Abstract
Background
The optimal time for the initiation of antiretroviral therapy for asymptomatic patients with human immunodeficiency virus (HIV) infection is uncertain.
Methods
We conducted two parallel analyses involving a total of 17,517 asymptomatic patients with HIV infection in the United States and Canada who received medical care during the period from 1996 through 2005. None of the patients had undergone previous antiretroviral therapy. In each group, we stratified the patients according to the CD4+ count (351 to 500 cells per cubic millimeter or >500 cells per cubic millimeter) at the initiation of antiretroviral therapy. In each group, we compared the relative risk of death for patients who initiated therapy when the CD4+ count was above each of the two thresholds of interest (early-therapy group) with that of patients who deferred therapy until the CD4+ count fell below these thresholds (deferred-therapy group).
Results
In the first analysis, which involved 8362 patients, 2084 (25%) initiated therapy at a CD4+ count of 351 to 500 cells per cubic millimeter, and 6278 (75%) deferred therapy. After adjustment for calendar year, cohort of patients, and demographic and clinical characteristics, among patients in the deferred-therapy group there was an increase in the risk of death of 69%, as compared with that in the early-therapy group (relative risk in the deferred-therapy group, 1.69; 95% confidence interval [CI], 1.26 to 2.26; P<0.001). In the second analysis involving 9155 patients, 2220 (24%) initiated therapy at a CD4+ count of more than 500 cells per cubic millimeter and 6935 (76%) deferred therapy. Among patients in the deferred-therapy group, there was an increase in the risk of death of 94% (relative risk, 1.94; 95% CI, 1.37 to 2.79; P<0.001).
Conclusions
The early initiation of antiretroviral therapy before the CD4+ count fell below two prespecified thresholds significantly improved survival, as compared with deferred therapy.
The use of antiretroviral therapy has dramatically reduced disease progression and death among patients with human immunodeficiency virus (HIV) infection,1,2 but the optimal time to begin therapy is uncertain.3,4 Current guidelines recommend treatment for asymptomatic patients who have a CD4+ count of less than 350 cells per cubic millimeter on the basis of accumulating observational data.5,6 However, these guidelines note the lack of data from randomized clinical trials regarding the timing of the initiation of antiretroviral therapy.3,4 Data from randomized trials are limited to an analysis of a subgroup of 477 patients7 from the Strategies for Management of Antiretroviral Therapy (SMART) trial (ClinicalTrials.gov number, NCT00027352),8 which suggested that deferring antiretroviral therapy until the CD4+ count fell below 250 cells per cubic millimeter increased the risk of progression to the acquired immunodeficiency syndrome (AIDS) or death, as compared with initiation of therapy at a CD4+ count of more than 350 cells per cubic millimeter.7,9
Several observational studies have examined the prognosis for patients who begin antiretroviral therapy at different CD4+ counts.5,6,10-16 However, these studies do not address the question of when to start antiretroviral therapy, since they do not have a comparison group of patients who deferred therapy.17,18 A few studies have compared patients with similar CD4+ counts who either initiated or deferred antiretroviral therapy,19-24 but these studies did not have the statistical power and methods18,25,26 to examine differences in outcomes, particularly among patients with higher CD4+ counts.
Since previous studies have enrolled small numbers of patients and had a relatively short follow-up,19-24,27 they have required the use of a combined end point of progression to an AIDS-defining illness or death. However, serious conditions that are not traditionally considered to be associated with AIDS have resulted in death and complications in HIV-infected patients,8,28 and the risk of these conditions is greater than the risk of AIDS among patients with a CD4+ count of more than 200 cells per cubic millimeter.29-33
Emerging data about the benefits of early antiretroviral treatment, including a better response to therapy and a preservation of immune function,34-39 suggest that the initiation of antiretroviral therapy earlier in the course of HIV infection may improve long-term outcomes. In a recently established collaboration of research groups in the United States and Canada, we examined all HIV-infected patients with a CD4+ count ranging from 351 to 500 cells per cubic millimeter and all patients with a CD4+ count of more than 500 cells per cubic millimeter who had received no previous antiretroviral therapy and had no history of an AIDS-defining illness to determine whether the initiation of antiretroviral therapy at early stages of HIV infection would be associated with better survival than deferred therapy.
Methods
Study Patients
The data for this study were collected as part of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiological Databases to Evaluate AIDS project. Details on the collaboration and sites have been published previously.40 Briefly, the NA-ACCORD consists of 22 research groups, representing more than 60 cites. The study was approved by local institutional review boards and used standardized methods of data collection, including routine surveillance of U.S. national and Canadian provincial death indexes. Each group of investigators submitted data regarding demographic characteristics, treatment, and clinical, laboratory, and vital status on enrolled patients.
We conducted parallel analyses on two distinct study groups of patients who received medical care between January 1996 and December 2005, had not had a previous AIDS-defining illness (according to 1993 criteria of the Centers for Disease Control and Prevention41), and had not undergone previous antiretroviral therapy. The patients were identified from a total of 67,527 patients who underwent screening. For the first analysis, we identified all patients who had a CD4+ count of 351 to 500 cells per cubic millimeter. For the second analysis, we identified patients who had a CD4+ count of more than 500 cells per cubic millimeter.
Study Definitions
Antiretroviral therapy was defined as a regimen containing at least three antiretroviral drugs, including a protease inhibitor, a nonnucleoside reverse-transcriptase inhibitor, or three nucleoside reverse-transcriptase inhibitors, including abacavir or tenofovir.
In a study design similar to that of a randomized clinical trial, we compared the rates of death from any cause in the group that initiated antiretroviral therapy when the CD4+ count was within one of the two thresholds of interest (early-therapy group) with the rate in the group that did not initiate antiretroviral therapy until the CD4+ count fell below these thresholds (deferred-therapy group). Follow-up ended at the month of death, at 1 year after the date of the last measurement of the CD4+ count, or on December 31, 2005, whichever occurred first.
Statistical Analysis
The baseline characteristics of patients who initiated antiretroviral therapy early were compared with those who deferred therapy with the use of Wilcoxon and chi-square tests for association. Among the patients in the deferred-therapy group who transitioned to a CD4+ count of either 350 cells or less or 500 cells or less per cubic millimeter, we identified and characterized the subgroup that initiated antiretroviral therapy.
We used multivariate Cox proportional-hazards models with strata for each cohort of patients and each baseline calendar year. Baseline was defined as the date of the first measurement of a CD4+ count either in the range of 351 to 500 or of more than 500 cells per cubic millimeter. To avoid immortal time bias,42 patients in the early-therapy group contributed person-time to the deferred-therapy group between baseline and the date of initiation of antiretroviral therapy. After the initiation of antiretroviral therapy, these patients contributed all person-time to the early-therapy group in an intention-to-treat approach that ignored subsequent changes in therapy. In addition to the primary analysis between the early-therapy group and the deferred-therapy group, adjustment was made for sex, age, and the CD4+ count at baseline. Data regarding other prognostic factors in patients with HIV infection (including the HIV RNA level,43 a history injection-drug use,35 the presence or absence of hepatitis C virus [HCV] infection,44,45 and race) were available for most of the patients. Thus, we separately fit models that adjusted for these covariates in prespecified subgroup analyses, treating patients with unknown status as a separate covariate group. Proportional-hazards assumptions were assessed graphically with the use of the methods of Grambsch and Therneau.46 A two-sided P value of 0.05 or less was considered to indicate statistical significance.
Since patients did not undergo randomization and data censoring was not performed in a random fashion, we used inverse-probability weighting methods.26,47 To investigate the sensitivity of our results to potential unmeasured confounding factors associated with the time to death and deferral of therapy,48 we conducted a set of Monte Carlo simulations49 (for details on both methods, see the Supplementary Appendix, available with the full text of this article at NEJM.org).
Results
Patients
In the first analysis, 8362 patients (who contributed 23,977 person-years of follow-up) met the inclusion criteria with a CD4+ count of 351 to 500 cells per cubic millimeter (351-to-500 CD4+ count). Of these patients, 2084 (25%) initiated antiretroviral therapy within 6 months after the first CD4+ count was within the range of interest, as compared with the remaining 6278 patients (75%), who deferred therapy until the CD4+ cell count fell below the range (Table 1). Of the patients in the deferred-therapy group, 2829 (45%) were not observed with a CD4+ count of 350 cells or less per millimeter; data from 22% of the patients were censored because the patients initiated antiretroviral therapy beyond the 6-month target window after the first CD4+ count ranged from 351 to 500 cells per millimeter. The other 3449 patients (55%) transitioned to a CD4+ count of 350 cells or less per cubic millimeter; of these patients, 803 initiated antiretroviral therapy within 6 months after the first CD4+ count of 350 cells or less per cubic millimeter.
Table 1.
Stratification of Patients According to CD4+ Count at Baseline.*
| Variable | 351-to-500 CD4+ Count | More-Than-500 CD4+ Count |
|---|---|---|
| Patients with a CD4+ count within prespecified range and no history of previous antiretroviral therapy or AIDS-defining disease — no. | 8362 | 9155 |
| Patients who initiated antiretroviral therapy within 6 mo — no. | 2084 | 2220 |
| Patients who deferred antiretroviral therapy — no. | 6278 | 6935 |
| Patients who deferred therapy and who either transitioned to a lower CD4+ count stratum or not | ||
| Transition — no. (no. of cells) | 3449 (≤350 cells/mm3) | 3881 (≤500 cells/mm3) |
| No transition — no. | 2829 | 3054 |
| Timing of initiation of therapy among patients who deferred therapy and who transitioned to a lower CD4+ count | ||
| Within 6 mo — no. | 803 | 539 |
| Not within 6 mo — no. | 2646 | 3342 |
The CD4+ count was measured in cells per cubic millimeter.
In the second analysis, 9155 patients (who contributed 26,439 person-years of follow-up) met the inclusion criteria with a CD4+ count of more than 500 cells per cubic millimeter (more-than-500 CD4+ count). Of these patients, 2220 (24%) initiated antiretroviral therapy within 6 months after the first CD4+ count was within the range of interest, as compared with the remaining 6935 patients (76%) who deferred therapy. Of the patients in the deferred-therapy group, 3054 (44%) were not observed with a CD4+ count of 500 cells or less per millimeter. The other 3881 patients transitioned to a CD4+ count below the threshold of interest. Of these patients, 539 (14%) initiated antiretroviral therapy within 6 months after the first CD4+ count of 500 cells or less per cubic millimeter. The proportion of patients who initiated antiretroviral therapy at a CD4+ count of more than 500 cells per cubic millimeter peaked at 15% in 1999 and had dropped to less than 10% by 2003.
Demographic and clinical characteristics of the patients in the two groups are shown in Table 2. In each of the groups with the two thresholds of CD4+ counts, patients in the early-therapy group were slightly older and were more likely to be white men. At study entry, the CD4+ count within each of the two ranges of interest was similar in the early-therapy group and the deferred-therapy group. Among patients with a 351-to-500 CD4+ count, the median count per cubic millimeter was 422 cells in the early-therapy group and 431 cells in the deferred-therapy group; among patients with a more-than-500 CD4+ count, the median count per cubic millimeter was 679 cells in the early-therapy group and 664 cells in the deferred-therapy group.
Table 2.
Characteristics of the Patients, According to the Timing of Initiation of Antiretroviral Therapy and CD4+ Count at Baseline.*
| Variable | 351-to-500 CD4+ Count | More-Than-500 CD4+ Count | ||
|---|---|---|---|---|
| Early-Therapy Group | Deferred-Therapy Group | Early-Therapy Group | Deferred-Therapy Group | |
| No. of patients | 2084 | 6,278 | 2220 | 6,935 |
| Follow-up person-years (no.) | 8353 | 15,624 | 8663 | 17,776 |
| Deaths within 1 yr after last CD4+ count (no.) | 137 | 238 | 113 | 198 |
| Age (yr) | ||||
| Median | 40 | 38 | 40 | 38 |
| Interquartile range | 34–48 | 32–45 | 33–48 | 32–45 |
| Male sex (%) | 84 | 75 | 82 | 73 |
| Race (%)† | ||||
| White | 45 | 40 | 49 | 42 |
| Black | 38 | 44 | 36 | 44 |
| Other | 17 | 16 | 15 | 14 |
| CD4+ count at baseline (cells/mm3) | ||||
| Median | 422 | 431 | 679 | 664 |
| Interquartile range | 387–460 | 391–468 | 580–840 | 573–811 |
| HIV RNA at baseline (log10 copies/ml)‡ | ||||
| Median | 4.2 | 4.1 | 3.5 | 3.7 |
| Interquartile range | 2.9–4.9 | 3.4–4.6 | 2.6–4.6 | 2.7–4.4 |
| Hepatitis C virus infection (%)§ | 27 | 33 | 25 | 35 |
| History of injection-drug use (%)¶ | 15 | 20 | 14 | 21 |
| No. of patients who initiated antiretroviral therapy | 2084 | 803 | 2220 | 539 |
| Year of initiation of antiretroviral therapy | ||||
| Median | 2000 | 2001 | 2000 | 2000 |
| Interquartile range | 1998–2001 | 1998–2003 | 1998–2001 | 1998–2003 |
| Interval from first CD4+ count to initiation of antiretroviral therapy (mo) | ||||
| Median | 3 | 3 | 2 | 3 |
| Interquartile range | 2–3 | 2–4 | 2–3 | 2–4 |
| CD4+ count at initiation of antiretroviral therapy (cells/mm3) | ||||
| Median | 422 | 286 | 679 | 410 |
| Interquartile range | 387–460 | 228–320 | 580–840 | 332–464 |
| Type of first antiretroviral therapy (%) | ||||
| Protease inhibitor–based | ||||
| Without boost | 48 | 40 | 50 | 43 |
| With boost | 9 | 11 | 7 | 9 |
| Nonnucleoside reverse-transcriptase inhibitor–based | 33 | 38 | 32 | 38 |
| Protease inhibitor and nonnucleoside reverse-transcriptase inhibitor–based | 4 | 2 | 4 | 3 |
| ≥3 Nucleoside reverse-transcriptase inhibitors | 6 | 9 | 6 | 8 |
| HIV RNA <500 copies/ml within 12 mo after initiation of antiretroviral therapy (%) | 75 | 72 | 81 | 71 |
The CD4+ count was measured in cells per cubic millimeter. P<0.05 for all comparisons between the early-therapy group and the deferred-therapy group except for the response to the initiation of antiretroviral therapy in the number of HIV RNA copies in patients with a 351-to-500 CD4+ count (P = 0.16) and the median year of initiation of antiretroviral therapy in patients with a more-than-500 CD4+ count (P = 0.72). HIV denotes human immunodeficiency virus.
Race was self-reported. Race was evaluated in 7657 patients with a CD4+ count of 351 to 500 cells per cubic millimeter and in 8462 patients with a CD4+ count of more than 500 cells per cubic millimeter.
Baseline HIV RNA levels were evaluated in 6425 patients with a CD4+ count of 351 to 500 cells per cubic millimeter and in 6566 patients with a CD4+ count of more than 500 cells per cubic millimeter.
The status of hepatitis C virus infection was evaluated in 4404 patients with a CD4+ count of 351 to 500 cells per cubic millimeter and in 5147 patients with a CD4+ count of more than 500 cells per cubic millimeter. Data on hepatitis C virus infection were not obtained for two cohorts of patients.
The status of injection-drug use was evaluated in 5744 patients with a CD4+ count of 351 to 500 cells per cubic millimeter and in 6087 patients with a CD4+ count of more than 500 cells per cubic millimeter. The use of injection drugs was not evaluated for one cohort.
In the subgroup of patients for whom data were available, fewer patients with a history of injection-drug use and HCV infection were in the early-therapy group. Patients in the early-therapy group were more likely to initiate a protease inhibitor–based regimen that did not include a ritonavir boost; in the early-therapy group and the deferred-therapy group, the proportions of patients who initiated a protease inhibitor–based regimen with a ritonavir boost were low. The proportions of patients who had an HIV RNA level of less than 500 copies per milliliter within 12 months after the initiation of antiretroviral therapy were clinically similar in the two groups, although among patients with a more-than-500 CD4+ count, there was a statistically significant difference.
Overall Risk of Death
Among patients with a 351-to-500 CD4+ count, there were 137 deaths in the early-therapy group and 238 deaths in the deferred-therapy group. The crude rate of death for patients in the early-therapy group was 1.6 deaths per 100 person-years. Among patients with a more-than-500 CD4+ count, there were 113 deaths in the early-therapy group and 198 deaths in the deferred-therapy group. The crude rate of death for patients in the early-therapy group was 1.3 deaths per 100 person-years. A crude rate of death could not be calculated for the deferred-therapy group because of data censoring to address “outside of protocol” violations.
The median time until data censoring was 3.7 years (interquartile range, 1.1 to 5.3) in the early-therapy group and 1.3 years (interquartile range, 0.7 to 2.9) in the deferred-therapy group among patients with a 351-to-500 CD4+ count and 3.7 years (interquartile range, 1.9 to 5.8) in the early-therapy group and 1.7 years (interquartile range, 0.9 to 3.5) in the deferred-therapy group among patients with a more-than-500 CD4+ count. At the end of the study, data on the cause of death had been obtained for 16% of the patients who had died. The majority of deaths in the early-therapy group and the deferred-therapy group were from non–AIDS-defining conditions, including hepatic, renal, and cardiovascular diseases and non–AIDS-defining cancers in both analyses.
351-to-500 CD4+ Count
Among patients with a baseline CD4+ count of 351 to 500 cells per cubic millimeter, the relative risk of death for deferred therapy, as compared with early therapy, was 1.69 (95% confidence interval [CI], 1.26 to 2.26; P<0.001), as calculated with weighted Cox regression analysis stratified according to cohort of patients and the year of the first CD4+ count of 351 to 500 cells per cubic millimeter (Table 3). An increased risk of death was associated with an older age at baseline (relative risk, 1.68 for each 10-year increment; 95% CI, 1.48 to 1.91; P<0.001) but not with the patient's sex or the baseline CD4+ count (Table 3). These results remained significant and of similar magnitude when data from each of the cohorts were systematically excluded. Graphical and statistical tests did not suggest any deviation from the proportionality assumption of any variable except for age. The results in the deferred-therapy group remained similar after adjustment for age with the use of a cubic regression spline. There were no significant interactions between the effect of deferred therapy and age, sex, baseline HIV RNA level, a history of injection-drug use, HCV infection, cohort, or calendar year; the latter was examined as a continuous or categorical variable.
Table 3.
Risk of Death Associated with Deferral of Antiretroviral Therapy, According to CD4+ Count at Baseline, with Adjustment for HIV RNA Level, Age, and Sex.*
| Variable | 351-to-500 CD4+ Count | More-Than-500 CD4+ Count | ||
|---|---|---|---|---|
| Relative Risk (95% CI) |
P Value | Relative Risk (95% CI) |
P Value | |
| Without inclusion of HIV RNA data | ||||
| Deferral of antiretroviral therapy | 1.69 (1.26–2.26) | <0.001 | 1.94 (1.37–2.79) | <0.001 |
| Female sex | 1.21 (0.89–1.64) | 0.24 | 1.85 (1.33–2.59) | <0.001 |
| Older age (per 10-yr increment) | 1.68 (1.48–1.91) | <0.001 | 1.83 (1.62–2.06) | <0.001 |
| Baseline CD4+ count (per 100 cells/mm3) | 1.13 (0.72–1.78) | 0.59 | 0.93 (0.87–0.99) | 0.03 |
| With inclusion of HIV RNA data | ||||
| Deferral of antiretroviral therapy | 1.63 (1.21–2.19) | 0.002 | 1.85 (1.20–2.86) | 0.006 |
| Female sex | 1.47 (1.02–2.12) | 0.04 | 1.35 (0.85–2.15) | 0.20 |
| Older age (per 10-year increment) | 1.89 (1.69–2.11) | <0.001 | 1.81 (1.58–2.07) | <0.001 |
| Baseline CD4+ count (per 100 cells/mm3) | 0.74 (0.55–1.00) | 0.06 | 0.97 (0.89–1.05) | 0.45 |
| Baseline HIV RNA level (per log10 copies/ml) | 1.11 (0.96–1.28) | 0.15 | 1.13 (0.96–1.33) | 0.14 |
The CD4+ count was measured in cells per cubic millimeter. Results were calculated with the use of Cox regression analyses with inverse probability-of-censoring weights. HIV denotes human immunodeficiency virus.
Some patients had a first CD4+ count of 351 to 500 cells per cubic millimeter in years before the routine availability of HIV RNA testing. When the analysis was restricted to patients for whom data were available regarding the baseline HIV RNA level, the relative risk of death for deferred therapy, as compared with early therapy, was similar to that in the primary analysis (relative risk, 1.63; 95% CI, 1.21 to 2.19; P = 0.002). However, the HIV RNA level at baseline was not an independent risk factor for death.
After the exclusion of one group of investigators that did not collect data regarding a history of injection-drug use and the exclusion of patients with a history of injection-drug use, the risk of deferred therapy, as compared with early therapy, remained significantly elevated (relative risk, 1.66; 95% CI, 1.02 to 2.70; P = 0.04). After adjustment for a history of injection-drug use, the risk of death in the deferred-therapy group was attenuated (relative risk, 1.28; 95% CI, 0.85 to 1.93; P = 0.23). Patients who had a history of injection-drug use had a higher risk of death than those with no such history (relative risk, 1.64; 95% CI, 1.10 to 2.44; P = 0.01).
After the exclusion of two groups of investigators that did not collect data regarding HCV infection, the risk of death in the deferred-therapy group was also similar to that in the primary analysis (relative risk, 1.71; 95% CI, 1.20 to 2.45; P = 0.003). The presence of HCV infection was associated with an increased risk of death (relative risk, 1.85; 95% CI, 1.07 to 3.23; P = 0.03). After the exclusion of patients with HCV infection, the risk of death for deferred therapy remained significantly elevated (relative risk, 1.52; 95% CI, 1.01 to 2.28; P = 0.04).
Female sex was associated with an increased risk of death in the analysis that included the HIV RNA level, but the risk was not significant after adjustment for either a history of injection-drug use or the presence of HCV infection. Race (white vs. all others) was not significantly associated with an increased risk of death, and the inclusion of race as a variable in the model did not have an effect on the results. A small proportion of patients in the deferred-therapy group transitioned to a CD4+ count of less than 200 cells per cubic millimeter, but the exclusion of these patients from the analysis did not change the results.
More-than-500 Cd4+ Count
Similarly, among patients who had a CD4+ count of more than 500 cells per millimeter, the risk of death among patients who deferred therapy, as compared with those with early therapy, increased by 94% (relative risk, 1.94; 95% CI, 1.37 to 2.79; P<0.001). After restriction of the analysis to patients for whom data on the baseline HIV RNA level were available, the relative risk for the deferred-therapy group was similar to that in the primary analysis (relative risk, 1.85; 95% CI, 1.20 to 2.86; P = 0.006).
After the exclusion of one group of investigators that did not collect data regarding a history of injection-drug use, the risk of death for deferred therapy remained significantly elevated (relative risk, 1.73; 95% CI, 1.08 to 2.78; P = 0.02). After the exclusion of patients who had a history of injection-drug use, the risk of death for deferred therapy was of similar magnitude (relative risk, 2.00; 95% CI, 1.15 to 3.46; P = 0.01). After the exclusion of two groups of investigators that did not collect data regarding the status of HCV infection, the risk of death for deferred therapy was also similar to that in the primary analysis (relative risk, 2.03; 95% CI, 1.37 to 3.01; P<0.001). After the exclusion of patients with HCV infection, the risk of death for deferred therapy remained significantly elevated (relative risk, 1.90; 95% CI, 1.14 to 3.18; P = 0.01).
Again, the risk of death was not significantly associated with race or the baseline HIV RNA level. Female sex was associated with an increased risk of death, but the risk was not significant after adjustment for the baseline HIV RNA level, a history of injection-drug use, or the presence of HCV infection. Very few patients in the deferred-therapy group transitioned to a CD4+ count of less than 350 cells per cubic millimeter. When data from these patients were censored at the first CD4+ count of less than 350 cells per cubic millimeter, the relative risk of death among patients in the deferred-therapy group with a 351-to-500 CD4+ count, as compared with patients in the early-therapy group with a more-than-500 CD4+ count, was 1.80 (95% CI, 1.24 to 2.62; P = 0.002).
Confounding by Unmeasured Covariates
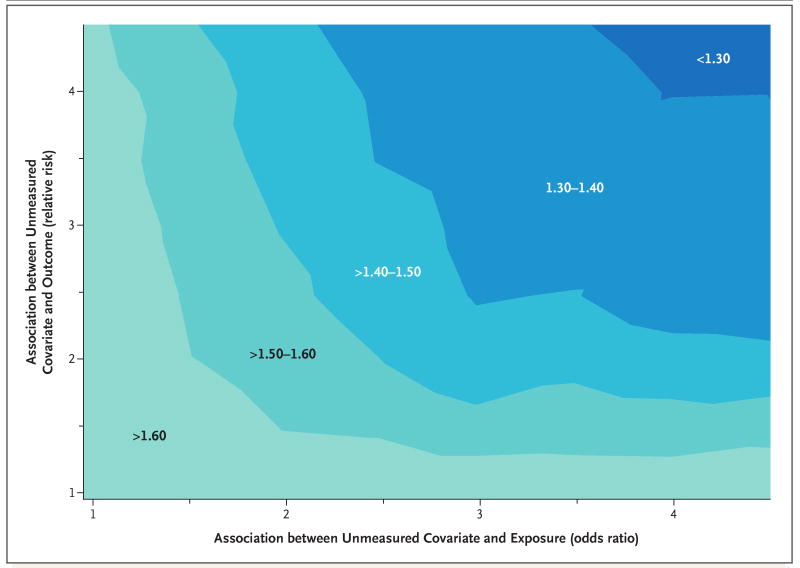
Finally, we evaluated the extent to which an unmeasured covariate might reduce the relative risk of death. Figure 1 shows the results of the analysis of patients with a 351-to-500 CD4+ count; similar results were seen for the analysis of patients with a more-than-500 CD4+ count. The plot shows that a confounding factor with a relative risk of death of 4.0 and an odds ratio for the deferral of therapy of 4.0 after adjustment for all included variables would reduce the estimated relative risk for deferred therapy to approximately 1.30.
Figure 1. Results of Sensitivity Analyses Examining the Effect of Possible Unmeasured Confounding Factors on the Association between Deferral of Antiretroviral Therapy and Death.
The contour plot shows that a confounding factor with a relative risk for death of 4.0 and an odds ratio for deferral of therapy of 4.0 after adjustment for all included variables would reduce the estimated relative risk for deferred therapy to approximately 1.30.
Discussion
The results of this study suggest that among patients with a 351-to-500 CD4+ count, the deferral of antiretroviral therapy was associated with an increase in the risk of death of 69%, as compared with the early initiation of therapy. Among patients with a more-than-500 CD4+ count, deferred therapy was associated with an increase in the risk of death of 94%. As has been shown in previous studies, an older age was an independent risk factor for death,5,50 as were a history of injection-drug use35 and the presence of HCV infection.45
Results regarding the risk of deferred therapy on mortality were robust after adjustment for these factors and after the exclusion of patients with a history of injection-drug use, who may have had a higher likelihood of deferring treatment, a poorer level of adherence to therapy, and a higher risk of death. The type of the initial antiretroviral regimen and the proportion of patients who achieved viral suppression as a measure of adherence were clinically similar in the early-therapy group and the deferred-therapy group. The increased risk of death for patients who deferred treatment was similar throughout the 10-year study period. The improved rate of survival among patients who initiate antiretroviral therapy at higher CD4+ counts probably can be attributed to multiple factors, including earlier control of viral replication and viral diversity and a greater immunologic benefit.34-37 Incomplete restoration of the CD4+ count is common among patients initiating antiretroviral therapy at lower CD4+ counts, despite viral suppression for up to 10 years while receiving therapy, and there may be persistent deficits in immunologic function even with substantial restoration of the CD4+ count.38,39
Studies in which patients were observed only after they began antiretroviral therapy could not provide data regarding AIDS events and deaths that occurred during deferral of therapy, a factor that introduced a lead-time bias.18 Some investigators have applied methods18 to impute these missing events,21,22,27 using data from patients with HIV infection during the era before the advent of potent antiretroviral therapy, but such methods may not fully address the lead-time bias. In contrast, our study was not subject to such lead-time bias. An additional strength of our study was the comprehensive ascertainment of mortality through linkage with national death registries in both the United States and Canada.
The benefits of initiating antiretroviral therapy earlier after HIV infection must be weighed against potential adverse effects of treatment. Newer antiretroviral therapies that are more potent, have fewer side effects, and have to be taken less frequently improve adherence and maintain viral suppression at lower levels of adherence51 than did previous regimens,52 which decreases the risk of drug resistance.53 Furthermore, starting therapy at progressively higher CD4+ counts has been shown to lower the risk of some toxic effects associated with antiretroviral therapy, including peripheral neuropathy, anemia, and renal insufficiency.54 However, all the potential side effects of long-term antiretroviral therapy are unknown.
The size and duration of our study enabled us to use death as the end point. This was an advantage, since death is a more definitive and all-inclusive outcome measure than progression to an AIDS-defining event, an end point that has been used in previous studies. Non–AIDS-defining conditions may occur at different rates among patients who defer therapy, as compared with those who initiate therapy early. HIV infection and decreasing CD4+ counts are associated with a higher risk of cardiovascular, liver, and renal diseases and non–AIDS-defining cancers, and treatment with antiretroviral therapy appears to reduce the risk of these conditions.8,29-33,55,56 Among patients for whom cause-of-death data were available, the majority of deaths were from causes that were non–AIDS-defining conditions.
There remains uncertainty about when to start antiretroviral therapy for asymptomatic patients with HIV infection.3,4 Ideally, such data would come from randomized trials.25 Since patients in our study did not undergo randomization, the decision to initiate or defer antiretroviral therapy could have been influenced by multiple factors. To address potential confounding, we adjusted the analyses for established risk factors for death in HIV disease (including age, the CD4+ count, the HIV RNA level, a history of injection-drug use, and the presence or absence of HCV infection), factors that may also affect the decision to defer treatment. Patients in the early-therapy group and the deferred-therapy group received similar initial antiretroviral therapy regimens. In addition, our analyses were adjusted for potential cohort effects and improvement in survival because of advances in therapies over time.
Our approach had several limitations. As with any observational study, even after adjustment for known prognostic factors, residual confounding may occur because of unmeasured socioeconomic and other factors that may be associated with both deferral of therapy and death. Whereas only a large, randomized trial can balance such unmeasured factors, our sensitivity analyses suggest that the effect size of unmeasured confounding would have to be uncommonly large to mitigate our results. In addition, we made assumptions in our analyses regarding the target and follow-up windows on the basis of clinical practice. However, different assumptions might have had an effect on the magnitude of the relative risk estimates, and such assumptions warrant future study. More careful assessment of the misclassification inherent in measures of injection-drug use and the presence of HCV infection will provide a better understanding of how such factors are associated with a risk of death.
Recommendations for the initiation of therapy for asymptomatic HIV-infected patients have undergone major shifts since the introduction of potent antiretroviral therapy more than a decade ago. In 1996, antiretroviral therapy was recommended for all HIV-infected patients with a CD4+ count of less than 500 cells per cubic millimeter,57 but concern about resistance,58 inadequate adherence,52 and toxic effects59 led to a shift to delay initiation of treatment until later stages of HIV disease. Significant advances in our understanding of the role of HIV infection in inflammation and immune activation resulting in potentially irreversible immune-system and end-organ damage have renewed the impetus for earlier treatment of HIV.60,61
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health and from the Agency for Healthcare Research and Quality.
We thank all the patients, physicians, investigators, and staff who were involved in this study.
APPENDIX
The investigators in the study were affiliated with the following research groups: G. Kirk (AIDS Link to the IntraVenous Experience); C. Benson, R. Bosch, A. Collier (Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials); R. Hogg, R. Harrigan, J. Montaner (HAART Observational Medical Evaluation and Research); J. Brooks (HIV Outpatient Study); K. Gebo (HIV Research Network); R. Moore (Johns Hopkins HIV Clinical Cohort); B. Rodriguez (John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University); M. Horberg, M. Silverberg (Kaiser Permanente Northern California); J. Goedert (Multicenter Hemophilia Cohort Study II); L. Jacobson (Multicenter AIDS Cohort Study); M. Klein (Montreal Chest Institute Immunodeficiency Service Cohort); S. Rourke, A. Rachlis (Ontario HIV Treatment Network Cohort Study); L. Calzavara (Polaris HIV Seroconversion Study); M. Kushel (Research in Access to Care for the Homeless Cohort); J. Gill (Southern Alberta Clinic Cohort); S. Deeks, J. Martin (Studies of the Consequences of the Protease Inhibitor Era); M. Saag, M. Mugavero, J. Willig (University of Alabama at Birmingham 1917 Clinic Cohort); J. Eron, S. Napravnik (University of North Carolina, Chapel Hill, HIV Clinic Cohort); M. Kitahata (University of Washington HIV Cohort); A. Justice, D. Fiellin (Veterans Aging Cohort Study); T. Sterling, S. Stinette, P. Rebeiro, D. Haas (Vanderbilt-Meharry Centers for AIDS Research Cohort); S. Gange (Women's Interagency HIV Study).
The committee members for the study were as follows: Executive Committee: R. Moore, M. Saag, S. Gange, M. Kitahata, R. McKaig, A. Freeman. Epidemiology/Biostatistics Core: S. Gange, B. Merriman, A. Abraham, B. Lau, K. Althoff, J. Zhang. Data Management Core: M. Kitahata, S. Van Rompaey, H. Crane, W. Lober, E. Webster, B. Simon. Administrative Core: R. Moore, A. Freeman, C. Lent.
Footnotes
Dr. Saag reports receiving consulting fees from Ardea Biosciences, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Monogram Biosciences, Pain Therapeutics, Panacos, Pfizer, Progenics, Roche Laboratories, Tibotec, Tobira Therapeutics, and Vicro and research support from Achillion Pharmaceuticals, Avexa, Boehringer Ingelheim, GlaxoSmithKline, Merck, Panacos, Pfizer, Progenics, Theratechnologies, and Tibotec; Dr. Hogg, receiving grant support from Merck; Dr. Deeks, receiving consulting fees from GlaxoSmithKline, Roche, Gilead, and Boehringer Ingelheim and grant support from Merck, Gilead, Bristol-Myers Squibb, and Pfizer; Dr. Eron, receiving consulting fees from Tibotec, Bristol-Myers Squibb, Merck, GlaxoSmithKline, and Pfizer, lecture fees from Roche, Bristol-Myers Squibb, Tibotec, and Merck, and grant support from GlaxoSmithKline, Merck, and Boehringer Ingelheim; Dr. Gill, receiving consulting fees from Gilead, GlaxoSmithKline, Abbott, Merck, Boehringer Ingelheim, Tibotec, and Pfizer and grant support from GlaxoSmithKline, Abbott, Tibotec, and Pfizer; Dr. Klein, receiving consulting fees from GlaxoSmithKline, Abbott, Pfizer, and Boehringer Ingelheim, lecture fees from Abbott, Gilead, Tibotec, Bristol-Myers Squibb, and GlaxoSmithKline, and research support from the Canadian HIV Trials Network, the Ontario HIV Treatment Network, and Schering-Plough Canada; Dr. Rodriguez, receiving consulting fees from Gilead and Bristol-Myers Squibb, lecture fees from Bristol-Myers Squibb, and grant support from STERIS; Dr. Rachlis, receiving consulting and lecture fees from GlaxoSmithKline, Abbott, Merck, Pfizer, Bristol-Myers Squibb, Gilead, and Tibotec and grant support from GlaxoSmithKline, Tibotec, Boehringer Ingelheim, Abbott, Merck, Pfizer, and Roche; Dr. Horberg, receiving grant support from Gilead, Abbott, and Bristol-Myers Squibb; Dr. Silverberg, receiving grant support from Pfizer, Merck, Gilead, the University-wide AIDS Research Program, and Community Benefit/Kaiser Permanente; Dr. Gebo, receiving consulting fees from Tibotec and grant support from the Johns Hopkins University Richard Ross Award; Dr. Benson, receiving consulting fees from GlaxoSmithKline, Pfizer, Merck, and Achillion and grant support from Gilead; Dr. Collier, receiving consulting fees from Merck, Pfizer, and GlaxoSmithKline and grant support from Schering-Plough, Tibotec-Virco, Gilead, Koronis, and Merck and having an equity interest in Bristol-Myers Squibb and Abbott; and Dr. Moore, receiving consulting fees from Bristol-Myers Squibb and GlaxoSmithKline, lecture fees from Gilead, and grant support from Pfizer, Merck, and Gilead. No other potential conflict of interest relevant to this article was reported.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Detels R, Muñoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 3.Rockville, MD: AIDSinfo; 2008. [March 31, 2009,]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents — January 29. at http://www.aidsinfo.nih.gov/Guidelines/Default.aspx?MenuItem=Guidelines. [Google Scholar]
- 4.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 5.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study: Swiss HIV Cohort Study. BMJ. 1997;315:1194–9. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–97. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 8.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 9.Hughes MD, Ribaudo HR. The search for data on when to start treatment for HIV infection. J Infect Dis. 2008;197:1084–6. doi: 10.1086/586712. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JE, Hanson DL, Cohn DL, et al. When to begin highly active antiretroviral therapy? Evidence supporting initiation of therapy at CD4+ lymphocyte counts <350 cells/microL. Clin Infect Dis. 2003;37:951–8. doi: 10.1086/377606. [DOI] [PubMed] [Google Scholar]
- 11.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 12.Cozzi Lepri A, Phillips AN, d'Arminio Monforte A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS. 2001;15:983–90. doi: 10.1097/00002030-200105250-00006. [DOI] [PubMed] [Google Scholar]
- 13.van Sighem AI, van de Wiel MA, Ghani AC, et al. Mortality and progression to AIDS after starting highly active antiretroviral therapy. AIDS. 2003;17:2227–36. doi: 10.1097/00002030-200310170-00011. [DOI] [PubMed] [Google Scholar]
- 14.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–8. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 15.May M, Porter K, Sterne JA, Royston P, Egger M. Prognostic model for HIV-1 disease progression in patients starting antiretroviral therapy was validated using independent data. J Clin Epidemiol. 2005;58:1033–41. doi: 10.1016/j.jclinepi.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Antiretroviral Therapy Cohort Collaboration. Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194:612–22. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 17.Phillips AN, Lepri AC, Lampe F, Johnson M, Sabin CA. When should antiretroviral therapy be started for HIV infection? Interpreting the evidence from observational studies. AIDS. 2003;17:1863–9. doi: 10.1097/00002030-200309050-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Li R, Anastos K, et al. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004;23:3351–63. doi: 10.1002/sim.1579. [DOI] [PubMed] [Google Scholar]
- 19.Sterling TR, Chaisson RE, Moore RD. HIV-1 RNA, CD4 T-lymphocytes, and clinical response to highly active antiretroviral therapy. AIDS. 2001;15:2251–7. doi: 10.1097/00002030-200111230-00006. [DOI] [PubMed] [Google Scholar]
- 20.Opravil M, Ledergerber B, Furrer H, et al. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count >350× 10(6)/ l. AIDS. 2002;16:1371–81. doi: 10.1097/00002030-200207050-00009. [DOI] [PubMed] [Google Scholar]
- 21.Anastos K, Barrón Y, Miotti P, et al. Risk of progression to AIDS and death in women infected with HIV-1 initiating highly active antiretroviral treatment at different stages of disease. Arch Intern Med. 2002;162:1973–80. doi: 10.1001/archinte.162.17.1973. [DOI] [PubMed] [Google Scholar]
- 22.Ahdieh-Grant L, Yamashita TE, Phair JP, et al. When to initiate highly active antiretroviral therapy: a cohort approach. Am J Epidemiol. 2003;157:738–46. doi: 10.1093/aje/kwg036. [DOI] [PubMed] [Google Scholar]
- 23.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Sterling TR, Chaisson RE, Moore RD. Initiation of highly active antiretroviral therapy at CD4+ T lymphocyte counts of >350 cells/mm3: disease progression, treatment durability, and drug toxicity. Clin Infect Dis. 2003;36:812–5. doi: 10.1086/367934. [DOI] [PubMed] [Google Scholar]
- 25.Lane HC, Neaton JD. When to start therapy for HIV infection: a swinging pendulum in search of data. Ann Intern Med. 2003;138:680–1. doi: 10.7326/0003-4819-138-8-200304150-00018. [DOI] [PubMed] [Google Scholar]
- 26.Robins J, Orellana L, Rotnitzky A. Estimation and extrapolation of optimal treatment and testing strategies. Stat Med. 2008;27:4678–721. doi: 10.1002/sim.3301. [DOI] [PubMed] [Google Scholar]
- 27.Jaén A, Esteve A, Miró JM, et al. Determinants of HIV progression and assessment of the optimal time to initiate highly active antiretroviral therapy: PISCIS Cohort (Spain) J Acquir Immune Defic Syndr. 2008;47:212–20. doi: 10.1097/qai.0b013e31815ee282. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. Erratum, Lancet 2002;360:1178. [DOI] [PubMed] [Google Scholar]
- 29.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 30.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–9. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 31.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 32.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV Outpatient Study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 33.Lau B, Gange S, Moore R. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44:179–87. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 34.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–62. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 35.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 36.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 37.Gras L, Kesselring AM, Griffin JT, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45:183–92. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 38.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 39.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 42.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–9. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 43.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 44.Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42:1481–7. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- 45.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. Erratum, Lancet 2001;357:1536. [DOI] [PubMed] [Google Scholar]
- 46.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 47.Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;98:237–42. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum PR. Observational studies. 2nd. New York: Springer; 2002. [Google Scholar]
- 49.Schneewiess S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 50.Phillips A, Pezzotti P. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18:51–8. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- 51.Gulick RM. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis. 2006;43:942–4. doi: 10.1086/507549. [DOI] [PubMed] [Google Scholar]
- 52.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. Erratum, Ann Intern Med 2002;136:253. [DOI] [PubMed] [Google Scholar]
- 53.King MS, Bernstein BM, Walmsley SL, et al. Baseline HIV-1 RNA level and CD4 cell count predict time to loss of virologic response to nelfinavir, but not lopinavir/ritonavir, in antiretroviral therapy-naive patients. J Infect Dis. 2004;190:280–4. doi: 10.1086/422037. [DOI] [PubMed] [Google Scholar]
- 54.Lichtenstein KA, Armon C, Buchacz K, et al. Initiation of antiretroviral therapy at CD4 cell counts ≥350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47:27–35. doi: 10.1097/QAI.0b013e31815acacc. [DOI] [PubMed] [Google Scholar]
- 55.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 56.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996: recommendations of an international panel. JAMA. 1996;276:146–54. [PubMed] [Google Scholar]
- 58.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 59.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.