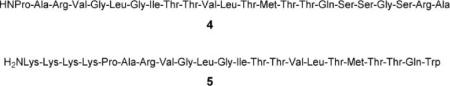Abstract
The development of low molecular weight anion transporters is an emerging topic in supramolecular chemistry. The major focus of this tutorial review is on synthetic chloride transport systems that operate in vesicle and cell membranes. The transporters alter transmembrane concentration gradients, and thus they have applications as reagents for cell biology research and as potential chemotherapeutic agents. The molecular designs include monomolecular channels, self-assembled channels and mobile carriers. Also discussed are the experimental assays that measure transport rates across model bilayer membranes.
1. Introduction
The most abundant anions in physiological solutions are chloride, bicarbonate and phosphate. The spatial distribution of these anions in cells and tissue is not even. For example, the typical extracellular and intracellular concentrations for chloride are 110 mM and 5–15 mM, respectively; furthermore, the intracellular concentrations vary with type of organelle. The ion concentration gradients are created and maintained by the concerted action of transport proteins that are buried in the relatively impermeable bilayer membranes. The chemical gradients act as energy sources to drive metabolic processes, and they also control electrical signaling in nerves, muscles and synapses. The focus of this tutorial review is the development of synthetic transporters for small inorganic anions, especially chloride. The transport action is expected to alter anion concentration gradients across biological membranes and thus induce physiological effects. Synthetic anion transporters can also be employed in other technologies such as membrane-based sensors and separation processes, but these applications are beyond the scope of this focused article and the reader is directed elsewhere for recent summaries.1,2
As a prelude to facilitated transport, consider the process of unassisted ion transport through a bilayer membrane. The membrane permeability coefficients for monovalent halide anions are in the range of 10−9−10−27 cm s−21, which is approximately two to three orders of magnitude faster than K+, a monovalent cation of similar size. The reasons for this difference in permeability are currently not clear, but there is evidence that metal cations absorb more strongly onto the phospholipid head groups at the membrane interface.3 In addition, there is a dipole layer at the interface of an unpolarized membrane (negative outside and positive inside). A major contributor to this dipole is thought to be the first layer of water molecules associated with the phospholipid head groups. The solvating water molecules are aligned so that their dipolar OH bonds are directed at the phosphate oxygens in the phospholipid head groups. Although this dipole layer is a barrier to anion entry into the membrane, it should be remembered that the membrane interior has a net positive charge, which is anion stabilizing. Thus, a major objective of any anion transport system is to promote anion movement through the repulsive dipole layer at the membrane interface.4
The mechanism of unassisted membrane transport for ionic and polar solutes has been examined experimentally and theoretically. In the case of neutral polar solutes such as water, there is evidence of a solubility-diffusion mechanism, where the molecules partition into the membrane and diffuse through the lipophilic interior. In the case of metal cations, it appears that transport occurs through transient water pores. Recent computer simulations using Molecular Dynamics methods indicate that a local charge imbalance creates an electric field, which induces a transient water pore and allows facilitated cation passage.5 A larger electric field is calculated to induce a larger pore and faster transport. This charge imbalance model suggests that association of ionic species at a membrane surface would promote pore formation and thus increase membrane transport.
Less attention has been paid to the mechanism of unassisted anion transport, but in the specific case of halide ions, it is known that permeabilities have only a modest dependence on the size of the unsolvated halide anion and the thickness of the bilayer membrane (the permeability of I− is four times faster than Br−, which is six times faster than Cl−). This experimental data is most consistent with a solubility-diffusion mechanism that envisions each halide in a hydrated state as it moves through the membrane (all three halides as hydrated anions have virtually the same radii of 3.31 Å).6 With regard to other anions, the order of membrane association and subsequent permeation follows the Hofmeister series, a solvation-based selectivity bias that is typically observed for liquid/liquid partitioning processes.7 Of the three most common physiological anions, Cl− is the most abundant, and is more lipophilic than bicarbonate and phosphate. Therefore simple synthetic transporters are likely to be inherently Cl−-selective.
2. Endogenous chloride transporters
Chloride is transported across cell membranes by a variety of different natural anion transport proteins, including anion exchangers, cation-dependent Cl−-cotransporters and Cl− channels. In many excitable cells, Cl− is normally distributed either below or close to equilibrium so that the chloride equilibrium potential (ECl) is near to that of the resting membrane potential.8 As a result, the function of Cl− channels in nerve and muscle cells is, like K+ channels, to stabilize the membrane potential. The opening of Cl− channels allows Cl− to enter the cell, the membrane voltage is clamped at ECl and the generation of action potentials is prevented. By way of contrast, in epithelial tissues, Cl− is accumulated intracellularly at a concentration above ECl by the action of cation-dependent Cl−-cotransporters such as the Na+,K+,2Cl−-cotransporter. The opening of Cl− channels in epithelia provides a pathway for Cl− exit from the cell and a key point at which to regulate the rate of transepithelial ion transport. In airway and intestinal epithelia, Cl− channels located in the apical membrane mediate Cl− secretion, whereas in renal epithelia, Cl− channels located in the basolateral membrane play an important role in Cl− reabsorption. Other major roles for Cl− channels include cell cycle regulation, stimulus-secretion coupling and the control of cell volume and intracellular pH. Thus, Cl− channels are essential for the maintenance of cell homeostasis.
Mutations in natural anion channels have profound effects on membrane transport. The best known example is the common life-shortening genetic disease cystic fibrosis, which is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel.9 Malfunction of CFTR disrupts salt and water transport across epithelia, causing ducts and tubes within the body to become blocked by thick sticky mucus. This leads to the wide-ranging symptoms of the disease, which include severe lung disease, exocrine pancreatic failure, intestinal blockage, male infertility and salty sweat. Currently, there is no cure for cystic fibrosis and the average life expectancy of cystic fibrosis patients is ~30 years. The early success of gene therapy, as an approach to correct the loss of transport function, has been hindered by technical problems that produce low transfection efficiency.
In recent years, a plethora of genetic diseases have been associated with anion channels (so called anion channelopathies).10 Best disease, a form of macular degeneration leading to vision loss, is caused by a malfunction of the Ca2+-activated Cl− channel bestrophin in the retinal pigment epithelium.11 The neurological disorders Startle disease and Angelman syndrome are associated with the malfunction of glycine and GABAA receptors, ligand-gated Cl− channels that inhibit neuronal activity.10 Of note, two forms of myotonia (muscle stiffness), nephrolithiasis (kidney stones), Bartter's syndrome type III (severe renal salt loss), osteopetrosis (bone disease) and a rare form of deafness are all caused by the loss of function of different members of the ClC family of Cl− transporters.12 Thus, the malfunction of pathways for anion transport have profound effects on biological systems and lead to a wide spectrum of disease states.
Functional studies have identified a rich variety of Cl− channels in different cells and tissues. While the molecular characterisation of Cl− channels is far from complete, the molecular identity of some types is now established.13 The main classes of Cl− channels may be summarised as follows:
Voltage-dependent Cl− channels
The largest family of Cl− transporters is the ClC family of proteins. Many ClC proteins have a ubiquitous distribution (e.g. ClC-2 and -7), whereas others have a more restricted tissue distribution (e.g. ClC-1, skeletal muscle and ClC-Ka, kidney). Some ClC proteins form low-conductance (~1 pS) plasma membrane Cl− channels gated by the membrane voltage (e.g. ClC-1, -2, -Ka and -Kb). However, other ClC proteins function as Cl−/H+ exchangers in intracellular organelles (e.g. ClC-4 and -5). ClC proteins are homodimeric, consisting of two subunits, each with its own independent Cl− transport pathway.
cAMP-activated Cl− channels
Cl− channels regulated by cAMP-dependent phosphorylation are widely expressed in epithelial tissues. They are also found in some non-epithelial cells, most notably cardiac myocytes. The identification of the defective gene responsible for cystic fibrosis led to the demonstration that CFTR is the epithelial Cl− channel regulated by cAMP-dependent phosphorylation. A CFTR is distinguished by its small conductance (6–10 pS), linear current–voltage (I–V) relationship, time- and voltage-independent gating behaviour, and regulation by phosphorylation, ATP-binding and hydrolysis. A CFTR is assembled from two motifs, each containing a membrane-spanning domain and a nucleotide-binding domain, which are linked by a unique regulatory (R) domain. The membrane-spanning domains form an anion-selective pore, while the nucleotide-binding domains and R domain control channel activity.
Ca2+-activated Cl− channels
Cl− channels regulated by an increase in the intracellular free concentration of Ca2+ are found in many cell types. These Cl− channels have a strongly outwardly rectifying I–V relationship and are gated both by intracellular free Ca2+ concentration and voltage. Channel activation is Ca2+-dependent, whereas channel deactivation is Ca2+-independent. A strong candidate for the molecular identity of the Ca2+-activated Cl− channel is bestrophin. However, the behaviour of bestrophin-generated Cl− currents are not identical to those of native Ca2+-activated Cl− channels, suggesting that bestrophin might assemble with other subunits to form the native channel.
Volume-regulated Cl− channels
Cl− channels regulated by cell swelling play a key role in regulatory volume decrease (RVD), the process by which mammalian cells recover their original volume following swelling. Volume-regulated Cl− channels have an outwardly rectifying I–V relationship and display time-dependent inactivation at large positive voltages. Because single-channel conductance outwardly rectifies (~70 pS at positive voltages and ~10 pS at negative voltages), rectification is an intrinsic property of the channel pore. The molecular identity of the volume-regulated Cl− channel is currently unknown.
Ligand-gated Cl− channels
The inhibitory neurotransmitters glycine and γ-aminobutyric acid (GABA) mediate their effects by interacting with specific receptors that form Cl−-selective channels. Glycine receptors are mainly found in the brain stem and spinal cord, whereas GABAA receptors are expressed in the brain. Both receptors have a pentameric structure, with each subunit composed of four α-helices that span the lipid bilayer, the second of which lines the channel pore. Current flow through this pore is characterised by multiple conductance levels, with the main conductance being 90 pS for glycine and 45 pS for GABAA receptors.
3. The need for synthetic anion transporters
Low molecular weight transporter molecules can exert powerful effects on biological systems by mimicking the action of natural ion channels. Aside from medical applications (see below), cationic ionophores such as valinomycin have proved to be invaluable tools for studies of membrane transport, biochemistry and physiology. Valinomycin is widely used to provide controls/comparisons for cation transport and to moderate membrane conductances, transmembrane voltages (through the application of a [K+] gradient) or cell volume (through osmotic flow). Similarly, gramicidin A is employed as a model system for biophysical studies on cation channels and their regulation by membrane lipids. These examples argue that anion-selective ionophores have significant potential as research tools to modify cell volume, pH, ionic composition and membrane voltage without disturbing metal cation concentrations. They also suggest that anionophores might prove valuable models for the molecular behaviour of natural anion channels.
One might expect that anionophores could be accessed from natural sources. Cation-transporting natural products are quite common, valinomycin and gramicidin A (see above) being good examples. Curiously, however, there are very few secondary metabolites with anion-transporting properties,14,15 and none of well-established utility. The best-studied are probably the prodigiosins14 (see Section 5.2), and these transport protons as well as anions. There is thus extensive scope for the design of purely synthetic anion-transporting systems.
If a disease is due to the malfunction of endogenous Cl− transport, substitution with an alternative transport system might have therapeutic potential. For example, one strategy to overcome the loss of Cl− channel function in cystic fibrosis is to exploit other pathways for anion transport in affected epithelial tissues (termed bypass therapy). Most work in this area has focused on the stimulation of native anion channels, especially the Ca2+-activated Cl− channel that is abundantly expressed in the respiratory airways, the major site of disease in cystic fibrosis. An alternative strategy is to create artificial anion channels that mediate transepithelial Cl− transport. Using a synthetic peptide-derived Cl− channel mimic (see below) and Madin Darby canine kidney epithelia, Tomich and co-workers provided proof of the principle of this approach.16 Importantly, they also demonstrated that synthetic anion transporter-mediated transepithelial Cl− secretion may be regulated by modulating the activity of basolateral membrane ion channels and transporters. These and other data17 raise the possibility that in the longer term, anionophore-based therapy for cystic fibrosis and other anion channelopathies might be efficacious.
3.1 Design criteria for successful anion transporters
It has been argued that the transmembrane segments of ion channels are structurally quite simple and do not have special folding patterns.18 Thus, structurally simple ion channels could have easily appeared in early evolution and may have exhibited relatively complex behavior patterns. Inspection of the X-ray structure of the ClC Cl− ion channel indicates that conduction and gating are achieved by a fascinating combination of protein structural features,19 but the basic role of the selectivity filter section of the protein is to provide hydrogen bond donors that displace the solvating water molecules around the Cl− anion. Therefore, synthetic compounds that can achieve this same function should be able to promote anion transport. It should be noted that the interface of a biological membrane is a sea of phospholipid head groups with anion phosphate diester residues that can compete for hydrogen bonding sites. If this happens, the transporters may promote phospholipid flip–flop as a competing transport outcome.20
Although the field of synthetic anion transporters is in an early stage, with most studies using model bilayer membranes, it is still worth considering the pharmacokinetic demands of final applications and how they may influence transporter designs. For example, if the aim is to invent therapeutic Cl− transporters that replace inactive channels in the airway epithelium, then methods of drug delivery have to be considered. For this specific application, the transporter compounds must be sufficiently water soluble for delivery but also be sufficiently lipophilic for insertion into a biomembrane. Therefore, a logical strategy is to make amphiphilic molecules that are water soluble but also able to diffuse readily across membranes.
4. Methods for measuring anion transport
The techniques used to detect and quantify anion transport fall into two categories: (a) methods employing vesicle suspensions, in which transport in and out of the vesicles is examined, and (b) conductance measurements on patches of synthetic or natural membranes, whole cells or sheets of tissue. The two types provide complementary information and are appropriate in different circumstances. For example, the vesicle-based methods observe membrane transport as a “bulk phenomenon” —macroscopic quantities of ions are transported across large areas of membrane (typically ~ 1 m2). In contrast, the conductance methods often use very small patches of membrane (~10−8 m2) and are highly sensitive; ion flow due to single channels is readily detected. The conductance methods also detect electrogenic transport, in which net charge transfer takes place across the membrane. In vesicles, electrogenic transport causes a potential difference, which rapidly stops further movement. Vesicles are therefore more suitable for studying charge-neutral processes such as M+X− cotransport (symport) or X−/Y− exchange (antiport).
4.1 Vesicle-based methods
Most methods employ unilamellar vesicles, which can be considered as “bubbles” of bilayer membrane that are 0.1–1 μm in diameter. They may be formed from lipid suspensions by a number of methods, such as extrusion through a nanoporous filter.21 Typically, the initial solution contains water-soluble components (ions, indicators) that are trapped inside the vesicles. Material which remains outside the vesicles is removed, e.g. by dialysis, and replaced by a different set of components. This sets up concentration gradients across the membrane that can be released by active transporters. Anion transport can be detected by various techniques. Especially common is the use of fluorescent dyes that respond to the appearance or disappearance of anions. Some examples are shown in Fig. 1. The pH-sensitive dye pyranine (1)22 (also called HPTS) provides a convenient method and has been quite widely used. However, it should be noted that a change in pH is an indirect consequence of anion transport and can also be brought about by cation transport, (e.g. exchange of H+ with M+). Lucigenin (2) is one of a number of dyes whose fluorescence is quenched by polarisable anions such as halides.23 It is therefore useful for detecting the exchange of halides with oxoanions such as nitrate. Safranin O (3) is a potential-sensitive dye that can detect the small amount of electrogenic transport possible in vesicular systems.24
Fig. 1.
The use of fluorescent dyes to detect anion transport. (a) Pyranine/HPTS: Transport of X− out of the vesicle is accompanied by cotransport of H+ or exchange of OH−. Deprotonation of pyranine (pKa = 7.2) causes an increase in fluorescence when excitation and detection wavelengths are suitably adjusted. (b) Lucigenin: Fluorescence of Lucigenin is quenched by halides (X−), but not by oxoanions such as nitrate, sulfate or phosphate (Y−). The experiment can detect the cotransport of M+X− or exchange of X− and Y−. (c) Safranin O: Fluorescence of the lipophilic, cationic dye increases when it associates with a membrane. Anion transport into the vesicle generates a membrane potential difference (i.e. an electric field) that drives the dye into the membrane, thus increasing fluorescence.
An alternative to fluorescence is the use of commercially available ion-selective electrodes. For example, vesicle-encapsulated chloride ions are invisible to a chloride-selective electrode but they can be detected if released by a transporter.25 The method is direct and technically straightforward, although kinetic experiments are limited by the relatively slow response time of the electrode. Furthermore, it is only applicable to outward transport of the analysed anion. This means that the transporter cannot be pre-incorporated in the membrane as the anion will leak during vesicle preparation. Finally, NMR spectroscopy can distinguish between anions inside and outside vesicles, provided a suitable shift reagent is present on one side of the membrane. For chloride anion, it known that paramagnetic Co2+ can shift the 35Cl resonance. However, lengthy acquisition times are required to observe the inherently weak signal.26 Thus, the method can directly confirm that Cl− transport has occurred but it is not very useful for kinetic studies.
4.2 Conductance measurements
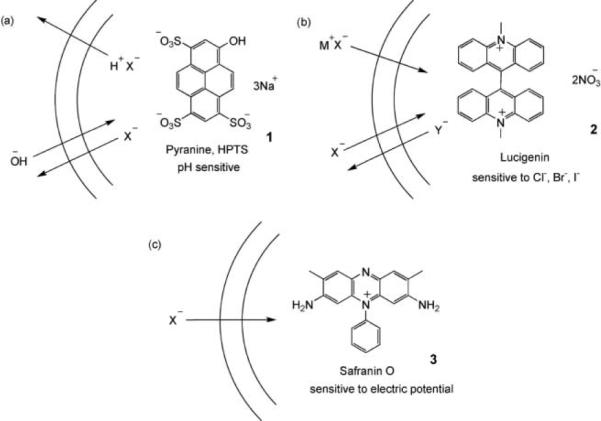
Conductance measurements are usually performed using one of two experimental formats: (a) the “planar lipid bilayer” or “black lipid membrane” (BLM) method,27 or (b) the “patch-clamp” technique.28 The planar lipid bilayer method employs an apparatus in which cups inserted into two chambers are separated by a partition containing a small hole, typically ~100 μm in diameter (Fig. 2). Electrolyte is placed in the two chambers and a lipid membrane is “painted” across the aperture as a hydrocarbon solution (typically decane). The decane and excess lipid diffuse away until just a thin layer, essentially a single bilayer, remains. The process can be observed visually because, as the membrane thins, it ceases to reflect light and appears black. The conductance of the membrane can be measured using electrodes placed on either side. Transporters can be added to the aqueous phases or incorporated directly into the lipids before the bilayer is formed.
Fig. 2.
Apparatus for planar lipid bilayer (black lipid membrane, BLM) experiments.
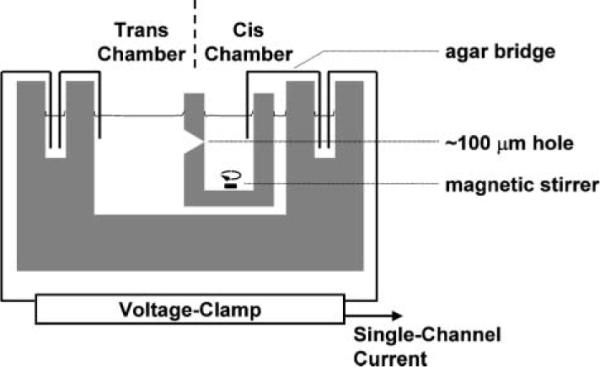
The patch-clamp method involves the use of a thin glass pipette (tip diameter ~2 mm) to capture a patch of membrane, creating a barrier between the interior and the exterior of the pipette. The conductance of the patch can then be measured, as for the planar lipid bilayer. This technique is widely used to study natural transport systems and is the mainstay of electrophysiology. As illustrated in Fig. 3, membrane patches can be excised from living cells with their protein content, which can then be readily studied. Measurements are often performed on single channels, revealing detailed information about their biophysical properties. The method is less commonly used for synthetic receptors in artificial membranes; nevertheless, such methods are available. One possibility, exploited by ourselves, is the application of giant unilamellar vesicles as surrogates for cells.29 An advantage of the patch-clamp approach is that synthetic transporters can be studied in the same apparatus as their natural counterparts, allowing direct comparisons to be made.
Fig. 3.
A patch-clamp experiment, as used in electrophysiology. (a) Pipette approaches cell. (b) Pipette makes contact with cell, surrounding one or more endogenous channels, and gentle suction is applied to the back of the pipette to form a high resistance (GΩ) seal. (c) The patch of membrane is excised from the cell by retracting the pipette so that the properties and regulation of the channel can be studied.
Finally, mention should be made of the Ussing chamber method.30 This employs oriented layers of polarised epithelial cells grown on filter supports and placed between current- and voltage-measuring electrodes. It is not applicable to the study of synthetic membranes, but is a good method for testing the effect of transporters on large-scale biological systems.
5. Progress so far; synthetic anion transporters from the literature
Synthetic ion transporters fall into two categories: (a) Ion-channel mimics, relatively static structures that provide an ion-conductance pathway, and (b) mobile carriers, small organic molecules that shuttle ions from one side to the other. Research on cation transporters has suggested that transport rates are generally higher for the channel mechanism, while it is easier to achieve good selectivities for the carrier mechanism.31 Anion transporters of both types have been developed, although channels have predominated thus far.
5.1 Synthetic anion channels
The first sustained attempt to develop synthetic anion transporters was initiated in the early 1990s by J. M. Tomich et al.16,32 Their design is based on a natural anion channel, the spinal cord glycine receptor (GlyR). As biochemical studies had suggested that the 23-mer M2 segment of this protein (M2GlyR, 4) lined the channel wall, so this peptide was synthesised and tested as an anion transporter using the planar lipid bilayer method.32 Channel activity was observed, and ascribed to tetrameric and pentameric aggregates (the natural channel is understood to be a pentamer). With KCl on either side, the channels were found to be 85% anion-selective. Although the amino acids in 4 are mostly neutral, it is notable that the ends are guarded by positively-charged arginines. Interestingly, when these were replaced with glutamates, the channels became cation-selective. Subsequently, the group have explored a range of variations on 4 with a view to developing practical therapies for cystic fibrosis. The addition of four lysines to either end improved the solubility properties whilst retaining channel activity. Further alterations, including truncations, have been tested and, at time of writing, sequence 5 is among the most promising.32
A second type of peptide-based channel, termed a “synthetic chloride membrane transporter” (SCMTR), has been studied by the group of Gokel.33 The prototype is 6, in which a short peptide sequence is flanked by two lipophilic membrane anchors; a benzyl group at the C-terminus and an acyl group bearing C18 units at the N-terminus. The GGGPGGG sequence was inspired by a conserved motif in the ClC family of chloride transporters. Compound 6 was tested for chloride transport across vesicle membranes using a chloride electrode (see above) and across planar lipid bilayers. It was found to transport anions and cations in the order Cl− ~ NO3− > SO42− >> K+, with a Cl− vs. K+ selectivity of >10. It also transported the fluorescent anionic dye carboxyfluorescein (8). Hill plots of rate vs. [6] implied that the species responsible for transport was a dimer. Gating was observed in the BLM conductance experiments, strongly suggesting channel formation. To explain these results, it was proposed that two molecules of 6 combine in the membrane, as shown by structure 9 in Fig. 4. The curvature of the peptide regions due to the prolines results in an aperture 7–8 Å in diameter. The disruption caused by the anchoring groups creates a pore extending through both leaflets of the membrane. The aperture is presumed to expand to allow the passage of 8 (~10 Å).
Fig. 4.
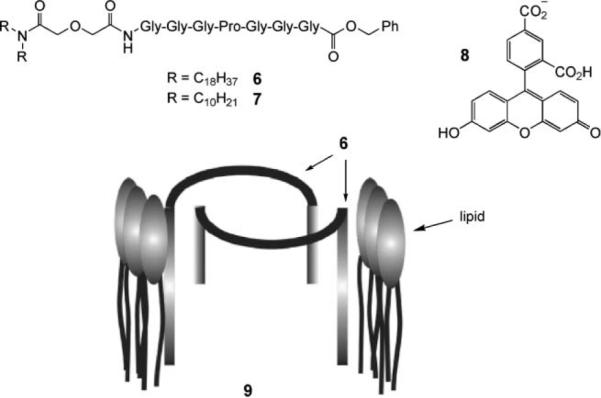
Gokel et al.'s dimeric peptide-based channel. Reproduced by permission of the Royal Society of Chemistry.
Since the initial reports on 6, variations have been made to the anchor groups, the central (4th) amino acid and the glycine units. In many cases, efficiencies have been increased, often unexpectedly. For example, merely shortening the N-terminal anchor to give 7 yields remarkable improvements in transport rates. However, other properties may also be affected, and it turns out that 7 shows no selectivity for Cl− vs. K+. Progress with this system will no doubt continue and methods for optimising both rates and selectivities are likely to emerge.
Another class of self-assembled channels are the octiphenyl-based β-barrels developed by Matile and co-workers.34 As illustrated in Fig. 5, these are held together by β-sheet structures created from short peptides attached to the p-octiphenyl scaffold. The side-chains in β-sheets point in alternating directions, so the peptide sequences can stabilise the structure by the alternation of polar and apolar substituents. The apolar side-chains (mainly Leucine) can point outwards, immersing themselves in membrane hydrocarbon, while the polar side-chains point into the water-filled pore. The approach can be used to make channels with various characteristics, depending on the nature of the polar substituents. For anion selectivity, the pore can be furnished with positively-charged centres. This has been achieved in three ways. Firstly, the channel derived from 10, previously shown to be cation-selective (due to the negative Asp side-chains), was modified by the addition of Mg2+. Unlike 10 itself, the resulting 10·Mg2+ complex was able to transmit the negatively-charged 8. Secondly, lysines were incorporated to give 11. The derived channels showed selectivity for Cl− vs. K+, with permeability ratios (PCl−/PK+) of ~3. Thirdly, both arginine and histidine were employed to make 12. In this case, the selectivity was pH-sensitive. At pH = 6, the pores were slightly cation-selective, but at pH = 4 (presumably after protonation of the histidines) they showed a PCl−/PK+ of ~4.
Fig. 5.
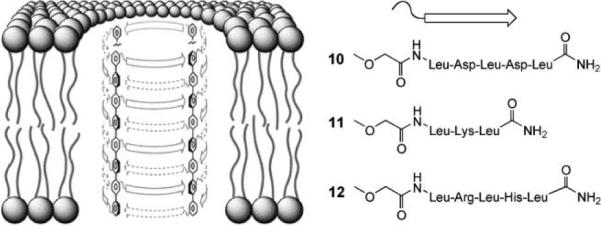
Matile et al.'s p-octiphenyl β-barrel pores. Octiphenyls are substituted with short peptides that can interdigitate to form β-sheets. Four such units combine to form a pore. Leucine side-chains point outwards into the apolar membrane environment, while the polar side-chains point inwards and line the surface of the pore. Reproduced by permission of the Royal Society of Chemistry.
The channels described thus far exploit biomimetic, peptide-based structural motifs. In contrast, the group of J. T. Davis have developed completely synthetic systems with no natural components.35 A first example was the calixarene 1,3-alt tetra-amide 13. This compound promotes anion transport across vesicle membranes, as detected by the pyranine/HPTS method (Fig. 1a) and across BLMs. The liposome studies showed selectivity for Cl− vs. HSO4−, while the BLM measurements showed gating, indicative of channel formation. Compound 13 was a poor chloride receptor but readily extracted HCl into chloroform. It was suggested that the calixarene self-assembles into a channel that transports both H+ and Cl−. The rates of the two processes can be different, to allow detection of current in the BLM experiments. The crystal structure of a complex involving the N-methyl analogue of 13, HCl and water suggested how association might take place in the membrane. More recently, the paco (partial-cone) analogue 14 was tested, along with the tert-butyl-substituted variant 15. Compound 14 promoted Cl−/NO3− exchange in vesicles, as measured by the Lucigenin method (Fig. 1b). Compound 15 was inactive and, interestingly, inhibited the action of 14, perhaps by forming non-productive heteroaggregates.
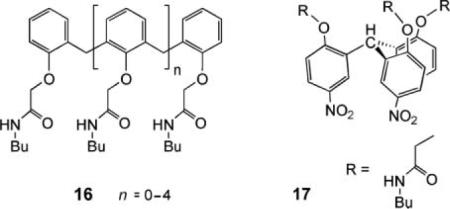
Also studied were the oligoamides 16, acyclic analogues of 13. These compounds were active, showing that the cyclic calixarene framework is not actually necessary. Indeed, the most effective, triamide 16 (n = 1), was roughly ten times more powerful than 13. In this case, the Safranin O method (Fig. 1c) was applied, showing that the triamide could create a potential 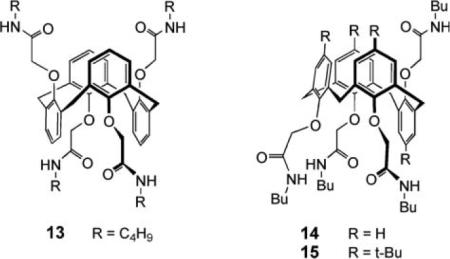 difference across a membrane. Again it is thought that 16 self-associates to form a channel. Finally, the C3-symmetric triamide 17 was very recently reported to promote H+/NO3− cotransport under vesicle conditions, where 14 promotes Cl−/NO3− exchange.36 The picture emerging from this work is that subtle changes in transporter structure can lead to significantly different membrane transport outcomes.
difference across a membrane. Again it is thought that 16 self-associates to form a channel. Finally, the C3-symmetric triamide 17 was very recently reported to promote H+/NO3− cotransport under vesicle conditions, where 14 promotes Cl−/NO3− exchange.36 The picture emerging from this work is that subtle changes in transporter structure can lead to significantly different membrane transport outcomes.
A logical approach to anion transport, already illustrated by β-barrels 10–12, is to provide a positively-charged pathway through the membrane. The Regen group have applied this strategy in the design of 18, one of the earliest synthetic anion transporters.37 The structure was inspired by squalamine, a naturally-occurring steroidal polyamine with anti-microbial properties. It was supposed that the ammonium–sulfate interaction would hold the molecule in a cyclic conformation, the steroidal surface would draw the molecule into a membrane with the ion pair at the surface, and individual units would then combine to form channels (Fig. 6). Ion transport was detected by the pyranine/HPTS assay (Fig. 1a) with NaCl present on either side of the membrane. As separate experiments had shown that 18 did not transport Na+, it was concluded that H+Cl− symport, or possibly Cl−/OH− exchange, was taking place. Transport was extremely sensitive to the thickness of the membrane, implying a self-assembled channel of a specific length. Transporter 18 has been further studied by a medicinal chemistry group, who have demonstrated enhanced chloride transport across cystic fibrosis epithelial cell membranes.38
Fig. 6.
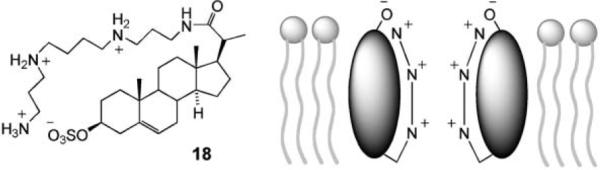
Regen's steroid-based ion channel.
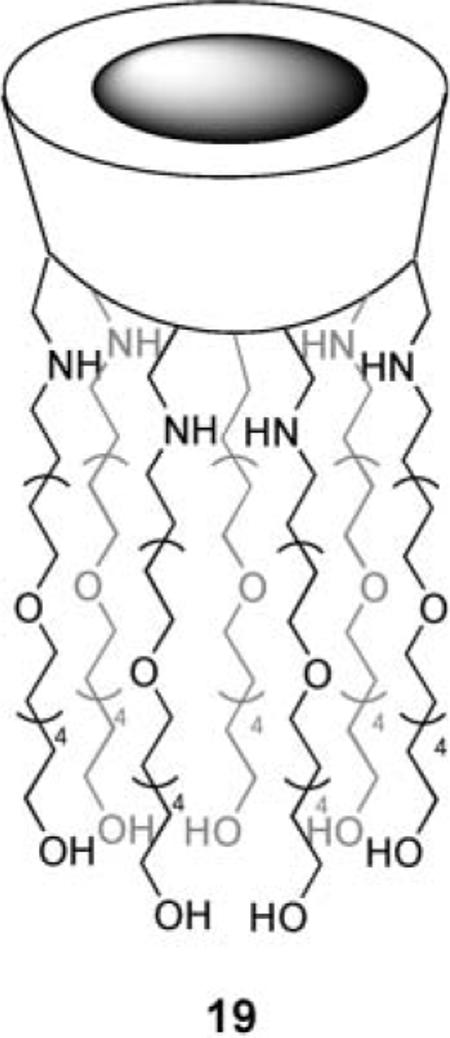
Finally, a recent system due to Gin et al. also exploits positive charges, this time in a monomolecular channel.39 Transporter 19 is derived from β-cyclodextrin by displacement of all seven primary hydroxyl groups with amino-terminated pentabutyleneglycol. The side-chains are long enough to span the membrane, while the protonated nitrogens create a portal with up to seven positive charges. Initially 19 was tested as a Na+ transporter and was quite active. However, a pH-based (HPTS) method revealed effects due to both anions and cations. Bromide and especially iodide were transported significantly faster than Na+. Rates were linear with [19], consistent with transport by the monomer.
5.2: Synthetic anion carriers
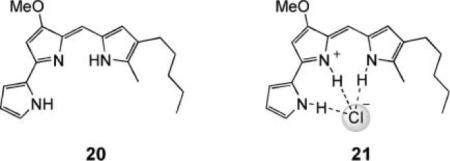
As mentioned earlier, anion transport capabilities are rare in natural products, in strong contrast to cation transport. Among the few known anionophores, the prodigiosins stand out as being small, non-peptidic molecules (the parent compound is structure 20) with a rich array of pharmaceutical activities (e.g. antibiotic, antitumor, immunosuppressive). At present, it is not clear how the prodigiosins induce their biological effects; however, one interesting question is whether any activity can be attributed to their ability to act as HCl transporters. They are known to inhibit the development of pH differences across biological membranes and to discharge pH differences in liposomal systems in a chloride-dependent process.14 In membranes formed from DPPC, which undergoes a temperature-dependent gel–liquid crystalline transition, the transport activity is quenched when the membrane is in the gel state.40 This is characteristic of the carrier mechanism, which requires that the transporter molecules should be able to move freely within the membrane (however, another possible explanation for this experimental outcome is decreased partitioning of the transporter into the gel phase membrane).41 Carrier-type transport of HCl accords with theoretical expectation. Protonation of 20 gives a cation, which is preorganised to bind chloride, as in 21. The latter is electroneutral, and lipophilic enough to diffuse across a membrane, delivering HCl to the other side.
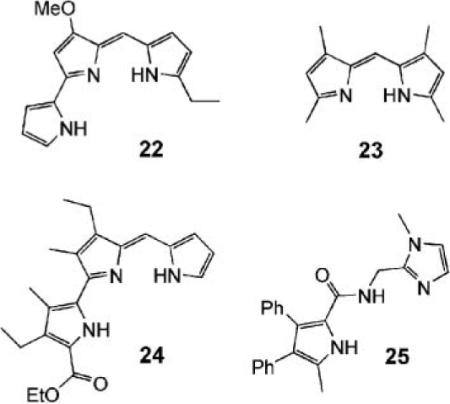
Two groups have recently reported functional analogues of prodigiosins. Sessler et al. have examined dipyrroles and tripyrroles such as 22–24.42 When protonated, all were good receptors for Cl− in acetonitrile, with Ka values in the region 105–106 M−1. The compounds were tested in liposomes with chloride inside and nitrate or sulfate outside. Chloride efflux was detected with an electrode and found to be pH-dependent, as expected for HCl symport. Although all the molecules were active, it is notable that 22, the most prodigiosin-like, was the most effective by some margin. Gale et al. have performed similar studies on pyrrolecarboxamide 25 with similar results.43
In principle, anion transport should not be difficult for a positively-charged carrier such as protonated prodigiosin. Cations are normally accompanied by anions, and any cation that is lipophilic enough to locate in a membrane should bring along an anionic partner. The design of electroneutral anion carriers presents an extra challenge. In this case, the carrier needs to both extract the anion from water and separate it from its cation. Thus far, only one class of molecules has been clearly shown to have this capability in bilayer membranes, the cholapods 26.17 The steroidal framework in 26, derived from inexpensive cholic acid, confers lipophilicity while pre-organising arrays of anion-binding hydrogen bond donor groups. As a result, the cholapods are soluble in non-polar media yet can possess highly polar binding sites, showing quite extreme affinities (up to 1011 M−21 for Cl− in CHCl3).44 It transpires that cholapods with affinities of ~107 M−1 upwards (for Cl− in CHCl3) are effective chloride transporters. A first series, 27, were studied in vesicles using the chloride electrode method. The steroids promoted efflux of Cl− when the external medium contained nitrate, but not when it contained only sulfate. This provided clear evidence of anion selectivity—if the transporter were active for both cations and anions, the external anion would be irrelevant. The cholapods must also be selective for Cl− and NO3− vs. SO42−; both Cl− and NO3− are transported, allowing exchange, but SO42− is not affected. As selective anion carriers, the cholapods should also be electrogenic, capable of altering membrane potentials. This was confirmed using the Safranin O method (Fig. 1c). Transport was halted in gel phase vesicles, supporting the carrier mechanism.
As might be expected, the effectiveness of 27 as carriers increased with their strength as anion receptors. Although the correlation could, in principle, be reversed for very powerful receptors (where anion release could be rate determining), this has not yet happened in practice. Cholapod 28 possesses 5 NH groups and electron-withdrawing para-nitrophenyl substituents that enhance hydrogen bond donor capability. Accordingly, it is among the strongest of the cholapod anion receptors. It is also the most powerful transporter, exchanging Cl− for NO3− in a few minutes at a 1: 25 000 ratio of lipid to 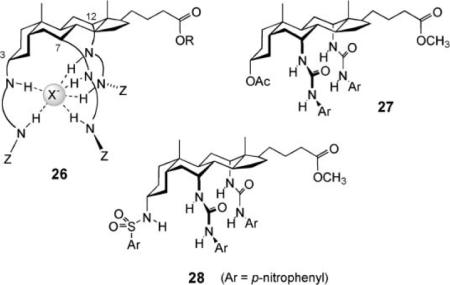 steroid. This and other evidence suggests there is still scope for improving transport rates by raising anion affinities.
steroid. This and other evidence suggests there is still scope for improving transport rates by raising anion affinities.
To conclude this section, it is interesting to compare the performance of the two classes of transporter; channels and carriers. As mentioned earlier, one might anticipate higher transport rates for the channels and higher selectivities for the carriers. Regarding selectivity, this expectation is probably met; most of the anion channels seem to transport cations to some extent, while the action of the carriers seems to be more specific. There is no evidence that the cholapods or the prodigiosin family transport metal cations. Regarding activity, it is difficult to make comparisons because of differences in the reporting of concentrations; sometimes transporter/lipid ratios are listed, sometimes overall concentrations in the aqueous-lipid mixture. However, perhaps surprisingly, there is no clear indication that the synthetic channels give higher rates than the carriers. As far as one can tell, cholapod 28 is comparable with the best of the former. This may suggest that channel optimisation is still in its early stages and that substantial improvements are still possible.
6. Future directions and challenges
It is likely that some of the synthetic anion transporters described in this article can be employed as reagents in biomembrane research. Future cell studies should prove that they can dissipate electrical and/or chemical gradients in the same way as cationophores. A more ambitious goal is to produce lead compounds for channel replacement therapies. At present, the few synthetic transporters that have been evaluated for Cl− transport in epithelial cells have yielded promising results. The next step is to study these compounds in animal models, but this work is specialized and consumes resources. Thus, an immediate goal is to establish which transporters would most likely be effective in a living animal. More cell transport studies should be conducted to clearly establish which transporter designs produce the highest fluxes. In addition, there are pharmacokinetic factors to be considered such as undesired toxicity, metabolic instability, tissue selectivity and target membrane residence time. In the specific case of cystic fibrosis, there is the possibility of the direct delivery of a synthetic transporter to the lung by inhalation, but penetration of the thick sticky mucus that blocks air passages remains a formidable technical challenge.
A contrasting therapeutic application with Cl− transporters is to produce compounds that are selectively toxic to specific cell lines, such as cancer or immune cells. For example, it will be interesting to see if HCl transporters can be produced with the intriguing pharmaceutical properties of the prodigiosin family of compounds. Alternatively, it is known that drug induced apoptosis of cancer cells involves the activation of K+ and Cl− efflux channels, which promotes cell volume decrease.45 Chloride channel blockers have been found to inhibit apoptosis, raising the following question: Can appropriately designed synthetic Cl− transporters act as adjuvants and promote therapy-induced apoptosis?
Finally, there is the topic of transporters for anions other than Cl−. For example, an interesting target with potential utility is a synthetic transport system for bicarbonate. A specific goal would be a mimic of chloride/bicarbonate exchangers, which play important roles in red blood cells and epithelial tissues. The design challenge here is to produce a transporter that can extract the very hydrophilic bicarbonate anion into the lipophilic interior of a bilayer membrane. Other anion transporters include a wide range of cotransporters that simultaneously move either H+ or Na+ and an anion such as sulfate, phosphate or carboxylate. The facilitated transport of organic anions derived from amino acids, sugars and nucleotides, as well their oligomers, is also a major technical challenge and efficient synthetic transporters would have significant impact on various areas of biomedical science.
Acknowledgements
Financial support for this work was provided by the BBSRC, the EPSRC, the University of Bristol, the CF Trust, NIH (USA) and the Walther Cancer Institute.
Biographies

Bradley D. Smith obtained a BSc (Hons) degree from the University of Melbourne and a PhD in 1988 from Penn State University. After postdoctoral training at Oxford University and then Columbia University, he moved to the University of Notre Dame in 1991. He is currently a Professor of Chemistry and Biochemistry with research interests in the fields of bioorganic and supra-molecular chemistry. One of the aims of his group is to design and synthesize organic molecules that affect the structure and function of biomembranes.

Anthony P. Davis gained a BA in Chemistry from Oxford University in 1977, then stayed on for a DPhil under Dr. G. H. Whitham and postdoctoral research with Prof. J. E. Baldwin. In 1981, he moved to the ETH Zürich to work with Prof. A. Eschenmoser, then in 1982 was appointed as a Lecturer in Organic Chemistry at Trinity College, Dublin. In September 2000 he moved to the University of Bristol, where he is Professor of Supramolecular Chemistry. His principal research interests are the biomimetic recognition of carbohydrates, anion recognition and transport, crystal engineering of nanoporous solids and the design of enantioselective receptors.

David N. Sheppard investigates the cystic fibrosis trans-membrane conductance regulator (CFTR) Cl− channel with the goal of developing rational new therapies for cystic fibrosis and related diseases. After obtaining a PhD from the University of Cambridge with Dr. F. V. Sepúlveda, he undertook postdoctoral research with Prof. F. Giraldez (Universidad de Valladolid) and Prof. M. J. Welsh (University of Iowa). Returning to the UK, he was a BBSRC Advance Research Fellow at the University of Edinburgh before becoming a Lecturer, then Senior Lecturer at the University of Bristol.
References
- 1.Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. Royal Society of Chemistry; Cambridge: 2006. [Google Scholar]
- 2.Moyer BA, Singh R, editors. ACS Symp. Ser. Kluwer; New York: 2004. Fundamentals and Applications of Anion Separations. [Google Scholar]
- 3.Berkowitz ML, Bostick DL, Pandit S. Chem. Rev. 2006;106:1527. doi: 10.1021/cr0403638. [DOI] [PubMed] [Google Scholar]
- 4.Eisenman G, Horn RJ. J. Membr. Biol. 1983;76:197. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- 5.Gurtovenko AA, Vattulainen L. J. Am. Chem. Soc. 2005;127:17570. doi: 10.1021/ja053129n. [DOI] [PubMed] [Google Scholar]
- 6.Paula S, Volkov AG, Deamer DW. Biophys. J. 1998;74:319. doi: 10.1016/S0006-3495(98)77789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisson AL, Clare JP, Taylor LH, Charmant JPH, Davis AP. Chem. Commun. 2003 doi: 10.1039/b305261c. 2246 and references therein. [DOI] [PubMed] [Google Scholar]
- 8.Hille B. Ionic Channels of Excitable Membranes. 3rd edn Sinauer Associates; Sunderland: 2001. [Google Scholar]
- 9.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. McGraw-Hill; New York: 2001. p. 5121. [Google Scholar]
- 10.Ashcroft FM. Ion Channels and Disease Channelopathies. Academic Press; San Diego: 2000. [Google Scholar]
- 11.Hartzell C, Putzier I, Arreola J. Annu. Rev. Physiol. 2005;67:719. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 12.Jentsch TJ, Maritzen T, Zdebik AA. J. Clin. Invest. 2005;115:2039. doi: 10.1172/JCI25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilius B, Droogmans G. Acta Physiol. Scand. 2003;117:119. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 14.Prodigiosins: Ohkuma S, Sato T, Okamoto M, Matsuya H, Arai K, Kataoka T, Nagai K, Wasserman HH. Biochemistry. 1998;334:731. doi: 10.1042/bj3340731. and references therein.
- 15.Duramycin: Sheth TR, Henderson RM, Hladky SB, Cuthbert AW. Biochim. Biophys. Acta. 1992;1107:179. doi: 10.1016/0005-2736(92)90345-m.; Pamamycin: Jeong EJ, Kang EJ, Sung LT, Hong SK, Lee E. J. Am. Chem. Soc. 2002;124:14655. doi: 10.1021/ja0279646.
- 16.Wallace DP, Tomich JM, Iwamoto T, Henderson K, Grantham JJ, Sullivan LP. Am. J. Physiol.: Cell Physiol. 1997;272:C1672. doi: 10.1152/ajpcell.1997.272.5.C1672. [DOI] [PubMed] [Google Scholar]; Wallace DP, Tomich JM, Eppler JW, Iwamoto T, Grantham JJ, Sullivan LP. Biochim. Biophys. Acta. 2000;1464:69. doi: 10.1016/s0005-2736(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 17.Koulov AV, Lambert TN, Shukla R, Jain M, Boon JM, Smith BD, Li H, Sheppard DN, Joos J-B, Clare JP, Davis AP. Angew. Chem., Int. Ed. 2003;42:4931. doi: 10.1002/anie.200351957. [DOI] [PubMed] [Google Scholar]; McNally BA, Koulov AV, Smith BD, Joos J-B, Davis AP. Chem. Commun. 2005:1087. doi: 10.1039/b414589e. [DOI] [PubMed] [Google Scholar]
- 18.Pohorille A, Schweighoffer K, Wilson MA. Astrobiology. 2005;5:1. doi: 10.1089/ast.2005.5.1. [DOI] [PubMed] [Google Scholar]
- 19.Dutzler R. FEBS Lett. 2004;564:229. doi: 10.1016/S0014-5793(04)00210-8. [DOI] [PubMed] [Google Scholar]
- 20.Smith BD, Lambert TN. Chem. Commun. 2003;18:2261. doi: 10.1039/b303359g. [DOI] [PubMed] [Google Scholar]
- 21.New RRC, editor. Liposomes: A Practical Approach. IRL Press; Oxford: 1990. [Google Scholar]
- 22.Clement NR, Gould JM. Biochemistry. 1981;20:1534. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- 23.Biwersi J, Tulk B, Verkman AS. Anal. Biochem. 1994;219:139. doi: 10.1006/abio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 24.Woolley GA, Kapral MK, Deber CM. FEBS Lett. 1987;224:337. [Google Scholar]
- 25.Schlesinger PH, Ferdani R, Liu J, Pajewska J, Pajewski R, Saito M, Shabany H, Gokel GW. J. Am. Chem. Soc. 2002;124:1848. doi: 10.1021/ja016784d. [DOI] [PubMed] [Google Scholar]
- 26.Riddell FG. In: Encyclopedia of Spectroscopy and Spectrometry. Lindon JC, Tranter GE, Holmes JL, editors. Academic Press; London: 1999. p. 1584. [Google Scholar]
- 27.Williams AJ. In: Ion Channels: A Practical Approach. Ashley RH, editor. IRL Press; Oxford: 1995. p. 43. [Google Scholar]
- 28.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Pfluegers Arch. 1981;391:85. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 29.Riquelme G, Lopez E, Garcia-Segura LM, Ferragut JA, Gonzalez-Ros JM. Biochemistry. 1990;29:11215. doi: 10.1021/bi00503a009. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Sheppard DN, Hug MJ. J. Cystic Fibrosis. 2004;3:123. doi: 10.1016/j.jcf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Cragg PJ. Sci. Prog. 2002;85:219. doi: 10.3184/003685002783238780. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy GL, Iwamoto T, Tomich JM, Montal M. J. Biol. Chem. 1993;268:14608. [PubMed] [Google Scholar]; Broughman JR, Mitchell KE, Sedlacek RL, Iwamoto T, Tomich JM, Schultz BD. Am. J. Physiol.: Cell Physiol. 2001;280:C451. doi: 10.1152/ajpcell.2001.280.3.C451. [DOI] [PubMed] [Google Scholar]; Broughman JR, Shank LP, Takeguchi W, Schultz BD, Iwamoto T, Mitchell KE, Tomich JM. Biochemistry. 2002;41:7350. doi: 10.1021/bi016053q. [DOI] [PubMed] [Google Scholar]; Shank LP, Broughman JR, Takeguchi W, Cook G, Robbins AS, Hahn L, Radke G, Iwamoto T, Schultz BD, Tomich JM. Biophys. J. 2006;90:2138. doi: 10.1529/biophysj.105.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlesinger PH, Ferdani R, Liu J, Pajewska J, Pajewski R, Saito M, Shabany H, Gokel GW. J. Am. Chem. Soc. 2002;124:1848. doi: 10.1021/ja016784d. [DOI] [PubMed] [Google Scholar]; Schlesinger PH, Ferdani R, Pajewski R, Pajewska J, Gokel GW. Chem. Commun. 2002:840. doi: 10.1039/b200126h. [DOI] [PubMed] [Google Scholar]; Schlesinger PH, Djedovic NK, Ferdani R, Pajewska J, Pajewski R, Gokel GW. Chem. Commun. 2003:308. doi: 10.1039/b211629b. [DOI] [PubMed] [Google Scholar]; Djedovic N, Ferdani R, Harder E, Pajewska J, Pajewski R, Weber ME, Schlesinger PH, Gokel GW. New J. Chem. 2005;29:291. doi: 10.1039/b417091c. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ferdani R, Pajewski R, Djedovic N, Pajewska J, Schlesinger PH, Gokel GW. New J. Chem. 2005;29:673. doi: 10.1039/b417808b. [DOI] [PMC free article] [PubMed] [Google Scholar]; You L, Ferdani R, Gokel GW. Chem. Commun. 2006:603. doi: 10.1039/b517713h. [DOI] [PubMed] [Google Scholar]
- 34.Das G, Onouchi H, Yashima E, Sakai N, Matile S. ChemBioChem. 2002;3:1089. doi: 10.1002/1439-7633(20021104)3:11<1089::AID-CBIC1089>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; Sakai N, Houdebert D, Matile S. Chem.-Eur. J. 2003;9:223. doi: 10.1002/chem.200390016. [DOI] [PubMed] [Google Scholar]; Sakai N, Sordé N, Das G, Perrottet P, Gerard D, Matile S. Org. Biomol. Chem. 2003;1:1226. doi: 10.1039/b210604c. [DOI] [PubMed] [Google Scholar]
- 35.Sidorov V, Kotch FW, Abdrakhmanova G, Mizani R, Fettinger JC, Davis JT. J. Am. Chem. Soc. 2002;124:2267. doi: 10.1021/ja012338e. [DOI] [PubMed] [Google Scholar]; Seganish JL, Santacroce PV, Salimian KJ, Fettinger JC, Zavalij P, Davis JT. Angew. Chem., Int. Ed. 2006;45:3334. doi: 10.1002/anie.200504489. [DOI] [PubMed] [Google Scholar]; Sidorov V, Kotch FW, Kuebler JL, Lam YF, Davis JT. J. Am. Chem. Soc. 2003;125:2840. doi: 10.1021/ja029372t. [DOI] [PubMed] [Google Scholar]; Seganish JL, Fettinger JC, Davis JT. Supramol. Chem. 2006;18:257. [Google Scholar]
- 36.Santacroce PV, Okunola OA, Zavalij PY, Davis JT. Chem. Commun. 2006:3246. doi: 10.1039/b607221f. [DOI] [PubMed] [Google Scholar]
- 37.Deng G, Dewa T, Regen SL. J. Am. Chem. Soc. 1996;118:8975. [Google Scholar]; Merritt M, Lanier M, Deng G, Regen SL. J. Am. Chem. Soc. 1998;120:8494. [Google Scholar]; Otto S, Osifchin M, Regen SL. J. Am. Chem. Soc. 1999;121:7276. [Google Scholar]
- 38.Jiang CW, Lee ER, Lane MB, Xiao YF, Harris DJ, Cheng SH. Am. J. Physiol.: Lung Cell Mol. Physiol. 2001;281:L1164. doi: 10.1152/ajplung.2001.281.5.L1164. [DOI] [PubMed] [Google Scholar]
- 39.Madhavan N, Robert EC, Gin MS. Angew. Chem., Int. Ed. 2005;44:7584. doi: 10.1002/anie.200501625. [DOI] [PubMed] [Google Scholar]
- 40.Seganish JL, Davis JT. Chem. Commun. 2005:5781. doi: 10.1039/b511847f. [DOI] [PubMed] [Google Scholar]
- 41.Otto S, Osifchin M, Regen SL. J. Am. Chem. Soc. 1999;121:10440. [Google Scholar]
- 42.Sessler JL, Eller LR, Cho WS, Nicolaou S, Aguilar A, Lee JT, Lynch VM, Magda DJ. Angew. Chem., Int. Ed. 2005;44:5989. doi: 10.1002/anie.200501740. [DOI] [PubMed] [Google Scholar]
- 43.Gale PA, Light ME, McNally B, Navakhun K, Sliwinski KE, Smith BD. Chem. Commun. 2005:3773. doi: 10.1039/b503906a. [DOI] [PubMed] [Google Scholar]
- 44.Davis AP, Joos J-B. Coord. Chem. Rev. 2003;240:143. [Google Scholar]; Clare JP, Ayling AJ, Joos J-B, Sisson AL, Magro G, Pérez-Payán MN, Lambert TN, Shukla R, Smith BD, Davis AP. J. Am. Chem. Soc. 2005;127:10739. doi: 10.1021/ja0524144. [DOI] [PubMed] [Google Scholar]; Davis AP. Coord. Chem. Rev. 2006 in press. [Google Scholar]
- 45.Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahshi N. J. Membr. Biol. 2006;209:21. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]