Abstract
AMPK is an evolutionarily conserved fuel-sensing enzyme that is activated in shortage of energy and suppressed in its surfeit. AMPK activation stimulates fatty acid oxidation, enhances insulin sensitivity, alleviates hyperglycemia and hyperlipidemia, and inhibits proinflammatory changes. Thus, AMPK is a well-received therapeutic target for metabolic syndrome and Type 2 diabetes. Recent studies indicate that AMPK plays a role in linking metabolic syndrome and cancer. AMPK is an essential mediator of the tumor suppressor LKB1 and could be suppressed in cancer cells containing loss-of-function mutations of LKB1 or containing active mutations of B-Raf, or in cancers associated with metabolic syndrome. The activation of AMPK reprograms cellular metabolism and enforces metabolic checkpoints by acting on mTORC1, p53, fatty acid synthase and other molecules for regulating cell growth and metabolism. In keeping with in vitro studies, recent epidemiological studies indicate that the incidence of cancer is reduced in Type 2 diabetes treated with metformin, an AMPK activator. Thus, AMPK is emerging as an interesting metabolic tumor suppressor and a promising target for cancer prevention and therapy.
Keywords: acetyl CoA carboxylase, AMPK, fatty acid synthase, LKB1, metabolic syndrome, metabolism, mTOR, p53, tumor suppressor, tumorigenesis
AMP-activated protein kinase acts as a fuel gauge that is activated under stresses such as hypoxia, ischemia, glucose deprivation and exercise [1]. Activation of AMPK stimulates fatty acid oxidation to generate more ATP to cope with acute energy demand and inhibits anabolic processes that consume ATP [1]. As a result, energy is preserved for acute cellular programs. In addition, AMPK activation enhances insulin sensitivity, inhibits hepatic glucose production, stimulates glucose uptake in muscle, inhibits fatty acid synthesis and esterification, and diminishes proinflammatory changes [2]. Thus, AMPK is a well-accepted target for the treatment of metabolic syndrome and Type 2 diabetes (for extensive reviews, refer to [1–3]). During the last 5 years, since our first review [3], great attention has been drawn to link AMPK and cancer, and substantial progress has been made. AMPK, by regulating a variety of downstream targets, such as mTORC1, p53 and fatty acid synthase (FASN), and associated metabolic processes, controls intracellular energy levels in order to maintain the cell growth rate at an appropriate level. Likewise, AMPK activation under metabolic stress or by pharmacological activators can regulate various processes, including cell cycle checkpoint, cell polarity, senescence, autophagy and apoptosis [4–7]. In this article, we aim to summarize recent evidence in support of the notion that AMPK serves as a metabolic tumor suppressor and discuss the implications of AMPK in cancer prevention and treatment.
Activation of AMPK
AMP-activated protein kinase belongs to a family of serine/threonine protein kinases and is highly conserved from yeast to human. It consists of three subunits: a catalytic subunit (α) and two regulatory subunits (β and γ) [8,9]. In mammals, each subunit of AMPK contains two to three isoforms (α1, α2; β1, β2; γ1, γ2 and γ3). When cells confront metabolic stress, the intracellular AMP level or the ratio of AMP to ATP is increased. AMP then binds to the γ-subunit, yielding two outcomes; first, it serves as allosteric activator and second, it protects AMPK against phosphatases to dephosphorylate threonine 172 in the activation loop of the catalytic α-subunit [9–11]. Another critical step for AMPK activation is the phosphorylation of threonine 172. Previous studies suggest that AMP binding enables the phosphorylation of threonine 172 by an upstream kinase [12,13]. However, this notion is challenged by recent in vitro studies showing that phosphorylation of AMPK by purified LKB1 complex is independent of AMP [11,14]. A possible explanation of this discrepancy is that the AMPK preparations used in previous studies may have been contaminated with phosphatases, of which the activity toward AMPK is inhibited by AMP.
AMP-activated protein kinase can also be activated by hormones and cytokines, including leptin (e.g., in skeletal muscle) and adiponectin secreted from adipocytes, IL-6 and ciliary neurotrophic factor (CNTF) [1,15]. Interestingly, leptin could exert opposite effects on AMPK depending on cell types. While it inhibits the enzyme in the arcuate and paraventricular regions of the hypothalamus of fasted mice, leptin activates α2 isotype of AMPK in skeletal muscle [16,17]. In addition, AMPK can be activated by a variety of pharmacological agents. The prototypical activator is 5-aminoimidazole-4-carboxamide 1-D-ribonucleoside (AICAR), a cell permeable agent that is phosphorylated and converted to ZMP, an AMP analog, after entering the cell. Importantly, two clinically used antidiabetic drugs, metformin and thia-zolidinediones (TZDs), have been known to activate AMPK [15]. Upon activation, AMPK phosphorylates a plethora of substrates and regulates their functions (Figure 1), which are far beyond the canonical ones that are known to promote fatty acid oxidation and simultaneously inhibit lipid synthesis [8,9].
Figure 1. Essential Roles of LKB1/AMPK in controlling cell growth and tumorigenesis.
LKB1/AMPK by regulating mTORC1, p53 and other important molecules control cellular processes, such as cell cycle checkpoint, apoptosis, autophagy and cell polarity. Dysregulation of LKB1/AMPK causes loss of these control points and thus leads to unrestrained growth. Only a few representative molecules that are directly relevant to growth control are listed here.
TZD: Thiazolidinedione.
Thus far, several kinases have been identified to phosphorylate threonine 172 on the catalytic α-subunit of AMPK leading to its activation. The first kinase is LKB1, which is originally found in liver and also known as serine/threonine kinase 11 (STK11) [13,18,19]. LKB1 is ubiquitously expressed and responsible for AMPK activation in most scenarios. The second kinase is calmodulin-dependent protein kinase-β (CaMKK-β), which phosphorylates AMPK in response to increases in intracellular Ca2+ levels instead of AMP [20,21]. Other kinases include TGF-β-activating kinase 1 (TAK1) and ataxia-telangiectasia mutated (ATM) [22–24]. Whether these kinases are bona fide kinases for AMPK remains to be further determined by genetic approach. Interestingly, a recent study has reported that TAK1 mediates TNF-related apoptosis-inducing ligand to activate AMPK, inducing autophagy independent of LKB1 and CaMMK [25].
AMPK mediates the tumor suppressive function of LKB1
LKB1 is a tumor suppressor [26], and its loss-of-function mutations, most of which cause a loss of kinase activity, are an etiological factor of Peutz–Jeghers syndrome, an autosomal dominant genetic disorder. This genetic syndrome is characterized by multiple hamartomatous polyps (benign overgrowth of differentiated tissues) in the gastrointestinal tract and a markedly increased risk of gastrointestinal adenocarcinomas [26]. In addition, somatic mutations of the LKB1 gene have been found in several other cancers, for example, in approximately 34% of lung adenocarcinomas, 19% of squamous cell carcinomas and 20% of cervical carcinomas and other cancers [27–30].
Although a complete ablation of LKB1 causes embryonic lethality in mouse models, its heterozygous deletion increases the incidence of tumor in the intestine and stomach [26] and predisposes animals to carcinogenesis induced by 7,12 dimethylbenz(α)anthracene, thereby developing squamous cell carcinoma of the skin and lung [31]. In addition, tissue-specific deletion of LKB1 in the endometrial epithelium of female mice or the prostate epithelium of male mice causes endometrial adenocarcinomas and prostate neoplasia, respectively [32,33]. AMPK plays an important role in mediating tumorigenic effects of LKB1. Thus, a hypomorphic mutation that decreases LKB1 markedly accelerates tumor development in PTEN+/− mice, whereas pharmacological AMPK activators significantly delay tumor onset [34]. In LKB1-deficient lung cancer cells, AMPK activity is suppressed and refractory to its pharmacological activators, leading to increased mTORC1 signaling, whereas the ability of AMPK to inhibit cell growth is restored when wild-type LKB1 is expressed [35,36].
Downstream targets of AMPK
The number of AMPK targets has grown rapidly in recent years and their biological functions have been discussed in great detail elsewhere [1,5,8,9]. In this article, we highlight only some of the targets that are well characterized and known to have important roles in tumorigenesis.
mTORC1 signaling
Many studies have established that mTOR is a major downstream target of AMPK (Figure 2) [5]. Early studies have shown that AMPK activated by stress or pharmacological activators inhibits protein synthesis via regulation of mTOR/S6K [37] and translation elongation factor 2 [38]. mTOR is a key component of mTORC1 and mTORC2, both of which have essential roles in PI3K signaling [39]. While mTORC2 phosphorylates Akt at Ser 473, contributing to its activation, mTORC1 integrates nutrients and growth signals to function downstream of PI3K/Akt. Upon stimulation of cells with growth factors, activated Akt phosphorylates tuberous sclerosis complex 2 (TSC2), which forms a complex with TSC1 and thus constitutes a GTPase-activating protein (GAP) for a small GTPase, RHEB, and an immediate activator of mTOR. This phosphorylation leads to inactivation of the GAP activity, causing activation of mTOR. Germinal mutations of TSC1 or TSC2 account for another autosomal genetic disease, in which patients develop hamartomas in multiple organs, such as the brain, lung, skin, heart and kidney [40].
Figure 2. Interaction of AMPK with the ERK and PI3K pathways.
Recent studies have demonstrated that the LKB1/AMPK axis functionally interacts with the mitogenic pathways: Atk could phosphorylate and inhibit AMPK; LKB1 is inhibited by oncogenic B-Raf in an ERK/RSK-dependent fashion; KSR associates with Raf/MEK, which is required for Raf/MEK activation. Recently, KSR2 has been shown to associate with AMPK and participate in its activation. It will be interesting to test if the KSR scaffolds, by tethering Raf/MEK and AMPK, play a role in determining cell growth or arrest; AMPK inhibits TORC1 by phosphorylating TSC2 and Raptor. This negative regulation is important, as TORC1 is a critical regulator for the growth of cancer cells bearing the loss-of-function mutations of PTEN or activating mutations of PI3K and Akt.
AMP-activated protein kinase has been shown to phosphorylate TSC2 at a different site to the Akt site, which primes phosphorylation at an adjacent site by GSK3, leading to activation of the TSC GAP activity [41,42]. In addition, AMPK has recently been shown to phosphorylate Raptor, a scaffold in the mTORC1 complex. The phosphorylation results in 14-3-3 binding and inactivation of mTORC1 [43]. Thus, AMPK exerts a dual control of mTORC1, so as to enforce a metabolic checkpoint in response to nutrient deprivation. The identification of mTORC1 as a downstream target of LKB1/AMPK is of great interest in cancer biology and therapy, inasmuch as the pathway is activated in a large number of tumors owing to oncogenic activation of receptor-tyrosine kinase, PI3K, and loss-of-function mutations of PTEN [44]. Indeed, deficiency of LKB1 in humans and mouse models of both Peutz–Jeghers syndrome and lung cancer is accompanied with deregulated mTORC1 activity and associated changes in gene expression, such as increased expression of SREBP1 and HIF-1α [45,46]. When tested, these tumors are sensitive to the inhibition of mTORC1 [35,46,47].
p53 & other tumor suppressors
Another important partner of AMPK is the tumor suppressor p53, with which AMPK is mutually regulated. On one hand, AMPK activation by AICAR or glucose deprivation leads to upregulation of p53 as well as its phosphorylation at Ser15 [48,49]. Mouse embryonic fibroblasts (MEFs) bearing wild-type p53 are arrested at G1/S phase by glucose deprivation, AICAR and expression of a constitutively activated mutant of AMPK, whereas the metabolic checkpoint is ineffective in the p53-deficient MEF cells [48]. We have shown that expression of a dominant negative mutant of the AMPK α1-subunit in prostate cancer cells accelerates their growth, concomitant with decreases in mRNA and protein levels of p53 [50]. On the other hand, AMPK is regulated by p53. The first example is the study showing that the β1-isoform of the regulatory subunit of AMPK is upregulated by p53 [51]. A second study has revealed that two downstream targets of p53, sestrin 1 and sestrin 2, are implicated in the activation of AMPK and concordant inhibition of mTOR [52]. These findings suggest that their mutual regulation enhances their tumor suppressive functions.
In addition, AMPK has been shown to phosphorylate and/or regulate several other molecules that regulate cell metabolism, growth, survival and autophagy, such as p300 histone acetyltransferase [53], FOXO3 [54] and the cell cycle inhibitor p27 [55,56]. It is not clear whether AMPK phosphorylation of p300 affects its histone acetyl transferase activity in addition to inhibition of p300 interaction with several nuclear receptors. AMPK phosphorylates FOXO3 and p27 at the same sites as Akt. However, phosphorylation by Atk elicits a negative effect on their function, in contrast to the positive net effect imposed by AMPK. Hence, it cannot be excluded that mechanisms other than direct phosphorylation account for AMPK’s effects on these molecules.
Enzymes for fatty acid & cholesterol synthesis
AMP-activated protein kinase was first identified as a kinase that phosphorylates and inhibits acetyl CoA carboxylase (ACC) and HMG-CoA reductase, rate-limiting enzymes for de novo synthesis of fatty acid and cholesterol, respectively. Both of these enzymes have important roles in tumorigenesis. In keeping with this, statins, HMG-CoA reductase inhibitors that lower cholesterol levels, have been suggested to prevent cancer in experimental models and reduce cancer risk in humans [57,58]. FASN, ACC and other enzymes required for de novo synthesis of free fatty acid and cholesterol are highly expressed in several types of cancers, including those arising from the breast, prostate, colon and ovary [59,60]. The increase of these enzymes is attributed to increased expression and maturation of SREBP-1 and SREBP-2, which are transcription factors. AMPK has been shown to inhibit SREBP1 [61]. However, the mechanism is not fully understood. One possible mechanism is through the inhibition of mTOR [45]. It has been reported that the inhibition of FASN activity by pharmacological agents or by siRNA attenuates proliferation of cancer cells and causes their apoptosis [62,63]. Thus, in light of these findings and the clinical observations that it is upregulated in cancer, FASN is regarded as a metabolic oncogene [60]. Interestingly, pharmacological inhibition of FASN in human ovarian cancer cells leads to a rapid activation of AMPK, followed by the induction of cytotoxicity [64]. The cytotoxic effect is suppressed by compound C, a chemical inhibitor of AMPK. Although further studies using molecular approaches are needed, these findings suggest that AMPK mediates the effect of pharmacological inhibition of FASN.
AMPK: journey from metabolic syndrome to cancer
Metabolic syndrome is a combination of metabolic disorders such as insulin resistance, hyperinsulinemia and proinflammatory and procoagulant changes that increase the risk of cardiovascular disease and diabetes. Studies from both human and rodents have shown that metabolic disorders, such as insulin resistance, obesity and Type 2 diabetes, are accompanied by decreases in AMPK activity, and that activation of AMPK by ACIAR, TZDs, metformin, polyphenols and exercise can correct or prevent these disorders [2,65–68]. Of note, AMPK activation stimulates fatty acid oxidation, which sounds paradoxical to its use in the treatment of diabetes and metabolic syndrome, as increased fatty acid oxidation may lead to ketosis. It should be pointed out that ketosis is not as severe for Type 2 diabetes as for Type 1 diabetes. In Type 1 diabetes, peripheral tissues burn more fat as an alternative fuel source to compensate for the shortage of glucose in the absence of insulin, which results in intolerable increases in ketone bodies. By contrast, in Type 2 diabetes and metabolic syndrome, the major problems are insulin resistance in liver, muscle and adipose tissue (e.g., increased hepatic glucose production and decreased glucose uptake in skeletal muscle), and increased proinflammatory response in the cardiovascular system [2]. AMPK activation has been shown to mitigate all of these abnormalities. In fact, no severe ketosis has been found as a side effect of metformin and TZDs in the treatment of Type 2 diabetes.
In recent years, metabolic syndrome has been found to be associated with cancer, and new evidence suggests that AMPK could play a bridging role, as illustrated in Figure 3.
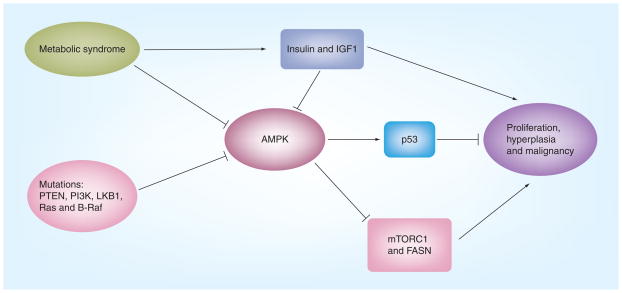
Figure 3. Suppression of AMPK results in loss of metabolic checkpoints.
AMPK could be suppressed in metabolic syndrome and by activated Akt owing to mutation of PTEN or PI3K, or activated ERK/RSK due to Ras or B-Raf, leading to a loss of metabolic checkpoints. Likewise, increased insulin and IGF1 associated with metabolic syndrome can inhibit AMPK and stimulate cell proliferation, hyperplasia and malignant growth of cancer cells.
AMPK: a key regulator to link metabolic syndrome & cancer
A large body of epidemiological studies has indicated that metabolic syndrome is a risk factor for many types of cancer and is associated with increased mortality and poor prognosis [69–72]. Likewise, the association between metabolic syndrome and tumorigenesis has also been demonstrated by experimental studies [73–75]. Using a tumor xenograft mouse model in which obesity and insulin resistance is induced with a high-fat diet, Yakar et al. have shown that tumor growth is significantly increased in obese mice [73]. A second line of evidence came from the study with a fatless mouse model, where the expression of the transgene A-ZIP/F-1, encoding a dominant negative protein that prevents the DNA-binding of B-ZIP transcription factors of both C/EBP and Jun families, is under the control of the adipose-specific aP2 enhancer/promoter [74]. The transgenic mice without white fat but with increased ectopic adiposity developed typical symptoms of metabolic syndrome and diabetes. The mice are susceptible to skin carcinogenesis induced by 7,12 dimethylbenz(α) anthracene, and the development of breast cancer is accelerated when they are crossed with transgenic mice expressing the SV40 large tumor antigen transforming sequences in their mammary glands. The increased carcinogenesis and tumorigenesis is probably attributed to the metabolic disorders engendered by hyperinsulemia. This possibility is corroborated by a recent elegant study by Fierz et al. [75]. In this study, a dominant negative mutant of the IGF1 receptor is specifically expressed in skeletal muscle of transgenic mice (MKR+/+), which results in insulin resistance and diabetes [76]. When the MKR+/+ mice are crossed with mice expressing the Polyoma Virus middle T oncongene in the mammary gland or are orthotopically inoculated with mouse tumor cells, tumor growth is markedly enhanced. Intriguingly, treatment of animals with CL-316243, a potent insulin sensitizing drug that specifically activates the β3-adrenergic receptor, significantly reduced the elevated insulin levels in MKR+/+ mice and concomitantly attenuated mammary tumor progression [75].
Thus far, both the animal and epidemiological studies support the contention that metabolic syndrome increases the risk of cancer and that correction of the metabolic disorders could be beneficial to cancer prevention and treatment. AMPK is a likely benevolent candidate to fulfill the latter mission. Indeed, this idea is supported by recent studies. First, retrospective investigations have reported that patients with Type 2 diabetes taking metformin display a reduced risk of cancer as compared with the normal population or with the patients who have never taken metformin [77,78], and a reduced trend of mortality compared with patients who take sulfonylureas or insulin [79]. In addition, reduced levels of adiponectin have been found in the plasma of patients with some cancers (e.g., breast and prostate cancers), and treatment of cancer cells with adiponectin attenuated their growth, an event that is blocked by the dominant negative mutant of AMPK [80–83]. Finally, a large number of studies have shown that maneuvers that activate AMPK, such as treatment with pharmacological agents (e.g., AICAR, metformin and TZDs), exercise and dietary restriction, can attenuate cancer cell growth in vitro and inhibit tumor development in vivo [34,36,84–87].
Dysregulation of AMPK in cancer
In addition to loss-of-function mutations of LKB1 in Peutz–Jeghers syndrome and cancers, AMPK could be suppressed by oncogenic mutations (Figure 2). Previous studies have shown that AMPK is phosphorylated and inhibited by insulin-activated Akt [88]. However, this has not been reported in cancer cells containing constitutively activated Akt. Additionally, the mRNA levels of AMPKα2 inversely correlate with clinical prognosis in breast and ovarian tumors, and are diminished in cancer cells by activated PI3K pathways [89]. Since Akt is activated in many cancer cells where PTEN is inactivated or PI3K is constitutively activated, it will be interesting to expand the study by examining more human tumor specimens. In fact, recent investigations, despite their limited number, shed light on this direction [90,91]. One report showed that AMPK activation is reduced in lung cancer specimens containing loss-of-function mutations of LKB1 [91]. A second study examined the activation status of AMPK in breast cancer specimens and showed that both phospho-AMPK and phospho-ACC signals were reduced, which inversely correlates with histological grade and axilliary node metastasis [90]. It has not been investigated if the reduction of AMPK activity correlates with metabolic status of the breast cancer patients.
Recently, two independent studies have indicated that LKB1 is inhibited by an active mutant of B-Raf, an event mediated by ERK1/2 [92,93]. Intriguingly, opposite changes between phosphorylation of ERK and AMPK were observed in melanoma specimens [92]. This finding may have an important clinical implication, inasmuch as activating mutation of B-Raf accounts for approximately 6% of human cancer, with the highest incidence in malignant melanoma (50–70%) [94]. Furthermore, ERK1/2 is constitutively activated in cancer cells containing Ras mutations, which is found in 20–30% of human cancers [95].
A more complex inter-relationship between the mitogenic and metabolic pathways is highlighted by a most recent discovery of the interaction between kinase suppressor of ras 2 (KSR2) and AMPK [96]. KSR1 and KSR2 are best known for their scaffold function for the Raf/MEK/ERK signaling cascade, facilitating the activation of Raf and MEK [97,98]. Costanzo-Garvey et al. have found that AMPK associates with KSR1 and KSR2 with more preference toward the latter [96]. Knockout of the mouse KSR2 gene causes obesity and insulin resistance. More interestingly, AMPK activation by AICAR is diminished in MEF cells isolated from the KSR2 knockout mouse. KSR2 binds to AMPK and Raf/MEK through different domains: while AMPK binds to the domain near or superimposed with the membrane targeting domain, the binding sites for Raf and MEK are located to a more carboxy terminal region of KSR2 [96,99]. Hence, it is tempting to speculate that KSR2 functions as a switching point of cell fate for mitogenesis and metabolic checkpoint if all these proteins coexist in the same complex. In proliferating cells, KSR2 brings an active MEK/ERK complex to AMPK, preventing its activation by LKB1 (as illustrated in Figure 2). Conversely, under metabolic stress, binding of AMPK to KSR2 prevents Raf/MEK to be targeted to the plasma membrane for their activation. Alternatively, KSR2 could form independent complexes with AMPK and Raf/MEK.
AMPK & glucose metabolism in cancer cells
One of the prominent traits of cancer cells is aerobic glycolysis, which was first described by Otta Warburg in the 1920s [100]. Regardless of adequate oxygen supply, cancer cells rely on the glycolysis that takes place in the cytosol over oxidative phosphorylation in the mitochondria, although the former is much less efficient at generating ATP. It is still not clear whether the switching of ATP-producing systems is causal or consequent to malignancy. Nonetheless, increased glycolysis offers many growth advantages to cancer cells in addition to adaptation of hypoxia tumor microenvironment [101]. For example, the high rate of glycolysis is not just required for the production of ATP to compensate for impaired oxidative phosphorylation in mitochondria, but it is also important to provide intermediates for anabolic processes, including biosynthesis of glycogen, amino acids, nucleic acids, and lipids [101,102]. Second, increased glycolysis in tumor cells is accompanied by increased stability of the mitochondrial membrane. This is partly ascribed to mitochondria membrane associated-hexokinase (HK) I or II, which is upregulated in tumors. As a result, leakage of cytochrome C into the cytosol and subsequent activation of caspases are prevented. As such, cancer cells are resistant to apoptosis invoked by the intrinsic pathway. Third, increased secretion of lactic acid creates an extracellular acidic milieu, which is advantageous for cancer cell invasion but toxic to normal cells.
Complex mechanisms are involved in the adaptation of cancer cells to glycolysis. Two important modulators relevant to AMPK are HIF-1 and p53 (Figure 4). HIF-1 is a transcription factor that is activated by hypoxic, oncogenic and other stresses [103]. HIF-1 is a heterodimer consisting of two subunits, α and β. The α-subunits are degraded under normoxic conditions owing to the sequential action of oxygen-dependent pro-lyl tryoxylases and the von Hippel-Lindau E3 ubiquitin ligase. Under hypoxic conditions, von Hippel-Lindau is inactivated, leading to stabilization of HIF-1α, which stimulates expression of modulators required for glycolysis, including glucose transporter 1, HK1 and HK2, lactate dehydrogenase and pyruvate dehydgrogenase kinase. The latter phosphorylates and inhibits pyruvate dehydrogenase, leading to a decrease in pyruvate entering mitochondria for oxidation. Therefore, the characteristic increases of glucose uptake and glycolysis in tumors distinguishes them from normal tissues, thereby offering a tracing advantage by 18F 2-fluoro-2-deoxy-D-glucose PET scanning, and these characteristic increases are also targeting points for cancer therapy [104].
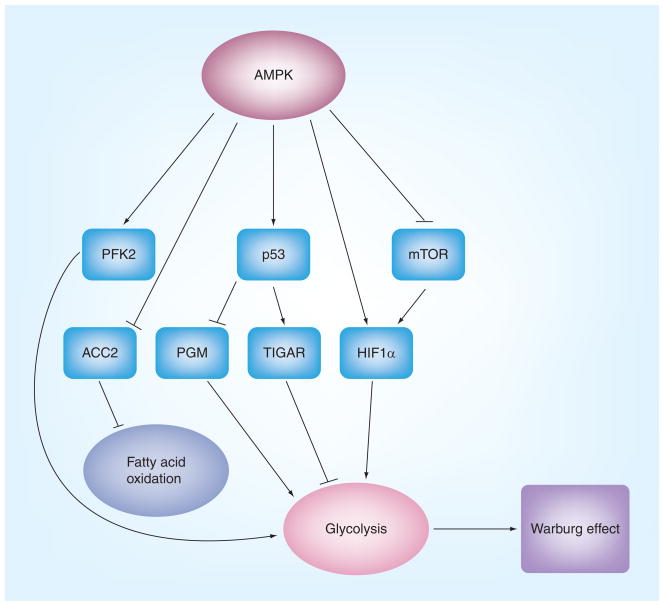
Figure 4. AMPK plays a role in reprogramming energy metabolism.
AMPK activation could have two effects on energy metabolisms: acute effect, AMPK that is activated under metabolic stress, such as hypoxia, ischemia and glucose deprivation, stimulates fatty acid oxidation and, in some circumstances, such as in the heart, enhances glycolysis to generate more ATP; chronic effect, AMPK may inhibit glycolysis via its action on mTOR and p53. Thus, suppression of AMPK in some cancer might result in increased glycolysis, which may contribute to the Warburg effect.
AMP-activated protein kinase has been implicated in the regulation of HIF1α. This may be mediated by its action on mTORC1 [105]. Thus, in LKB1- or AMPK-deficient fibroblasts, the levels of HIF1α and its downstream targets are elevated, which is diminished by rapamycin [46]. A similar increase of HIF1α is also found in the epithelia of gastrointestinal hamartomas from LKB1+/− mice [46]. These studies suggest that AMPK suppresses glycolysis in tumor cells by inhibiting mTOR. However, AMPK has also been shown to stimulate glycolysis in ischemic hearts by activating a cardiac specific enzyme, phosphofructo kinase 2 (PFK2) [106]. In addition, studies have asserted that AMPK activation upregulates HIF-1α and VEGF expression in hypoxia [107,108]. Thus, it needs to clarify whether these findings are reflective of acute stress response and examine whether the net effect of these opposite regulations by AMPK depends on cellular context and metabolic state of the cells.
Another paradigm of AMPK regulation of glycolysis is its possible action on p53. In addition to regulating cell growth and apoptosis, p53 has been recently implicated in the regulation of glycolysis and mitochondrial oxidative phosphorylation through at least three mechanisms [109]. First, p53 positively regulates the expression of the protein synthesis of cytochrome C oxidase 2 (SCO2), a key regulator of cytochrome C oxidase complex that is essential for mitochondrial respiration [110]. Second, p53 stimulates transcription of TP53-induced glycolysis and apoptosis regulator (TIGAR), a molecule that shares functional similarities with the bisphosphatase domain of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase [111]. Thus, the upregulation of TIGAR leads to a decrease in the levels of fructose-2,6-phosphates, thereby inhibiting glycolysis. Third, p53 downregulates the expression of phosphoglycerate mutase, another key enzyme in the glycolytic pathway [112]. Collectively, the net effect of p53 mutation is to enhance glycolysis. Therefore, AMPK, via regulating p53, could inhibit aerobic glycolysis and thus diminish the utilization of glycolytic intermediates for the biosynthesis of building blocks of cancer cells (e.g., protein, lipid and nucleic acids). However, data directly linking AMPK, p53 and glycolysis are currently still in scarcity.
Implication of AMPK in cancer prevention & treatment
Many studies have shown that exercise, AICAR and metformin, all of which cause AMPK activation, reduce insulin secretion and IGF1 production and alleviate hyperlipidemia and hyperglycemia [50,65,113–118]. We have provided direct evidence that AICAR suppresses the expression of IGF1 and its receptor in prostate cancer cells, while expression of the dominant negative mutant of AMPK causes their upregulation [50]. In addition, AMPK reduces reactive oxidation species, which can cause DNA damage and thus induce mutagenesis [3]. Hence, these studies suggest an important role of AMPK in tumor prevention. As noted earlier, this notion is supported by several recent epidemiological investigations in patients with Type 2 diabetes receiving metformin treatment and experimental studies using animal models.
AMP-activated protein kinase might also be a promising target for cancer therapy, as many recent studies have shown that pharmacological activators of AMPK, such as metformin, phenformin, AICAR and A769662, inhibit or delay the onset of tumors in animal models. Interestingly, one clinical study assessed the response of breast cancer patients with diabetes to nonadjuvant chemotherapy in combination with metformin or with nonmetformin antidiabetic drugs and demonstrated that the patients receiving metformin showed complete pathological responses [119]. This finding is intriguing and warrants further investigation. Indeed, an expanded use of metformin as an adjuvant in the treatment of breast cancer patients with or without diabetes is progressing at the Phase III clinical trial [120]. As an adjuvant of cancer therapy, several factors may determine the efficacy of pharmacological activators of AMPK and response to the treatment. For example, the presence of LKB1 is critical for the response of cancer cells to AMPK-targeted treatment. AMPK is unable to be activated by many pharmacological agents in the absence of LKB1 unless they directly bind to and activate AMPK. In some cancer cells, failure of AMPK activation may be beneficial to therapy, as it sensitizes the cells to apoptosis induced by chemotherapeutic agents [121]. Second, the cells containing hyperactive downstream targets of AMPK might be especially sensitive to AMPK activators when LKB1 is present. These include cancer cells containing hyperactivated mTORC1 and upregulated FASN [34,36,64]. In addition, the status of p53 might also be a determining factor. In keeping with this, a recent study has shown that metformin induces apoptosis of p53-null HCT116 colon cancer cells and selectively suppresses the growth of xenografts derived from these cells [87]. By contrast, metformin or AICAR fails to inhibit the tumor growth of HCT116 cells containing wild-type p53 but, instead, elicits autophagy [87]. However, another study has reported that p53 plays a critical role in mediating AMPK-induced apoptosis of thymocytes in response to glucose deprivation [122]. These results may reflect differences in cell types, but points to different treatment regimens needed to be considered when the genetic context varies.
Conclusion
In summation, AMPK appears to serve as a metabolic tumor suppressor to keep cell metabolism and growth at appropriate levels. Thus, in response to energy stress in the microenvironment of tumors, AMPK activation may lead to the reprogramming of cellular metabolism and elicits a metabolic checkpoint on the cell cycle through its action on mTORC1, p53 and other modulators for cell growth and survival. The checkpoint presumably allows a cell to fix the problems. Thus, when it is lost, the cell will undergo two directions of fate: apoptosis or unrestrained growth. In this regard, it is of no doubt that AMPK is a promising target for cancer prevention. Moreover, many lines of evidence suggest that AMPK could also serve as a target for cancer therapy, which will require more support from both preclinical and clinical investigations. The clinical study is just at its beginning, and hopefully, more exciting outcomes are expected to come along in the near future.
Future perspective
A great deal of experimental studies support the notion that AMPK is an important mediator of LKB1 in inhibiting cancer cell growth and tumorigenesis. It is noteworthy, however, that unlike LKB1, AMPK may not have emerged as a tumor suppressor by genetic studies, which is probably attributed to redundancy between the multiple isoforms [123,124]. Thus, to further delineate the tumor suppressive function of AMPK, it will be interesting to cross the AMPK α1 or α2 null mice or transgenic mice expressing the dominant negative mutant of AMPK α-subunit with p53 or PTEN-null mice and test whether ablation of AMPK accelerates tumor development or whether deletion of the AMPK α-genes increases the susceptibility to carcinogenesis. Another important direction will be to gain more evidence on AMPK dysregulaton in tumor microenvironment caused by metabolic or genetic changes. Inspired by the epidemiological studies showing the reduced risk of cancer and improved prognosis in breast cancer patients with diabetes who have taken metformin and in vitro studies using animal models with AMPK activators, extended studies of metformin use as an adjuvant to cancer therapy will hopefully be launched soon. An attractive reason to consider AMPK as a therapeutic target is the fact that two pharmacological activators, metformin and TZDs, have already been used in the clinical treatment of Type 2 diabetes, and tolerance of their side effects has been documented. Since metformin inhibits Complex I of the respiratory chain, one of its side effects is the production of lactic acidosis. Owing to the fatal acidosis side effect, phenformin, a potent analog of metformin, has been removed from the market in the USA. Although metformin also produces this side effect, it is to a much lower degree that is within the tolerable range. Furthermore, it is reasonable to expect more AMPK activators with higher specificity and potency to appear in the market in the near future. We should be aware that, as tumors have high degrees of heterogeneity, different genetic backgrounds may determine the sensitivity and efficacy, and the combination of AMPK activators with other treatment regimens will always be a better choice. Lastly and not surprisingly, as tremendous interest is invested, more AMPK targets and new functions will be identified.
Executive summary
AMPK serves as a critical downstream substrate of LKB1, a tumor suppressor whose mutations are found in Peutz–Jeghers syndrome and cancer.
AMPK has emerged as a therapeutic target for metabolic syndrome and Type 2 diabetes.
AMPK can be suppressed in metabolic syndrome, which is a risk factor for cancer, and by oncogenic activation of the Raf/ERK/RSK pathway.
Activation of AMPK by energy shortage (e.g., hypoxia and low levels of nutrients) reprograms cellular metabolism and enforces a metabolic checkpoint on the cell cycle. Loss of such a checkpoint could lead to unrestrained cell growth.
AMPK may function as a metabolic tumor suppressor regulating glucose, lipid and protein metabolism. It does so by acting on mTORC1, p53 and FASN.
AMPK is a promising target for cancer prevention and therapy.
Acknowledgments
The authors thank Ms Rong Tao and Miss Laura Stevens for checking typographical and grammatical errors.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work is supported by an NIH grant (R01CA118918 to Zhijun Luo). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Zhijun Luo, Email: zluo@bu.edu, Department of Biochemistry, Boston University School of Medicine, 715 Albany Street, Evans 645, Boston, MA 02118, USA, Tel.: +1 617 414 1033, Fax: +1 617 414 1646.
Mengwei Zang, Email: mwzang1@bu.edu, Department of Medicine, Boston University School of Medicine, 650 Albany Street, Boston, MA 02118, USA, Tel.: +1 617 638 2799, Fax: +1 617 638 7113.
Wen Guo, Email: wguo@bu.edu, Department of Medicine, Boston University School of Medicine, 670 Albany Street #211, Boston, MA 02118, USA, Tel.: +1 617 638 8279, Fax: +1 617 638 8217.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Steinberg GR, Kemp BE. AMPK in health and Disease. Physiol Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 2.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3(4):340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 3.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3(4):381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 5.Shackelford DB, Shaw RJ. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196(1):55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 7.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89(3):777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 9.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–S59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 10.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase Studies using bacterially expressed human protein phosphatase-2C α and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377(3):421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 11.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 13▪.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. One of the first two studies to independently show that LKB1 is an AMPK kinase. The study also shows that LKB1 requires accessory subunits STRAD and MO25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281(43):32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 17.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 18▪.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA. 2003;100(15):8839–8843. doi: 10.1073/pnas.1533136100. One of the first two studies to independently show that LKB1 is an AMPK kinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. One of the earliest studies to report that LKB1 is an AMPK kinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2(1):21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Xie M, Zhang D, Dyck JR, et al. A pivotal role for endogenous TGF-β-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA. 2006;103(46):17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281(35):25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Kusakai G, Kishimoto A, et al. IGF-1 phosphorylates AMPK-α subunit in ATM-dependent and LKB1-independent manner. Biochem Biophys Res Commun. 2004;324(3):986–992. doi: 10.1016/j.bbrc.2004.09.145. [DOI] [PubMed] [Google Scholar]
- 25.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28(6):677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62(13):3659–3662. [PubMed] [Google Scholar]
- 28.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 29.Wingo SN, Gallardo TD, Akbay EA, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4(4):E5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikediobi ON, Davies H, Bignell G, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5(11):2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 2008;68(1):55–63. doi: 10.1158/0008-5472.CAN-07-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contreras CM, Gurumurthy S, Haynie JM, et al. Loss of LKB1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 2008;68(3):759–766. doi: 10.1158/0008-5472.CAN-07-5014. [DOI] [PubMed] [Google Scholar]
- 33.Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR. LKB1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68(7):2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 34▪.Huang X, Wullschleger S, Shpiro N, et al. Important role of the LKB1–AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–221. doi: 10.1042/BJ20080557. Demonstrates that AMPK mediates LKB1 to inhibit tumorigenesis. [DOI] [PubMed] [Google Scholar]
- 35.Carretero J, Medina PP, Blanco R, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26(11):1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 36.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321(1):161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 37.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 38.Horman S, Browne G, Krause U, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12(16):1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Gomez MR. Phenotypes of the tuberous sclerosis complex with a revision of diagnostic criteria. Ann NY Acad Sci. 1991;615:1–7. doi: 10.1111/j.1749-6632.1991.tb37742.x. [DOI] [PubMed] [Google Scholar]
- 41.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 42▪.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. Shows cooperation between the Wnt signaling and AMPK sensing pathways in controlling mTOR. [DOI] [PubMed] [Google Scholar]
- 43▪.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. Describes that AMPK phosphorylates Raptor and inhibits mTOR, illustrating another mechanism by which AMPK dictates protein synthesis, metabolic checkpoint and cell growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 45.Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Shackelford DB, Vasquez DS, Corbeil J, et al. mTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz–Jeghers syndrome. Proc Natl Acad Sci USA. 2009;106(27):11137–11142. doi: 10.1073/pnas.0900465106. Shows that AMPK inhibits glycolysis via its action on mTOR, which may play a role in controlling the Warburg effect in tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 48▪.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–293. doi: 10.1016/j.molcel.2005.03.027. Characterizes in great detail the fact that activation of AMPK by energy deprivation activates p53, inducing the metabolic checkpoint. [DOI] [PubMed] [Google Scholar]
- 49▪.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287(2):562–567. doi: 10.1006/bbrc.2001.5627. First report to show that AMPK phosphorylates and activates p53 and induces cell cycle arrest. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Huang W, Tao R, et al. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009;28(18):1993–2002. doi: 10.1038/onc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Z, Hu W, de Stanchina E, et al. The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67(7):3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. p53 downstream targets also regulate AMPK, resulting in inhibition of mTOR. Thus, the study integrates the growth and metabolic controls by tethering p53–sestrin–AMPK–mTOR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276(42):38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 54.Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 55.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1–AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9(2):218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 56.Short JD, Houston KD, Dere R, et al. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. Cancer Res. 2008;68(16):6496–6506. doi: 10.1158/0008-5472.CAN-07-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz MS. Therapy insight: potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol. 2005;2(2):82–89. doi: 10.1038/ncponc0097. [DOI] [PubMed] [Google Scholar]
- 58.Farwell WR, Scranton RE, Lawler EV, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100(2):134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 59.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 60.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91(1):47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 61.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun. 2001;285(2):217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- 63.De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63(13):3799–3804. [PubMed] [Google Scholar]
- 64.Zhou W, Han WF, Landree LE, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67(7):2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 65.Zang M, Xu S, Maitland-Toolan KA, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55(8):2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 66.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and Type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55(8):2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 67.Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with Type 2 diabetes: a time-course and dose–response study. Diabetes. 2007;56(3):836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kraegen EW, Saha AK, Preston E, et al. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290(3):E471–E479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 69.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 70.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and Type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 71.Nawroth P. Diabetes, obesity, insulin resistance: different pathways to cancer? Exp Clin Endocrinol Diabetes. 2009;117(10):561–562. doi: 10.1055/s-0029-1241797. [DOI] [PubMed] [Google Scholar]
- 72.Percik R, Stumvoll M. Obesity and cancer. Exp Clin Endocrinol Diabetes. 2009;117(10):563–566. doi: 10.1055/s-0029-1241870. [DOI] [PubMed] [Google Scholar]
- 73▪▪.Yakar S, Nunez NP, Pennisi P, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147(12):5826–5834. doi: 10.1210/en.2006-0311. Demonstrates that high-fat-diet-induced obesity and insulin resistance are associated with increased susceptibility with carcinogenesis, suggesting a link between the metabolic syndrome and cancer. [DOI] [PubMed] [Google Scholar]
- 74▪▪.Nunez NP, Oh WJ, Rozenberg J, et al. Accelerated tumor formation in a fatless mouse with Type 2 diabetes and inflammation. Cancer Res. 2006;66(10):5469–5476. doi: 10.1158/0008-5472.CAN-05-4102. Also suggests a link between the metabolic syndrome and Type 2 diabetes with increased incidence of tumors using a fatless mouse model. [DOI] [PubMed] [Google Scholar]
- 75▪▪.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, Leroith D. Insulin sensitizing therapy attenuates Type 2 diabetes-mediated mammary tumor progression. Diabetes. 2009 doi: 10.2337/db09-1291. Epub ahead of print. Further demonstrates the link between Type 2 diabetes and tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim CH, Pennisi P, Zhao H, et al. MKR mice are resistant to the metabolic actions of both insulin and adiponectin: discordance between insulin resistance and adiponectin responsiveness. Am J Physiol Endocrinol Metab. 2006;291(2):E298–E305. doi: 10.1152/ajpendo.00319.2005. [DOI] [PubMed] [Google Scholar]
- 77▪▪.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. First report to indicate that patients using metformin have reduced risk of cancer, which supports in vitro studies and stimulates further clinical investigations using metfomin as an adjuvant for cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with Type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with Type 2 diabetes who use sulfonylureas or insulin: response to Farooki and Schneider. Diabetes Care. 2006;29(8):1990–1991. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 80.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86(3):S858–S866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 81.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98(2):370–379. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim KY, Baek A, Hwang JE, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69(9):4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 83.Sugiyama M, Takahashi H, Hosono K, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34(2):339–344. [PubMed] [Google Scholar]
- 84.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68(13):5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280(47):39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 86.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 87▪.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. Suggests that genetic context may determine the response of tumor to AMPK activators. [DOI] [PubMed] [Google Scholar]
- 88.Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281(9):5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 89.Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13(1):11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hadad SM, Baker L, Quinlan PR, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conde E, Suarez-Gauthier A, Garcia-Garcia E, et al. Specific pattern of LKB1 and phospho-acetyl-CoA carboxylase protein immunostaining in human normal tissues and lung carcinomas. Hum Pathol. 2007;38(9):1351–1360. doi: 10.1016/j.humpath.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 92▪.Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33(2):237–247. doi: 10.1016/j.molcel.2008.12.026. Shows that oncogenic activation of ERK/RSK can lead to the inhibition of LKB1. The study suggests that LKB1/AMPK might be inactivated by oncogenes in addition to loss-of-function mutations of LKB1, which warrants further investigation into whether LKB1/AMPK is suppressed in cancers containing oncogenic mutations of ras and BRAF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93▪.Esteve-Puig R, Canals F, Colome N, Merlino G, Recio JA. Uncoupling of the LKB1–AMPKα energy sensor pathway by growth factors and oncogenic BRAF. PLoS One. 2009;4(3):E4771. doi: 10.1371/journal.pone.0004771. Shows that oncogenic activation of ERK/RSK can lead to the inhibition of LKB1. The study suggests that LKB1/AMPK might be inactivated by oncogenes in addition to loss-of-function mutations of LKB1, which warrants further investigation into whether LKB1/AMPK is suppressed in cancers containing oncogenic mutations of ras and BRAF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 95.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 96▪.Costanzo-Garvey DL, Pfluger PT, Dougherty MK, et al. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10(5):366–378. doi: 10.1016/j.cmet.2009.09.010. Shows that KSR2, a well-known scaffold for the Raf/MEK pathway, associates with AMPK. Knocking out KSR2 induces obesity and insulin resistance and hampers AMPK activation. Thus, it suggests that KSR and AMPK might be control points for mitogenesis and energy homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kortum RL, Lewis RE. The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol Cell Biol. 2004;24(10):4407–4416. doi: 10.1128/MCB.24.10.4407-4416.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dougherty MK, Ritt DA, Zhou M, et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34(6):652–662. doi: 10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claperon A, Therrien M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26(22):3143–3158. doi: 10.1038/sj.onc.1210408. [DOI] [PubMed] [Google Scholar]
- 100.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 101.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18(4):165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 105.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18(6):598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277(34):30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 107.Hwang JT, Lee M, Jung SN, et al. AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1α expression in DU145 cells. Carcinogenesis. 2004;25(12):2497–2507. doi: 10.1093/carcin/bgh253. [DOI] [PubMed] [Google Scholar]
- 108.Lee M, Hwang JT, Yun H, et al. Critical roles of AMP-activated protein kinase in the carcinogenic metal-induced expression of VEGF and HIF-1 proteins in DU145 prostate carcinoma. Biochem Pharmacol. 2006;72(1):91–103. doi: 10.1016/j.bcp.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 109.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17(6):286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 111.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006;126(1):30–32. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 112.Kondoh H, Lleonart ME, Gil J, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65(1):177–185. [PubMed] [Google Scholar]
- 113.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lamontagne J, Pepin E, Peyot ML, et al. Pioglitazone acutely reduces insulin secretion and causes metabolic deceleration of the pancreatic β-cell at submaximal glucose concentrations. Endocrinology. 2009;150(8):3465–3474. doi: 10.1210/en.2008-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Leo V, La Marca A, Orvieto R, Morgante G. Effect of metformin on insulin-like growth factor (IGF) I and IGF-binding protein I in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85(4):1598–1600. doi: 10.1210/jcem.85.4.6560. [DOI] [PubMed] [Google Scholar]
- 116.Tsuboi T, da Silva Xavier G, Leclerc I, Rutter GA. 5′-AMP-activated protein kinase controls insulin-containing secretory vesicle dynamics. J Biol Chem. 2003;278(52):52042–52051. doi: 10.1074/jbc.M307800200. [DOI] [PubMed] [Google Scholar]
- 117.Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375(Pt 1):1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371(Pt 3):761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119▪.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. doi: 10.1200/JCO.2009.19.6410. First clinical study to assess the response of breast cancer to chemotherapy in diabetic patients receiving metformin, showing some positive results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27(20):3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 121.Kim HS, Hwang JT, Yun H, et al. Inhibition of AMP-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. J Biol Chem. 2008;283(7):3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 122.Okoshi R, Ozaki T, Yamamoto H, et al. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283(7):3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 123.Viollet B, Athea Y, Mounier R, et al. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki A, Lu J, Kusakai G, Kishimoto A, Ogura T, Esumi H. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol Cell Biol. 2004;24(8):3526–3535. doi: 10.1128/MCB.24.8.3526-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]