Abstract
Purpose
Pigment epithelial-derived factor (PEDF) is a potent angiogenesis inhibitor with multiple other functions, some of which enhance tumor growth. Our previous studies mapped PEDF anti-angiogenic and pro-survival activities to distinct epitopes. This study was aimed to determine the minimal fragment of PEDF, which maintains anti-angiogenic and anti-tumor efficacy.
Experimental Design
We analyzed antigenicity, hydrophilicity, and charge distribution of the angioinhibitory epitope (the 34-mer) and designed three peptides covering its C-terminus, P14, P18 and P23. We analyzed their ability to block endothelial cell (EC) chemotaxis and induce apoptosis in vitro and their anti-angiogenic activity in vivo. The selected peptide was tested for the anti-tumor activity against mildly aggressive xenografted prostate carcinoma and highly aggressive renal cell carcinoma. To verify that P18 acts in the same manner as PEDF, we used immunohistochemistry to measure PEDF targets, VEGFR2 and CD95L expression in P18-treated vasculature.
Results
P14 and P18 blocked endothelial cell chemotaxis; P18 and P23 induced apoptosis. P18 showed the highest IC50 and blocked angiogenesis in vivo: P23 was inactive and P14 was pro-angiogenic. P18 increased the production of CD95L and reduced the expression of VEGFR-2 by the endothelial cells in vivo. In tumor studies, P18 was more effective in blocking the angiogenesis and growth of the prostate cancer then parental 34-mer; in the renal cell carcinoma P18 strongly decreased angiogenesis and halted the progression of established tumors.
Conclusions
P18 is a novel and potent anti-angiogenic biotherapeutic agent, which has potential to be developed for the treatment of prostate and renal cancer.
INTRODUCTION
Angiogenesis (the formation of new capillaries from established ones) is a necessary pre-requisite for the exponential tumor growth. Angiogenesis is regulated by the balance between positive and negative regulators, inhibitors and stimuli, in the tumor microenvironment also termed angiogenic switch: in the normal tissue, the prevalence of inhibitors maintains vascular quiescence, while in tumors the balance is tipped in favor of the stimuli [1]. Angiogenic switch can be flipped back to the “off position”: by sequestering angiogenic stimuli with appropriate neutralizing antibodies, by blocking their signaling receptors using small molecule inhibitors, or by employing natural anti-angiogenic molecules. The advent of therapies targeting pro-angiogenic vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF-BB) or their receptors (Avastin, Sunitinib, Sorafenib) [2,3] underscores the therapeutic value of anti-angiogenics but also highlights the possibility of overcoming the withdrawal of a single angiogenic factor [4]. Studies of past two decades suggest that natural inhibitors of angiogenesis act as endothelial-specific tumor suppressors [5]; their specificity for remodeling endothelium and the interference with multiple pro-angiogenic factors makes natural inhibitors and their derivatives an attractive alternative to the blockade of the individual angiogenic stimuli [6,7].
PEDF is a potent and versatile angiogenesis inhibitor [8]: it is active against wide range of angiogenic stimuli, including VEGF, bFGF, PDGF-BB and IL-8 [8]. The loss of PEDF is common for multiple human cancers: recent studies indicate the link between decreased PEDF expression, higher microvascular density (MVD) and aggressive, metastatic phenotype in the carcinomas of prostate, pancreas, gliomas, and non-small cell lung cancer (reviewed in [9]). PEDF overexpression in melanoma, prostate and ovarian carcinoma, pancreatic cancer, osteosarcoma, Wilm’s tumor and neuroblastoma cell lines causes dramatic reductions in MVD, tumor burden and metastases [9,10] suggesting PEDF as a promising therapeutic target. In addition to its anti-angiogenic activity, PEDF acts as survival factor for the neural crest cells; it also stimulates expansion of the stem cell niche in the brain and induces differentiation towards neuronal phenotype in multiple cell types [11,12].
We have previously mapped PEDF anti-angiogenic function to the N-terminal surface epitope (the 34-mer amino acid residues 24–57) [13]. PEDF pro-survival and neurodifferentiating functions have been mapped to the adjacent epitope (44-mer residues 58–101) [10,14,15]. Combined with the results of binding studies, data by us and others indicate that PEDF can bind two non-identical receptors that trigger distinct signaling cascades, leading to different functional outcomes [13]. Recently one PEDF receptor, a phospolypase A2, has been identified, which is likely to transmit PEDF pro-survival and differentiating signals [16]; the anti-angiogenic PEDF receptor remains elusive.
While overexpression of the native PEDF is effective in delaying growth, treatment with native protein is less feasible due to antigenicity and stability issues, as well as prohibitive production costs. In addition, pro-survival function of exogenously added PEDF may oppose its anti-tumor effect: indeed, both PEDF at high concentrations and 44-mer are pro-angiogenic [13,17] suggesting the existence of the second, low-affinity endothelial receptor for the 44-mer epitope. Neuroendocrine (NE) differentiation of cancer cells due to the 44-mer may also augment tumor growth and angiogenesis, since NE cells secrete high levels of growth factors and cytokines, which are also pro-angiogenic [18]. Thus using PEDF derivatives, which retain only anti-angiogenic activity, could be more practical. Small peptides (less then 20 amino acids) are usually less likely to generate immune response; they are also more effectively absorbed through the oral mucosa by passive diffusion [19]. Although small peptides might have short half life in vivo, that obstacle can be overcome by chemical modifications. In the study below we identified minimal active peptide within the anti-angiogenic 34-mer epitope (P18). P18 in vitro activity was similar to that of the 34-mer. In vivo, however P18 inhibited angiogenesis and the growth of moderately aggressive prostate cancer and of highly aggressive renal cell carcinoma, while the 34-mer produced no significant effect. P18 altered the expression of the two PEDF signaling targets critical for its anti-angiogenic action, VEGF receptor (VEGFR) [20] and CD95 ligand (CD95L) [13] in P18 treated animals: the proportion of the VEGFR-positive microvessels was decreased in response to P18, while the percent of vascular structures positive for CD95L became significantly higher, suggesting that P18 shares molecular targets and signaling cascades with parental PEDF.
Materials and Methods
Cells and Reagents
Human umbilical vein and microvascular endothelial cells (HUVECs and HMVECs, respectively, from Lonza, Bazel, Switzerland) were maintained at 5% CO2 in MCDB131 medium (Sigma, St. Louis, MO) supplemented with endothelial cell bullet kit, 5% FBS (Lonza) and 1% penicillin-streptomycin mix (P/S); the cells were used at passages 6–9. Prostate carcinoma cells PC-3 (American Tissue Type Culture Collection, Manassas, VA) were maintained at 5% CO2 in RPMI (Mediatech, Inc, Manassas, VA), 10% FBS, 1% P/S. Renca cells (a gift from Dr. Chung Lee, Northwestern University Urology Department) were maintained at 5% CO2 in DMEM (Mediatech, Inc, Manassas, VA), 10% FBS.
VEGF and bFGF were purchased from R&D Systems (Minneapolis, MN) and Matrigel from BD Biosciences (San Jose, CA).
Three nested peptides covering the C-terminus of PEDF anti-angiogenic epitope (the 34-mer [13]) were synthesized to order at GenScript Corp (Piscataway, NJ, > 95% purity) and characterized by mass spectrometry: P14 (residues 43–57 of PEDF molecule), P18 (residues 39–57) and P23 (residues 34–57). The peptides were acetylated at the N-termini and amidated at the C-termini for stability. Stock solutions (6 mM) were made in DMSO and stored at −20°C. Working dilutions were made in maintenance media or in PBS and used immediately.
Endothelial cell chemotaxis assay was performed as previously described [21]: briefly, EC were starved overnight in serum-free medium supplemented with 0.1 % bovine serum albumin (BSA, Sigma), harvested, suspended at 1.5×106/mL, plated on the lower side of gelatinized microporous membranes (8 µm pores, Nuclepore, Whatman Inc, Florham Park, NJ) in the inverted modified Boyden chambers (Applied Biosystems, Foster City, CA) and allowed two hours to adhere at culture conditions (37°C, 5% CO2). The test substances were diluted in MCDB131, 0.1% BSA and added to the upper wells of the chamber, the cells incubated for additional 3–4 hours. The membranes were then recovered fixed, stained and mounted on slides. Migrated cells in each well were counted in 10 random high power (400X) fields. Each sample was tested in quadruplicate for statistical evaluation. Basic FGF (20ng/mL), used to induce chemotaxis, served as a positive control, MCDB131 with 0.1% BSA was considered a negative control (random, non-directional migration).
Viability assay
Tumor cells were seeded at 1×103 per well in the 96-well tissue culture plate and treated with increasing peptide concentrations from 100 pMol to 1 µMol. After 48 hours the number of viable cells was assessed using MTT assay kit (Biotium Inc., Hayward CA) according to the manufacturer’s instructions.
Endothelial cell apoptosis was measured using TUNEL assay kit (Chemicon, Temecula, CA) as recommended by manufacturer. Confluent HMVEC cells grown on cover slips were treated overnight with PEDF or PEDF peptides, with or without protective bFGF (20 ng/ml) in MCDB131 supplemented with low serum (0.2% FBS). The cells were counterstained with propidium iodide (PI); 3–5 10X fields were analyzed using epi-fluorescent microscope: total cell number (PI positive cells) and TUNEL-positive cells were quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA) and % apoptotic cells calculated.
Animals
Six to eight weeks old female athymic nude mice (nu/nu) or C57BLJ6 mice (Harlan, Indianapolis, IN) were kept in pathogen-free environment; autoclaved water and gamma-irradiated commercial diet were provided ad libitum. All manipulations were performed under sterile conditions, according to the institutional guidelines and in compliance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health).
Mouse corneal angiogenesis assay was performed as previously described [22]: briefly, the peptides were incorporated in slow-release Hydron Sucralfate pellets (~ 1 µl) containing bFGF (250ng/ml). The pellets were implanted in the cornea of C57/BL6J. bFGF served as positive control. Angiogenesis was monitored by slit-lamp microscopy and the pictures taken on day 5–7 post implantation. Ingrowth of the blood vessels into avascular cornea towards pellets was scored as positive response. The results are reported as positive corneas of total implanted.
Directed In Vivo Angiogenesis Assay (DIVAA)
Small cylindrical silicon angioreactors were filled with basement membrane extract (BME), containing pro-angiogenic factors (bFGF/VEGF mix) and the peptides (1µM) were implanted subcutaneously in both flanks of nude mice (two reactors per side, 4 reactors per animal). After 12 days the reactors were excised and the contents processed as recommended by manufacturer. The cells were labeled with FITC-lectin and fluorescence measured. Fluorescence intensity reflects the number of EC that entered the angioreactors and is a quantitative measure of angiogenic response. All reagents for the assay were provided in DIVAA Inhibition kit (Trevigen, Inc, Gaithersburg, MD).
Matrigel plug assay was performed as described elsewhere [23]. Matrigel (BD Biosciences, San Jose, CA) was mixed with bFGF (250 ng/ml), with or without PEDF peptides (1 or 10 nM, as indicated) and injected subcutaneously in the median abdominal area of nude mice. After 10 days the plugs were excised, snap-frozen in liquid nitrogen and sectioned to a 5 µm thickness.
Immunohistochemistry
Frozen sections were fixed in sequential changes of acetone - acetone and chloroform - acetone at −20°C; the sections were stained with anti-CD31 rat anti-mouse antibody (4µg/ml, BD Biosciences, San Jose, CA) followed by the donkey anti-rat Rhodamin Red-X conjugate (1:200 dilution, Jackson ImmunoResearch Labs, West Grove, PA) to detect blood vessels. Rabbit anti-mouse-human CD95, or CD95L antibodies (Calbiochem, Gibbstone, NJ) were used at 2.5 µg/ml and visualized using donkey anti-rabbit secondary antibodies, conjugated with Cy5 (1:100, Jackson ImmunoResearch). To detect VEGFR-2 we used rabbit anti-mouse antibodies (Santa Cruz, Santa Cruz, CA) and donkey anti-rabbit Cy2-conjugated secondary antibodies (Jackson Immunoresearch). In situ TUNEL assay was performed using Apoptag staining kit (Chemicon International Inc., Temecula, CA) according to the manufacturer’s instructions.
Tumorigenicity assay
Hormone refractory human prostate cancer cells, PC-3 were implanted by subcutaneous injections in the right flank region of female nude mice (2×106 cells per site). The treatment was commenced after the tumors became palpable and measurable. Highly aggressive mouse renal carcinoma cells (Renca) were implanted in the same manner (1×106 cells per site) and allowed to reach 100–200 mm3. Vehicle PBS or PEDF peptides (P18 and the 34-mer, 10 mg/kg) were administered by intra-peritoneal injections every two days. Tumor progression was monitored 25 days for the PC-3 cells and 10 days for Renca cells; tumors were measured every 2–3 days and the volumes calculated as length × width2 × 0.523 [24]. Renca tumors were also excised and weighted at the completion of experiment.
Statistical Analysis
All numerical data were analyzed with GraphPadPrism 5.0 (GraphPad Software Inc, San Diego, CA) using Student’s T-test, One-Way analysis of variance (ANOVA) or Repeated Measures ANOVA where appropriate. P values below 0.05 were considered statistically significant.
RESULTS
Short derivatives of the PEDF 34-mer epitope block endothelial cell chemotaxis and survival in vitro
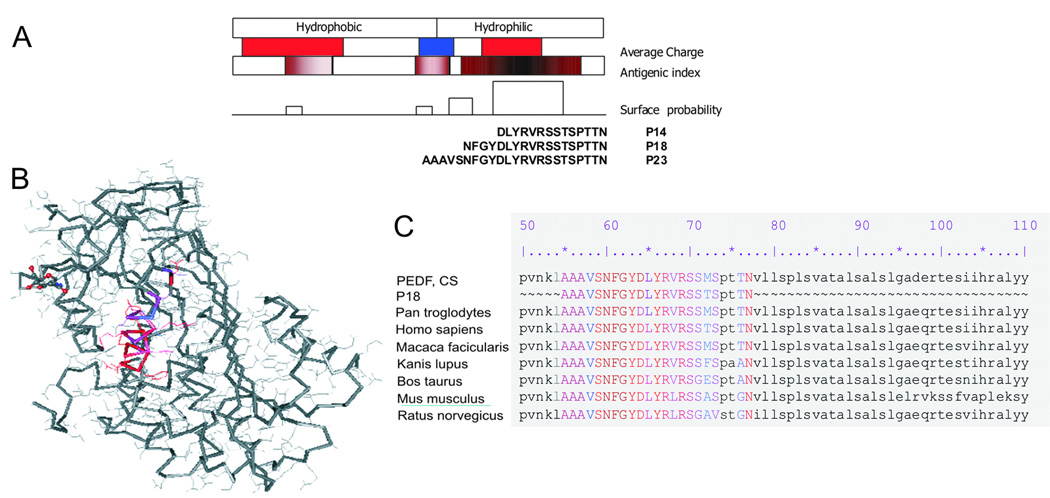
The angioinhibitory activity of PEDF was mapped to the highly conserved 34-mer surface epitope [13]. We analyzed the 3-dimensional structure of the 34-mer fragment using Protean software (Lasergene, DNA Star package, DNA Star Inc., Madison, WI) for the hydrophobic regions and charge distribution (Fig. 1A). The hydrophilic C-terminus with highly charged central area had the highest potential for the intramolecular interactions, including the target receptor. We designed three short overlapping peptides covering the 34-mer C-terminus termed according to their length P14 (amino acids 43–57 of the PEDF molecule), P18 (residues 39–57) and P23 (residues 34–57) (Fig. 1A). All the 23 amino acids are at the surface of the molecule and likely to interact with the putative angiogenic receptor (Fig. 1B); high degree of homology between species (Fig. 1C) in this part of the PEDF molecule also suggests its functional importance.
Figure 1. Design of the short peptide derivatives from PEDF 34-mer epitope.
(A) Protean analysis of the 34-mer sequence for surface presentation, hydrophilicity profile and antigenic index. Three nested peptides covering hydrophilic stretch of the 34-mer are shown. (B) Crystal structure of the PEDF molecule: 23-amino acid hydrophilic part of the 34-mer surface epitope is highlighted (generated using Cn3D4.1 software). Positive and negatively charged stretches are shown in red and blue, respectively. (C) Alignment of the 23-aa hydrophilic stretch with available PEDF sequences (GenBank) shows high conservation among the species.
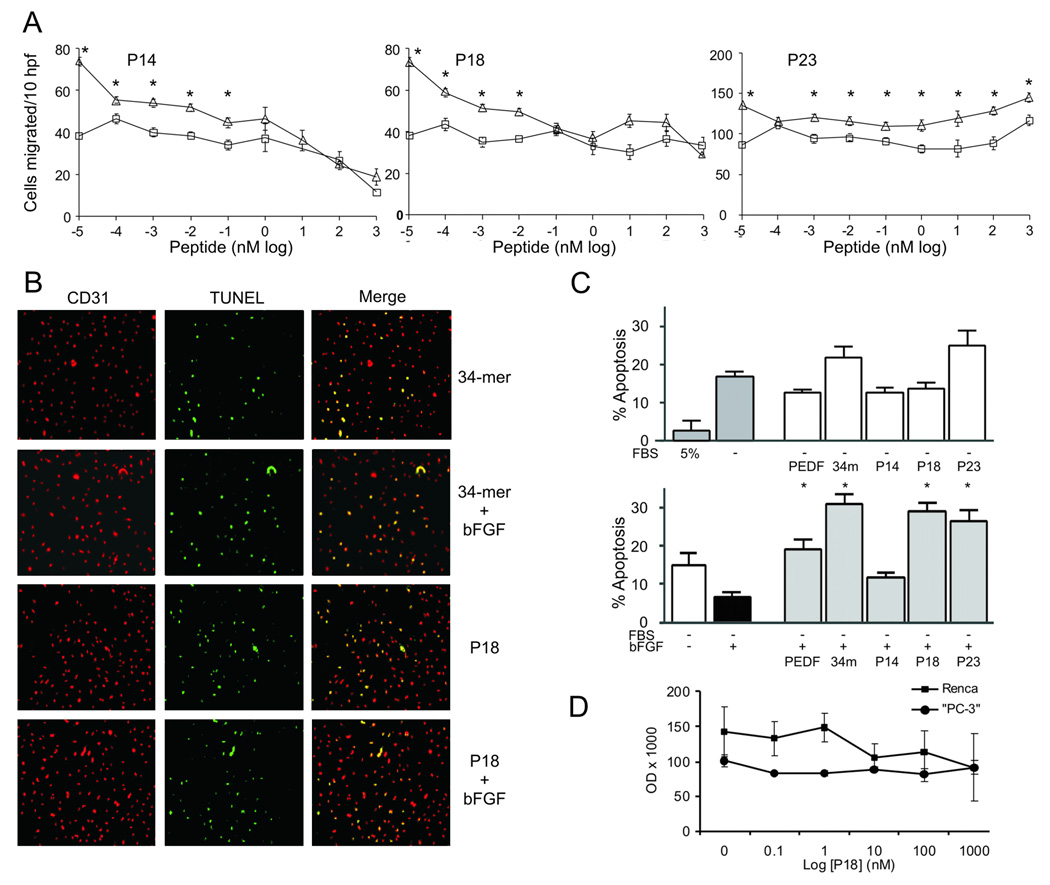
As an indicator of anti-angiogenic activity, we measured the ability of the peptides to inhibit endothelial cell (EC) chemotaxis along the gradient of angiogenic stimulus [25]. Dose response analysis (Fig. 2A) has yielded IC50 of 10 pMol and 1 pMol for the P14 and P18, respectively. The P23 had no inhibitory effect. As was shown previously [8,13], PEDF and the 34-mer have IC50 of 400 pMol and 100 pMol, respectively. P14 was slightly cytotoxic in a 4-hour assay at concentrations above 10 nM; P18 and P23 showed no cytotoxic activity (data not shown).
Figure 2. In vitro screening of the short peptide derivatives from PEDF 34-mer epitope.
(A) Representative endothelial cell (HUVEC) chemotaxis assays: P14, P18 and P23 were tested at increasing concentrations alone (□) or in combination with bFGF (Δ). The assay was performed independently for each peptide; each peptide concentration was tested in quadruplicate to achieve statistical significance and the experiment repeated three times. Values that differ significantly from the background control (BSA alone, P<0.001) are indicated with asterisks. Note the inhibition of bFGF-induced chemotaxis by P14, P18 but not P23. hpf, high-powered fields. (B-D) The induction of endothelial cell (HMVEC) apoptosis by PEDF peptides: the cells were plated on coverslips and incubated overnight with P14, P18 and P23 in low-serum medium alone, or in the presence of protective bFGF. Apoptosis was detected by TUNEL, PEDF (10 nM) and the 34-mer (100 nM) were used as control. Representative images are shown (B). (C) Neither of the peptides significantly augmented apoptosis by serum withdrawal (P=0.06, top). However, P18 and P23 overcame the protective effect of bFGF (P<0.008, bottom, asterisks indicate P<0.05). Note the lack of apoptosis in the presence of P14. Values are shown as mean +/−SD. (D) P18 had no effect on tumor cells. PC-3 and Renca cells were treated with increasing concentrations of P18 and the numbers of viable cells evaluated by MTT assay. Note the lack of significant differences in cell numbers with increasing P18 concentration.
PEDF and the 34-mer block angiogenesis by inducing endothelial cell apoptosis [13,26]. We therefore compared the apoptotic effects of P14, P18 and P23 with that of the parental 34-mer. In the microvascular endothelial cells (HMVECs), overnight serum deprivation (0.2% FBS) causes significant levels of apoptosis, which was relieved by protective bFGF (Fig. 2C). In low serum, the 34-mer and P23 enhanced stress-induced apoptosis, while PEDF and P18 had no effect (Fig. 2C). As was expected, in the presence of protective bFGF the 34-mer was pro-apoptotic, P18 and P23 caused apoptosis in the bFGF-induced ECs (Fig. 2C). P14 failed to induce EC apoptosis (Fig. 2C), although it was effective in migration assay (Fig. 2B). The effect of the P18 was restricted to the endothelial cells: the survival rates of PC-3 and Renca cells were not significantly inhibited by P18 at concentrations up to 1 µMol (Fig. 2D).
P18 blocked angiogenesis and induced endothelial cell apoptosis in vivo
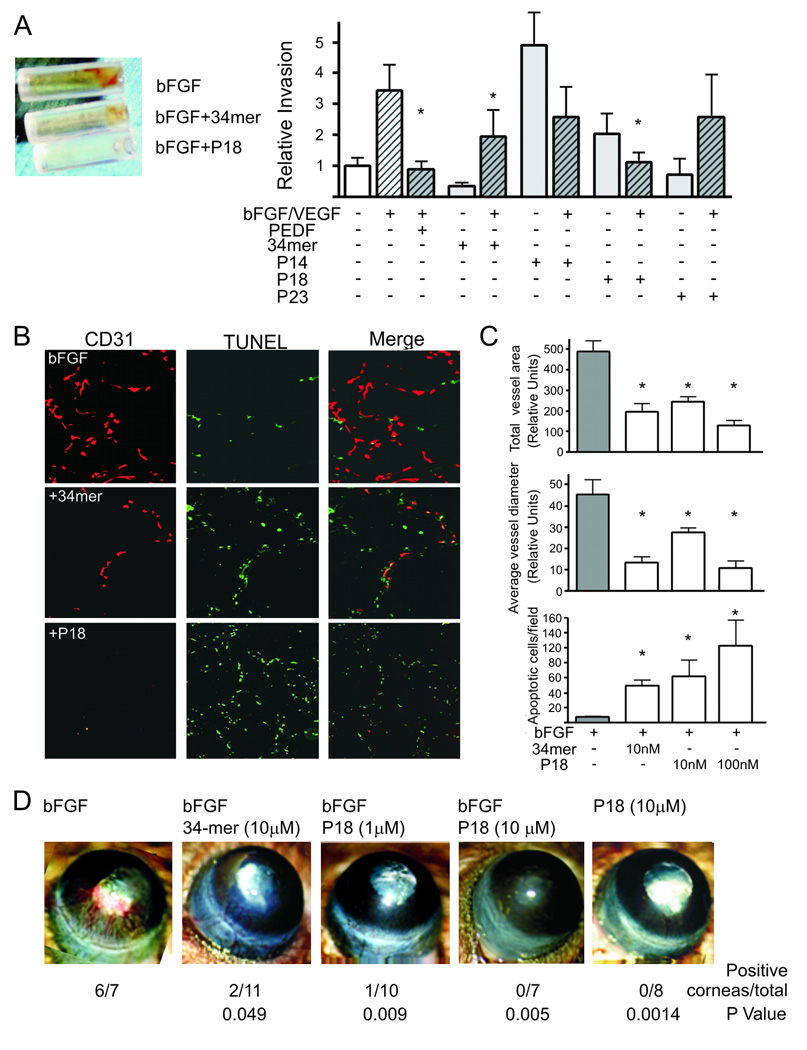
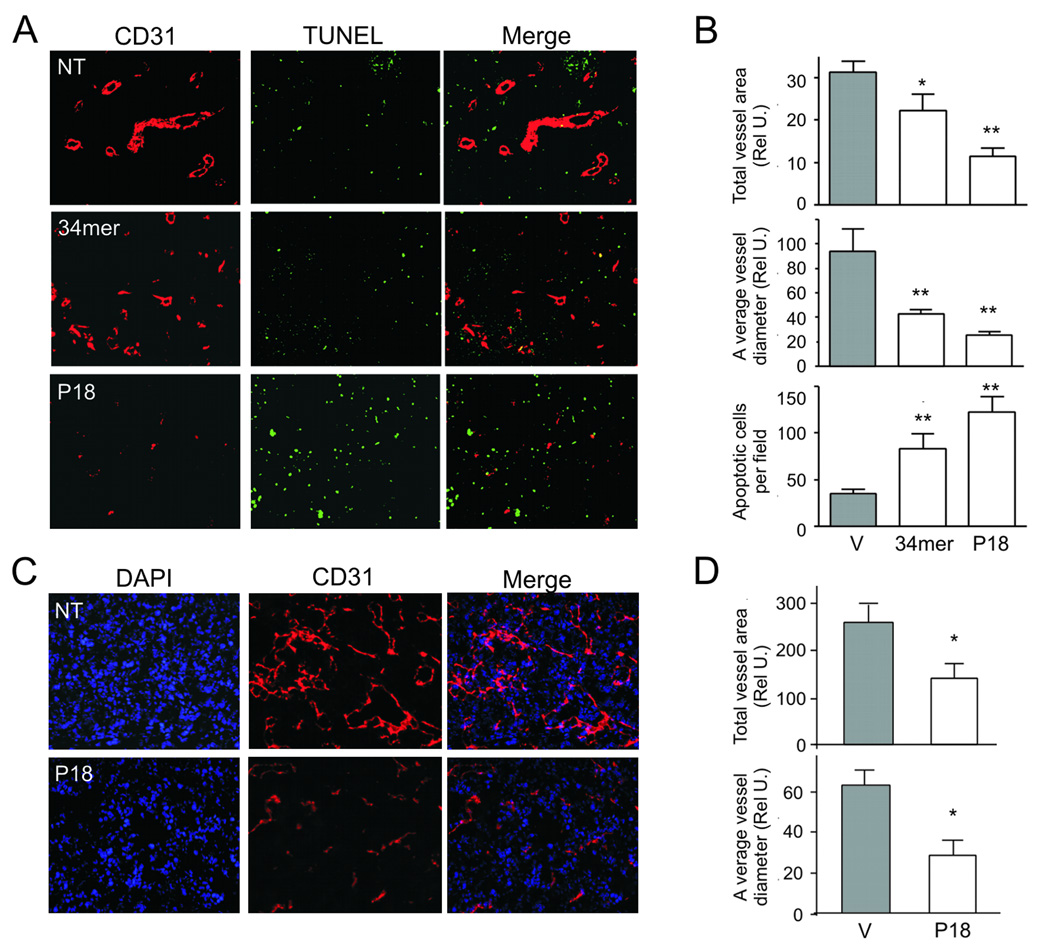
Based on their anti-angiogenic function in vitro, we tested P18, P14 and P23 in the three in vivo assays for angiogenesis: corneal micropocket assay, Directed in Vivo Angiogenesis Assay (DIVAA) and Matrigel plug assay. In a more quantitative DIVAA assay (Fig. 3A), bFGF/VEGF increased angiogenesis ~ 3 fold increase over negative control (BME, P=0.01). PEDF, 34-mer and P18 blocked bFGF/VEGF action. PEDF and P18 were the most effective reducing level of vascularization to the level of BME (P=0.006 and 0.01, respectively). P18 showed stronger inhibitory effect than the 34-mer. P14 and P23 had little effect on angiogenic response elicited by the FGF/VEGF combination (Fig. 3A), however P14 alone was pro-angiogenic (P<0.05). Matrigel plug assay is similar to the DIVAA, but performed on a larger scale: it provides an opportunity to section the plugs and evaluate the morphology of the neovessels and EC apoptosis in addition to the MVD (Fig. 3. B, C). As in DIVAA, both 34-mer and P18 at 10 nM caused a 3-fold decrease in MVD measured as total CD31-positive area (P<0.001) (Fig. 3C, top). We also evaluated mean diameter of the neovessels and detected approximately 4-fold decrease (P<0.01, Fig. 3C, middle). The response to P18 was clearly dose-dependent. Moreover, while both 34-mer and P18 induced significant levels of EC apoptosis, as was measured by in situ TUNEL, the apoptotic effect of P18 was significantly more robust at 100 nM (P<0.01, Fig. 3C, bottom). In the cornea assay, P18 was able to block bFGF-induced angiogenesis to the extent similar to the 34-mer (Fig. 3D).
Figure 3. In vivo testing of the anti-angiogenic properties of PEDF short peptides.
(A) DIVAA: the invasion of the vasculature into open-ended angioreactors filled with the basement matrix (left) is measured by lectin staining of the endothelial cells liberated from the reactors at the completion of experiment (10 days). Three independent experiments were performed, normalized per negative control and average fluorescence values calculated. Angiogenic activity was expressed as relative invasion of the ECs (right). The peptides were tested at 10 nM alone (gray bars) or in the presence of bFGF/VEGF mix (hatched bars). PEDF (20 nM) and the 34-mer (100 nM) were used as controls. Note the induction of angiogenesis by P14 and lack of inhibitory activity by P23. Values significantly lower than bFGF-induced invasion are indicated with asterisks. (B, C) P18 was tested at 1 and 10 nM in the conventional 11-day Matrigel assay with and without bFGF (100 ng/ml). Ten Matrigel plugs were tested per condition. (B) At the time of harvest, Matrigel plugs were snap-frozen, sectioned and stained for the endothelial marker CD31 (red). Apoptosis was detected by in situ TUNEL (green). Merged images are shown to visualize apoptotic endothelial cells. The 34-mer (10 nM) was used to control for the inhibitory activity. (C) Vascularization (total vessel area, top and average vessel diameter, middle) and apoptosis (bottom) were quantified by MetaMorph software (Molecular Devices, Sunnivale, CA). Asterisks indicate values significantly different from bFGF (P<0.01). Statistical significance was calculated by One Way Anova and Dunnette’s Multiple Comparison Test. (D) P18 angioinhibitory activity was verified in the mouse cornea assay for angiogenesis. The 34-mer was used to control for the inhibitory activity. Representative cornea, final scoring (positive cornea of total implanted) and statistical significance are shown. Statistical significance was calculated by Fisher’s Exact Test.
P18 inhibits the growth and angiogenesis of prostate and renal cancer xenografts
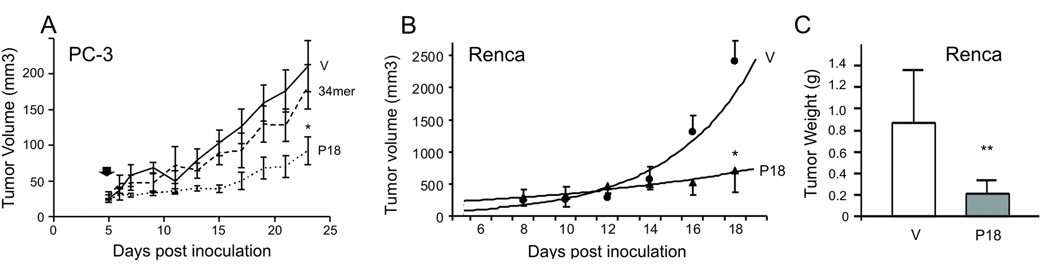
The exponential growth of solid tumors is contingent on angiogenesis. We therefore tested the effect of the P18 on tumor growth and angiogenesis. Androgen-independent human prostate cancer cells, PC-3, were injected subcutaneously into the flanks of nude mice. When palpable tumors were established, the animals were treated with the 34-mer, P18 or control vehicle. P18 administered every two days at 10 mg/kg strongly inhibited the growth of PC-3 xenografts, while the 34-mer had no significant effect (Fig. 4A). The differences were statistically significant (P<0.001) as was calculated by Repeated Measures Anova. P18 also halted the growth of highly aggressive renal cell carcinoma (RCC): P18 treatment of mice bearing established tumors 150–200 mm3 formed by mouse Renca RCC cells caused a roughly 4-fold reduction in tumor volume and weight after 10 days of treatment compared to the vehicle control (Fig. 4B, C, P<0.004 and P<0.0002, respectively). An approximately 3-fold decrease in tumor volume in P18 treated animals bearing PC-3 tumors (Fig. 4A, P<0.001) was accompanied by a dramatic decrease in angiogenesis (Fig. 5A, B): total vascular area and average vessel diameter were reduced by 2.7-fold (Fig. 5B, top; P<0.05) and 4.8-fold (Fig. 5B, middle; P<0.001), respectively. Decreased vascularization was paralleled by a nearly 3 fold increase (P<0.001) in the apoptotic cells, both endothelial cells and tumor cells proper (Fig. 5B, bottom). We observed similar reduction in vascularization of the more aggressive RCC tumor grafts (Fig. 5C, D); the decreased cellularity of the P18-treated tumors (Fig. 5C) likely reflects increased apoptosis.
Figure 4. Inhibition of tumor growth by P18.
(A) Treatment of the xenografted human prostate cancer (PC-3 cells, flank model) with the 34-mer and P18. P18 or the 34-mer were given at 10 mg/kg every two days, after the tumors became visible and palpable. Note significant delay in the growth of the P18-treated tumors (*, P<0.001). Tumor volumes were calculated as V= length × width2 × 0.52. Data from the two independent experiments (5 animals/group) were pulled together and analyzed with Repeated Measures ANOVA. (B) Treatment of the xenografted mouse renal cell carcinoma (Renca, flank model, 10 animals/group). The tumors were allowed to establish (100–200 mm3), P18 was administered and the tumor volumes calculated as in (A). *, P<0.004. (C) Tumors were excised and weighted at the endpoint of experiment. **, P<0.0002.
Figure 5. Inhibition of tumor angiogenesis by P18.
(A, B) Prostate tumor xenografts from the mice treated with 34-mer, P18 or vehicle control (V) were sectioned and stained for CD31 endothelial marker (red). Apoptosis was visualized by in situ TUNEL (green). Representative sections are shown (A); (B) total vascular area (top), average vessel diameter (middle) and the number of apoptotic cells per 10X (bottom) field were determined using MetaMorph software. Asterisks indicate statistically significant differences compared to bFGF as was measured by One Way ANOVA followed by Dunnett's Multiple Comparison Test (*, P<0.01, **, P<0.001). (C, D). Renca tumors treated with P18 or vehicle (V) were sectioned and stained for CD31, to visualize microvasculature (red). The sections were counterstained with DAPI (blue) to visualize nuclei. Represenative sections are shown (C). (D) Vascular area (top) and average vessel diameter (bottom) were determined as above. Note dramatic decrease in the vascularization and decreased cellularity of the P18 treated tumors.
Interestingly, the 34-mer treated prostate cancer xenografts also showed decreases in microvessel density and diameter, as well as increased apoptosis; however, these changes were less dramatic than in P18-treated tumors (Fig. 5A, B).
P18 in vivo targets correlate with PEDF angioinhibitory signaling
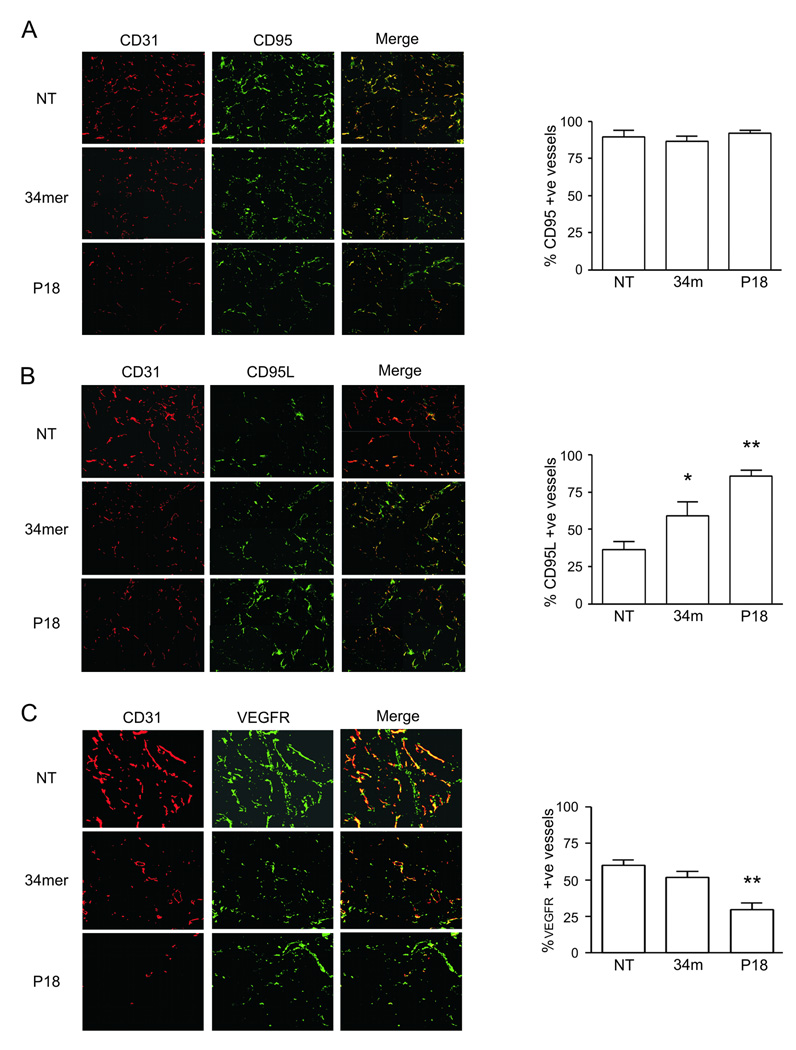
PEDF and the 34-mer block angiogenesis by inducing EC apoptosis. Key events leading to the PEDF-dependent apoptosis are the induction of intrinsic cell death pathway, where PEDF elicits the production of the CD95 ligand (CD95L), which binds to the VEGF/bFGF stimulated CD95 on the activated ECs and thus initiates caspase-8 and caspase-3 dependent apoptosis [27]. On the other hand, PEDF activates membrane-bound gamma-secretase and thus reduces the levels of the endothelial VEGF receptor (VEGFR-1) [20]. To ascertain that P18 recapitulates the main events typical for PEDF anti-angiogenic epitope we analyzed the vasculature in P18-treated and control (bFGF) Matrigel plugs for expression of CD95, CD95L and VEGFR. As expected of the endothelium activated with bFGF, the majority of the microvessels stained positive for CD95 in control plugs, regardless of the presence of the 34-mer or P18 (Fig. 6A). In contrast, CD95L was high in the vasculature treated with the 34-mer or P18 (60% and 83% positive microvessels, respectively) but not in the control plugs (32% positive vessels, P<0.001, Fig. 6B). The differences between the 34-mer and P18 were also statistically significant (P<0.01,Tukey’s Multiple Comparison Test). Finally, in control 60% of the vessels were positive for VEGFR; 34-mer caused no significant reduction (51%), while in P18 treated plugs only 29% of the vessels were VEGFR positive with (P<0.001 and P<0.01 vs. control and 34-mer, respectively) (Fig. 6C).
Figure 6. P18 and PEDF share molecular targets on remodeling vasculature.
Matrigel plugs containing bFGF (100 ng/ml) alone or in combination with the 34-mer or P18 were snap-frozen, sectioned and stained for CD31 (red), to visualize the vasculature, and for three critical PEDF vascular targets: CD95 (A), CD95L (B) and VEGFR (green). Total number of vascular structures and the number of microvessels positive for CD95, CD95L and VEGFR, respectively, was quantified using MetaMorph software; the percent of the structures positive for each respective marker was calculated and compared between treatments. Asterisks indicate statistically significant difference with control and other treatments (P<0.05, One way Anova followed by Tukey's Multiple Comparison Test).
DISCUSSION
In this study we sought to identify minimal PEDF derivative, which retains anti-angiogenic activity of the whole molecule. In our previous studies we have mapped PEDF anti-angiogenic activity to the 34-mer N terminal surface epitope. Here we further narrowed down this region to 18 amino acids, the residues 39–57 on the PEDF molecule. The longer P23 peptide (residues 34–57) failed to block endothelial cell chemotaxis but induced apoptosis; shorter P14 (residues 41–57) blocked chemotaxis but had no effect on apoptosis. Neither P23, nor P14 showed anti-angiogenic activity in vivo: moreover, P14 had stimulatory activity of its own. Of all the peptides only P18 exhibited activity and specificity similar or superior to that of the parental 34-mer. P18 inhibited chemotaxis and induced apoptosis in the activated endothelial cells similar to the parental PEDF and the 34-mer. In vivo, P18 blocked bFGF and VEGF-dependent angiogenesis in the cornea, in subcutaneous Matrigel plugs and in DIVAA.
P18 specific activity was superior compared to the native PEDF and the 34-mer in vitro, in the chemotaxis and apoptosis assays (IC50 values for P18, 34-mer and PEDF are 1 pMol, 10 pMol and 1 nMol, respectively). In vivo, P18 was also more effective than the 34-mer: it was 10 times more potent at inducing apoptosis of the vascular endothelium and at reducing MVD in the Matrigel plugs and, more importantly, in prostate cancer model. It is possible that shorter P18 maintains conformational stability in solution better than the longer 34-mer peptide.
In our previous studies we have demonstrated that PEDF and the 34-mer block angiogenesis in the activated endothelium via the cascade, which causes accumulation of the message and increased expression of CD95L, a death ligand for the intrinsic death receptor [13,27]. This receptor, CD95, is, in turn directed to the surface only in the inducer activated endothelial cells [27]. Moreover, P18 shares another target with PEDF: earlier study showed that PEDF-dependent activation of gamma-secretase leads to VEGFR cleavage and decreased angiogenesis [20]. Thus we generated short anti-angiogenic peptide derivative that reproduced main signaling functional and signaling evens that lead to cessation of angiogenesis by parental PEDF with high specific activity and, like PEDF affects only remodeling endothelium.
Parental PEDF has been shown to affect growth and invasion of the non-endothelial cells: it induces apoptosis in cultured glioma, osteosarcoma and prostate cancer cells and the anoikis of melanoma cells [28–30]. Moreover it suppresses invasion of the tumor cells, including melanoma and glioma [31,32]. However, PEDF effect on proliferation and invasion of the osteosarcoma cells was mediated by the more distal peptides, distinct from the 34-mer: residues 78–102 predominantly inhibited proliferation, while residues 90–114 increased adhesion to collagen type-1 and residues 387–411 were important for the inhibition of Matrigel invasion [33]. Furthermore, amino acids 40–64 of the PEDF molecule cause osteogenic differentiation in osteosarcoma cells [33]. This is consistent with the pro-differentiating activity of the 44-mer PEDF fragment (residues 58–101) [13,14]. Thus the direct effect of P18 on the tumor cells is less likely than its angiogenesis-mediated effects: we evaluated the effect of P18 and of P34 on proliferation of the PC-3 cells and found no significant changes.
P18 inhibited the growth of androgen-independent prostate cancer and of more aggressive renal cell carcinoma in vivo, in the subcutaneous xenograft model at 10 mg/kg: at this dose, the 34mer had no significant effect on tumor growth. In concert, P18 was more potent at blocking tumor angiogenesis and caused higher levels of intratumoral apoptosis. These findings also reflect superior potency of the P18 in comparison with the 34-mer.
In conclusion, we generated short peptide from PEDF anti-angiogenic region, which retains only PEDF anti-angiogenic function and is active in low picomolar range of concentrations. This peptide, P18 inhibits tumor growth and angiogenesis with high potency, and is a novel, highly effective biotherapeutic agent for cancer treatment, to be used alone or in combination with other agents.
STATEMENT OF TRANSLATIONAL RELEVANCE
Angiogenesis is requisite for the exponential tumor growth: anti-angiogenic therapies, like Avastin, have emerged as important new modalities for cancer treatment. Pigment epithelial-derived factor (PEDF) is a potent naturally occurring inhibitor of angiogenesis: PEDF re-expression in multiple tumor types including melanoma, prostate and ovarian cancer delays the onset of primary tumors and decreases metastases. However, clinical use of the native PEDF would be costly and complicated due to its multiple biological activities and antigenicity. We previously identified PEDF anti-angiogenic epitopes. Here we designed peptides covering its most potent anti-angiogenic region, the 34-mer. Using series of the in vivo and in vitro angiogenesis assays we screened these peptides and selected one, P18, which demonstrated efficacy against relatively mild prostate cancer and highly aggressive renal cell carcinoma. P18 is a short peptide with specific activity in a low nanomolar range, and a candidate biological drug for clinical testing.
ACKNOWLEDGEMENTS
This work was supported by the NIH grants RO1 HL068033, RO1 HL077471 (OV), DOD PCRP of the CDMRP fellowship W81XWH-06-1-0103 (YM) and by an award from Arbus Pharmaceuticals (RB, CP, OV, and VS).
REFERENCES
- 1.Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp. 1991;22:339–347. [PubMed] [Google Scholar]
- 2.Desai AA, Stadler WM. Novel kinase inhibitors in renal cell carcinoma: progressive development of static agents. Curr Oncol Rep. 2005;7:116–122. doi: 10.1007/s11912-005-0037-6. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 4.Mariani SM. Anti-angiogenesis: the challenges ahead. MedGenMed. 2003;5:22. [PubMed] [Google Scholar]
- 5.Sund M, et al. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci U S A. 2005;102:2934–2939. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Endogenous angiogenesis inhibitors. APMIS. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 8.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 9.Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol Med. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Garcia NI, Volpert OV, Jimenez B. Pigment epithelium-derived factor as a multifunctional antitumor factor. J Mol Med. 2007;85:15–22. doi: 10.1007/s00109-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 11.Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Biosci. 2005;10:2131–2149. doi: 10.2741/1686. [DOI] [PubMed] [Google Scholar]
- 13.Filleur S, et al. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744. [DOI] [PubMed] [Google Scholar]
- 14.Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv Exp Med Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 15.Smith ND, Schulze-Hoepfner FT, Veliceasa D, Filleur S, Shareef S, Huang L, Huang XM, Volpert OV. Pigment epithelium-derived factor and interleukin-6 control prostate neuroendocrine differentiation via feed-forward mechanism. J Urol. 2008;179:2427–2434. doi: 10.1016/j.juro.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notari L, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 17.Apte RS, Barreiro RA, Duh E, Volpert O, Ferguson TA. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Ophthalmol Vis Sci. 2004;45:4491–4497. doi: 10.1167/iovs.04-0172. [DOI] [PubMed] [Google Scholar]
- 18.Amorino GP, Parsons SJ. Neuroendocrine cells in prostate cancer. Crit Rev Eukaryot Gene Expr. 2004;14:287–300. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.40. [DOI] [PubMed] [Google Scholar]
- 19.Sulochana KN, Ge R. Developing antiangiogenic peptide drugs for angiogenesis-related diseases. Curr Pharm Des. 2007;13:2074–2086. doi: 10.2174/138161207781039715. [DOI] [PubMed] [Google Scholar]
- 20.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 21.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 23.Passaniti A, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 24.Wang L, Schmitz V, Perez-Mediavilla A, Izal I, Prieto J, Qian C. Suppression of angiogenesis and tumor growth by adenoviral-mediated gene transfer of pigment epithelium-derived factor. Mol Ther. 2003;8:72–79. doi: 10.1016/s1525-0016(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 25.Polverini PJ, Bouck NP, Rastinejad F. Assay and purification of naturally occurring inhibitor of angiogenesis. Methods Enzymol. 1991;198:440–450. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 26.Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513–1522. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 28.Ek ET, Dass CR, Contreras KG, Choong PF. Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin Exp Metastasis. 2007;24:93–106. doi: 10.1007/s10585-007-9062-1. [DOI] [PubMed] [Google Scholar]
- 29.Doll JA, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 30.Garcia M, et al. Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res. 2004;64:5632–5642. doi: 10.1158/0008-5472.CAN-04-0230. [DOI] [PubMed] [Google Scholar]
- 31.Ek ET, Dass CR, Contreras KG, Choong PF. Pigment epithelium-derived factor overexpression inhibits orthotopic osteosarcoma growth, angiogenesis and metastasis. Cancer Gene Ther. 2007;14:616–626. doi: 10.1038/sj.cgt.7701044. [DOI] [PubMed] [Google Scholar]
- 32.Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325–332. doi: 10.1038/sj.cgt.7700675. [DOI] [PubMed] [Google Scholar]
- 33.Ek ET, Dass CR, Contreras KG, Choong PF. PEDF-derived synthetic peptides exhibit antitumor activity in an orthotopic model of human osteosarcoma. J Orthop Res. 2007;25:1671–1680. doi: 10.1002/jor.20434. [DOI] [PubMed] [Google Scholar]