial areas of the ARC (Williams et al., 2009), as assessed by acute electrophysiological responses. This segregation may not be absolute, as Al-Qassab and colleagues (2009) reported electrophysiological recordings in 3 POMC neurons showing responsiveness to both leptin and insulin. Interestingly, they reported that the PI3K subunit p110β was required for the acute effects of insulin and leptin while the p110α isoform was required for only the acute effects of insulin. Thus, while different channel distribution is likely to be responsible for the selective responsiveness of POMC neurons to leptin or insulin, the activation of specific signaling cascades may be required as well. It is also possible that some POMC neurons targeted by insulin do not show changes in membrane potential and firing rate, perhaps including leptin-activated POMC neurons in which insulin exerts long-term genomic responses. Therefore, these recent electrophysiological findings do not exclude the potential for crosstalk between the insulin and leptin signal transduction pathways (Mirshamsi et al., 2004; Niswender et al., 2003; Niswender et al., 2001), including parallel PI3K activation and inhibition of FoxO to promote POMC-expression (Belgardt et al., 2008; Kitamura et al., 2006). Hence, deletion of both LepRs and IRs may have additional effects on POMC-transcription. Nevertheless, our results suggest the existence of functional redundancy of the actions of leptin and insulin on POMC neurons in the context of the control of glucose homeostasis.
These studies have demonstrated that insulin and leptin signaling within POMC neurons do not serve the same function in body weight regulation. Similar to a previous report (Shi et al., 2008), female mice lacking LepRs from POMC neurons showed increased adiposity accompanied by consistently decreased energy expenditure. We also saw suppression of ambulatory activity levels in these females, while previous reports showed merely a trend towards reduced wheel running. Nevertheless, mouse models with reduced melanocortin system activation such as MC3r and MC4r-deficient mice (Butler, 2006; Chen et al., 2000; Ste Marie et al., 2000) show substantially reduced activity. Additionally, restoration of LepRs in POMC neurons rescues the hypoactivity seen in mice lacking LepRs (Huo et al., 2009). In contrast, additional deletion of IRs from POMC neurons decreased body weight and adipose tissue in mice lacking POMC LepRs. These data suggest the anorectic effects of central insulin action (Baskin et al., 1987; Woods et al., 1979) are not mediated by POMC neurons. These findings may reflect differing roles of POMC neuronal populations that sense leptin or insulin in the modulation of adiposity. Indeed, we have recently shown that deletion of IRs from the brain in adulthood induces a pronounced loss of white adipose tissue (WAT) with a concomitant increase in circulating triglyceride levels, suggesting a role for central insulin signaling in the prevention of lipodystrophy and the expansion of adipocyte size (Koch et al., 2008). Our results suggest that these actions may be at least partially mediated by insulin-sensitive POMC neurons. Recent studies argue that WAT expansion serves a protective role in the face of excess energy intake (Gray and Vidal-Puig, 2007; Virtue and Vidal-Puig, 2008). Thus POMC neuronal populations may promote an adaptive response to a positive energy balance by increasing overall energy expenditure and promoting appropriate fat storage in WAT.
Our results confirm that POMC neurons are an important target for the actions of insulin and leptin in maintaining normal glucose homeostasis. Mice lacking insulin and LepRs in POMC neurons show a marked effect on HGP, no longer responding to high insulin levels to suppress HGP. These findings are in accord with of German and colleagues (German et al., 2009) who have demonstrated that replacement of LepRs in the ARC nucleus of LepR deficient rats improved peripheral insulin sensitivity via enhanced suppression of HGP independent of any change in insulin-stimulated glucose uptake or disposal. Interestingly, they could block the effect by selective hepatic vagotomy. Our results suggest that both leptin- and insulin-sensitive subpopulations of POMC neurons play a crucial role in the control of HGP, and that insulin as well as leptin action on these POMC neurons can suppress HGP. Indeed, redundancy in such a crucial function as the avoidance of beta-cell damage from excess glucose production should be anticipated.
Leptin and insulin action in the brain is required for coordinated reproduction (Bruning et al., 2000; Burks et al., 2000; de Luca et al., 2005; Keen-Rhinehart et al., 2005; Kowalski et al., 2001; Okamoto et al., 2004; Salvi et al., 2006), effects believed to be due to the ability of leptin and insulin to indirectly modulate GnRH release (Donato et al., 2009; Hill et al., 2008a; Leshan et al., 2009; Tortoriello et al., 2007). We have not measured GnRH pulse levels across the estrous cycle in Pomc-Cre, Leprflox/flox IRflox/flox mice. However, unlike mice lacking IRs in all neurons (Bruning et al., 2000), diestrous LH levels are normal in our mice, arguing against a diagnosis of simple hypothalamic hypogonadism.
Interestingly, our POMC double receptor knockout model recapitulates many characteristics associated with polycystic ovary syndrome (PCOS), including ovarian abnormalities, insulin resistance, and, notably, hyperandrogenism. Hyperinsulinemia may be the primary factor driving increased ovarian androgen production in PCOS patients (Adashi et al., 1981; Barbieri et al., 1986; Dunaif et al., 1990; Geffner et al., 1986; Soldani et al., 1994), though androgen excess in turn may promote further insulin resistance (Corbould, 2008). Mice lacking LepRs and IRs in POMC neurons may display a similar progression, as insulin resistance is detectable in females at two months of age and reproductive difficulties do not appear before 4 months. Given the population of lean PCOS patients with hyperinsulinemic androgen excess (Chang et al., 1983; Dunaif et al., 1989; Dunaif et al., 1992), it is interesting to note that our double deletion mouse is leaner than POMC LepR-only deleted mice and yet shows reproductive impairment not present in the latter.
In conclusion, our results establish that POMC neurons that respond to insulin and leptin regulate systemic glucose homeostasis via control of HGP and reveal a novel function for this system in maintaining fertility.
Experimental Procedures
Targeted Leptin and IR Deletion and Assessment of Energy Homeostasis
Care of all animals and procedures was approved by the UT Southwestern Medical Center Institutional Animal Care and Use Committees. All genotypes were on a mixed C57BL/6J;129S6/SvEv background. Experimental mice were compared to littermate controls carrying only the floxed alleles (Leprflox/flox IRflox/flox mice) and in some cases, to mice with a single receptor deletion (Pomc-Cre, Leprflox/flox or Pomc-Cre, IRflox/flox mice). Study animals were derived from crosses between animals that were heterozygous for the floxed LepR, homozygous for the floxed IR and carried the POMC-cre allele and animals that were homozygous for both floxed receptors. Thus mice lacking LepRs in POMC expressing cells were not used during the breeding of experimental animals.
In situ hybridization for POMC (35S) mRNA was performed as described earlier (Elias et al., 1999). Whole cell patch-clamp recordings from POMC neurons were performed as previously detailed (Cowley et al., 2001; Hill et al., 2008b). Food intake and body weight measurements were made using established protocols. Physical activity, V02, and RER were monitored using a combined indirect calorimetry system (TSE Systems GmbH, Bad Homburg, Germany) (Pfluger et al., 2008). See supplemental methods for additional information.
Hormone and Glucose Regulation Assays
Plasma corticosterone levels were obtained between 1400 and 1600 h following the protocol described by Popova and colleagues (Hill et al., 2008b; Popova et al., 2006). Briefly, psychosocial stress was induced in 8 mice of each strain by aggregation for 30 min in groups of four animals after 3-day isolation in individual cages. Blood samples were taken from the trunk in heparin-free tubes after decapitation within 30 seconds of handling. The corticosterone concentration was measured from serum by EIA (AssayDesigns Correlate-EIA Corticosterone kit) according to the manufacturer’s instructions. Additional hormone levels were assayed according to established protocols; see Supplemental Methods for additional information.
Hyperinsulinemic-euglyclemic clamps were performed as previously described (Nawrocki et al., 2006). In all GTTs and insulin tolerance tests (ITTs), subsets of age-matched mice with similar body weights were chosen to avoid the confounding influence of body weight on results. Additional details appear in Supplemental Methods.
Foxo Translocation Measurements in Hypothalamic Slices
We used a reporter mouse to monitor PI3K-Akt signaling in specific populations of neurons in hypothalamic slice cultures based on FoxO1 nucleocytoplasmic shuttling. The reporter, FoxO1 fused to green fluorescent protein (FoxO1GFP), is expressed under the control of a ubiquitous promoter silenced by a loxP flanked transcriptional blocker. Thus, expression of the reporter in selected cells depends on the action of Cre recombinase. Image pixel intensity, as a measurement of fluorescence intensity, was measured within specific regions of the neuron (cytoplasmic (in the soma) and nuclear) as well as in regions outside the cell (background) with the AxioVision 4.1 software. Neurons positive for cytoplasmic FoxO1GFP were defined as those with an N:C ratio of <1:2. See Supplemental Methods for additional details.
Mouse Islet Isolation
All pancreatic islets were obtained from 3 month old, male mice and were harvested in early morning with mice in the fed state. The mouse pancreas was perfused and digested with liberase R1 (Roche, Indianapolis, IN). Islets were then isolated using Ficoll gradient centrifugation and hand selection under a stereomicroscope for transfer to RPMI 1640 medium (11.1 mM glucose) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA), culture conditions routinely used to avoid apoptotic cell death and preserve optimal glucose-stimulated insulin capacity. Three mice were used per group to isolate islets that would be pooled and then distributed evenly among multiple wells (6 islets per well) for each assay condition. To determine the effect of glucose, mouse islets were incubated in glucose free secretion assay buffer for 1 hour, and then shifted to 5 mM (low glucose) or 17.5 mM glucose (high glucose). After 1 hour of incubation, insulin secretion from the islets was measured by EIA (Crystal Chem). All experiments were performed three times.
Reproductive Phenotyping
Mating success rates were determined by pairing experimental mice with unrelated control mice (known to be fertile) for two months or until a litter was produced. Pairs were monitored regularly for signs of visible pregnancy. Ovaries were removed at autopsy and fixed for 72 hours 4% paraformaldehyde. The tissues were then embedded in paraffin, cut into 20-microm sections on a sliding microtome, and stained with hematoxylin/eosin. The number of degenerating ova present in representative sections throughout the ovaries was tabulated by a blinded observer for each of the genotypes. Qpcr was performed using established protocols (Bookout and Mangelsdorf, 2003).
Statistics
The data are reported as mean ± SEM. All statistical analyses were performed using Prism (version 5.0) software. ITT and GTTs were analyzed by comparing the mean of the Area Under the Curves by t-test. Groups of more than two and individual weight-gain time points were analyzed by a Bonferroni’s post-hoc test following a one-way ANOVA. When planned comparisons had been part of the experimental design a Bonferroni post-hoc analysis was used to assess selected pairs of means. T-tests were used to compare results between groups of two. P < 0.05 was considered statistically significant.
Highlights
Redundant glucose regulation is exerted by subpopulations of POMC neurons.
Leptin and insulin-sensitive POMC neurons control hepatic glucose production.
Leptin and insulin lead POMC neurons to increase energy use and fat storage, respectively.
Subfertility in these mice results from hyperinsulinemia and –androgenemia linked ovarian defects.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Jason Anderson for technical assistance, Aktar Ali, Laura Brule and Damalie Namponye for Metabolic Phenotyping Core support (supported by 1PL1DK081182 and 1UL1RR024923), and Drs. SR Hammes, and RE Hammer for interpretive assistance. Ovarian histology was provided by the UTSW Molecular Pathology Core Laboratory and the University of Toledo Advanced Microscopy & Imaging Center Facility (supported by the Cancer Biology Fund of the University of Toledo Foundation). Radioimmunoassays were performed Dr. AF Parlow (Harbor-UCLA REI) and Aleisha Schoenfelder (UVA Ligand Assay and Analysis Core; NICHD/NIH (SCCPRI) Grant U54-HD28934). This work was supported by NIH grants 1F32DK066972 and K99HD056491 to JWH, PO1 DK56116 to BBL and JKE, and R01DK53301, RL1DK081185 and support from the American Diabetes Association and a Smith Family Foundation Pinnacle Program Project Award to JKE as well as funds by the German research foundation (DFG1492-7/1) to JCB. The authors declare no competing financial interests.
Nonstandard abbreviations used
- i.p.
intraperitoneally
- ARC
arcuate nucleus
- PCOS
polycystic ovarian syndrome
- POMC
proopiomelanocortin
- GnRH
gonadotropin releasing hormone
- CART
cocaine and amphetamine regulated transcript
- RIA
radioimmunoassay
- IRMA
immunoradiometric assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62:904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- Baskin D, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, et al. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- Baskin Dg, Figlewicz DP, Woods SC, Porte D, Jr, Dorsa DM. Insulin in the brain. Annu Rev Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel A, Schwartz NB. Pituitary LH content and reproductive tract changes during the mouse oestrous cycle. J Reprod Fertil. 1969;19:215–222. doi: 10.1530/jrf.0.0190215. [DOI] [PubMed] [Google Scholar]
- Bookout AF, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burks DJ, de Mora JF, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Cheung C, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, et al. The hypothalamic arcuate nucleus: A key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metabolism. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–532. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The Ventral Premammillary Nucleus Links Fasting-Induced Changes in Leptin Levels and Coordinated Luteinizing Hormone Secretion. J Neurosci. 2009;29:5240–5250. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990;70:699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffner ME, Kaplan SA, Bersch N, Golde DW, Landaw EM, Chang RJ. Persistence of insulin resistance in polycystic ovarian disease after inhibition of ovarian steroid secretion. Fertil Steril. 1986;45:327–333. [PubMed] [Google Scholar]
- Gelling R, Morton GJ, Morrison CD, Niswender KD, Myers MG, Jr, Rhodes CJ, Schwartz MW. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S, Vidal-Puig A. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65 doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Hill JW. Gene Expression and the Control of Food Intake by Hypothalamic POMC/CART Neurons. The Open Neuroendocrinology Journal. 2010;3:21–27. [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008a;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008b;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Jacobson PB, von GTW, Ohman L, Osterland M, Wang J, Zinker B, Wilcox D, Nguyen PT, Mika A, Fung S, et al. Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. J Pharmacol Exp Ther. 2005;314:191–200. doi: 10.1124/jpet.104.081257. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Kalra SP, Kalra PS. AAV-mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides. 2005;26:2567–2578. doi: 10.1016/j.peptides.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard J, Badman M, Balthasar N, Coppari R, Mori H, Lee C, Elmquist J, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kowalski Tj, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee E, Sawetawan C, Bird I, Rainey WE, Carr BR. The effects of insulin on 3 beta-hydroxysteroid dehydrogenase expression in human luteinized granulosa cells. J Soc Gynecol Investig. 1995;2:535–541. doi: 10.1016/1071-5576(94)00061-5. [DOI] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford MLJ. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, 3rd, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab. 2007;292:E1348–1357. doi: 10.1152/ajpendo.00466.2006. [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Maslova LN, Morosova EA, Bulygina VV, Seif I. MAO A knockout attenuates adrenocortical response to various kinds of stress. Psychoneuroendocrinology. 2006;31:179–186. doi: 10.1016/j.psyneuen.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Porte D, Jr, Baskin DG, Schwartz MW. Leptin and insulin action in the central nervous system. Nutr Rev. 2002;60:S20–29. doi: 10.1301/002966402320634797. discussion S68–84, 85–27. [DOI] [PubMed] [Google Scholar]
- Salvi R, Castillo E, Voirol MJ, Glauser M, Rey JP, Gaillard RC, Vollenweider P, Pralong FP. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–826. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 2008;294:E630–639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello D, McMinn J, Chua SC. Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes (Lond) 2007;31:395–402. doi: 10.1038/sj.ijo.0803392. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue S, Vidal-Puig A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Marked segregation of acute leptin and insulin effects in distinct populations of arcuate POMC neurons. Neuron tbd. 2009 doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Marked segregation of acute leptin and insulin effects in distinct populations of arcuate POMC neurons. Neuron. 2010 doi: 10.1523/JNEUROSCI.3118-09.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol. 2006;573:395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Zsombok A, Smith BN. Rapid Inhibition of Neurons in the Dorsal Motor Nucleus of the Vagus by Leptin. Endocrinology. 2006 doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Xu A, Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- Zhang G, Veldhuis JD. Insulin drives transcriptional activity of the CYP17 gene in primary cultures of swine theca cells. Biol Reprod. 2004;70:1600–1605. doi: 10.1095/biolreprod.103.019646. [DOI] [PubMed] [Google Scholar]
- Zinker B, Mika A, Nguyen P, Wilcox D, Ohman L, von GTW, Opgenorth T, Jacobson P. Liver-selective glucocorticoid receptor antagonism decreases glucose production and increases glucose disposal, ameliorating insulin resistance. Metabolism. 2007;56:380–387. doi: 10.1016/j.metabol.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Zurvarra FM, Salvetti NR, Mason JI, Velazquez MM, Alfaro NS, Ortega HH. Disruption in the expression and immunolocalisation of steroid receptors and steroidogenic enzymes in letrozole-induced polycystic ovaries in rat. Reprod Fertil Dev. 2009;21:827–839. doi: 10.1071/RD09026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.