Abstract
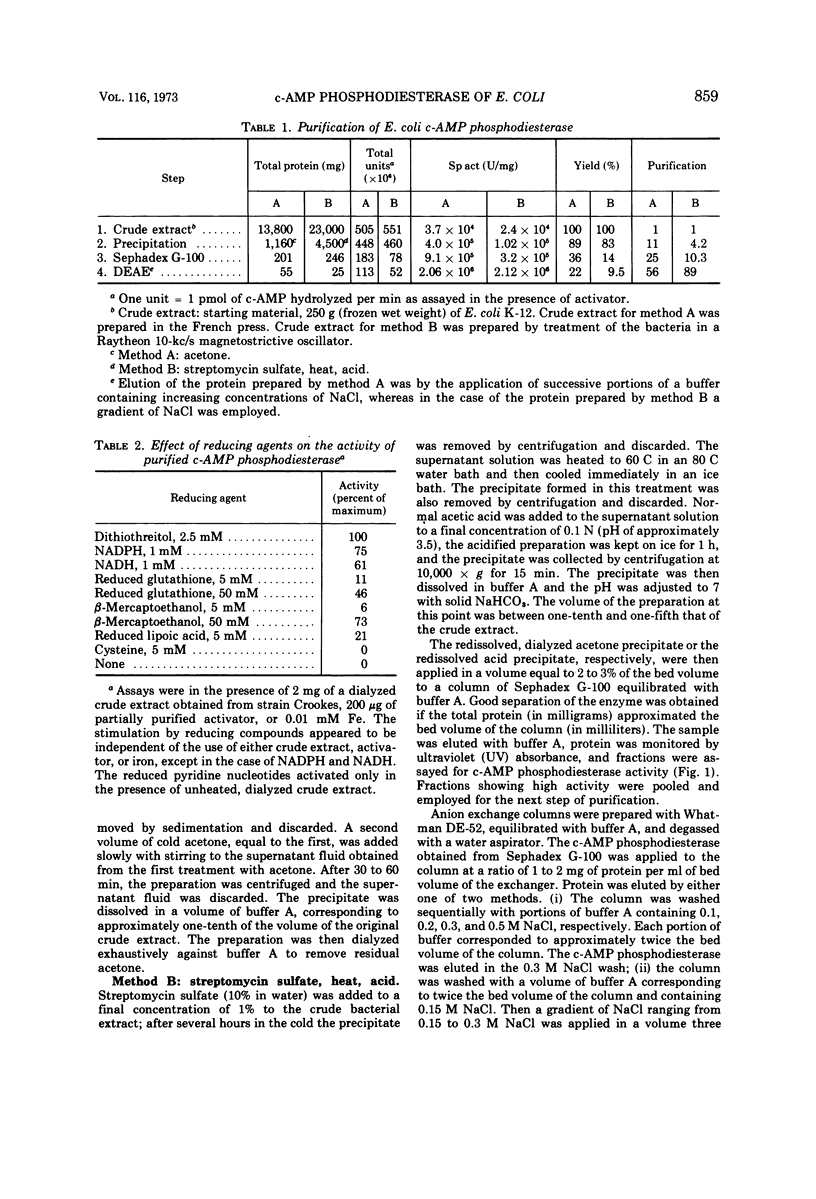
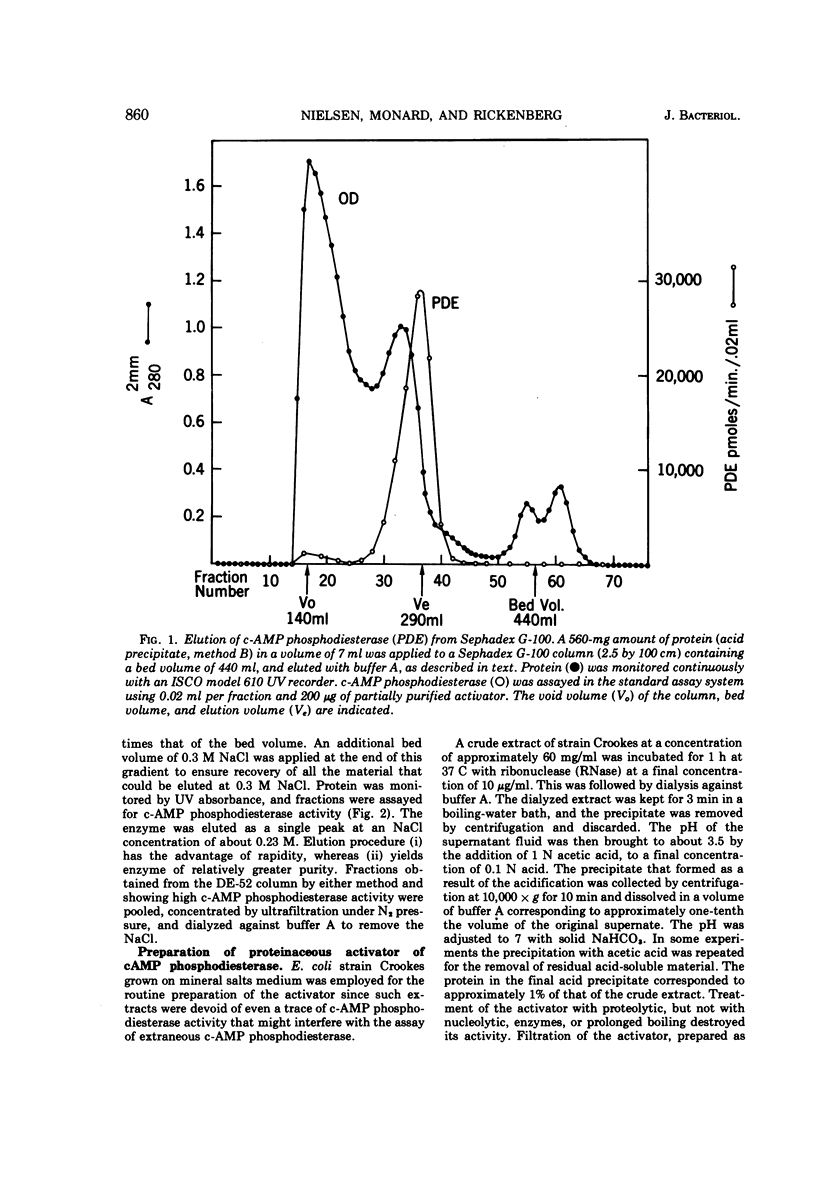
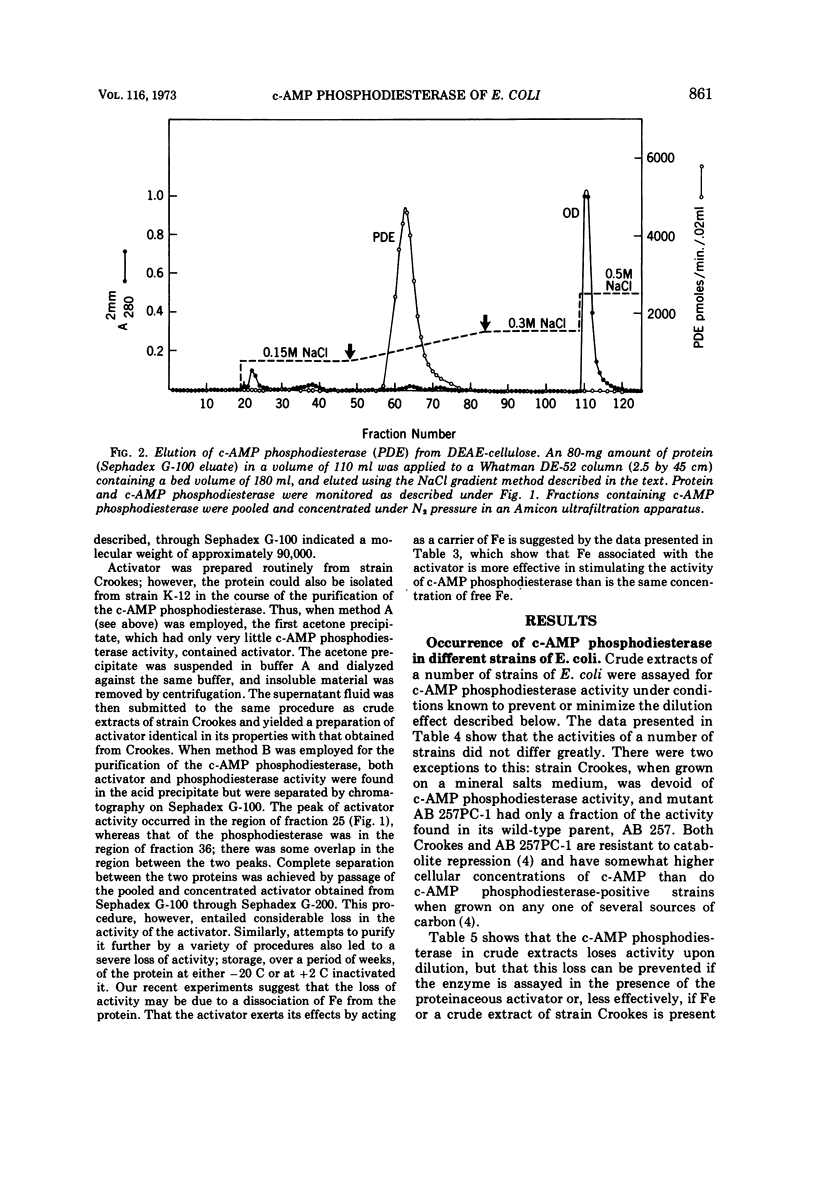
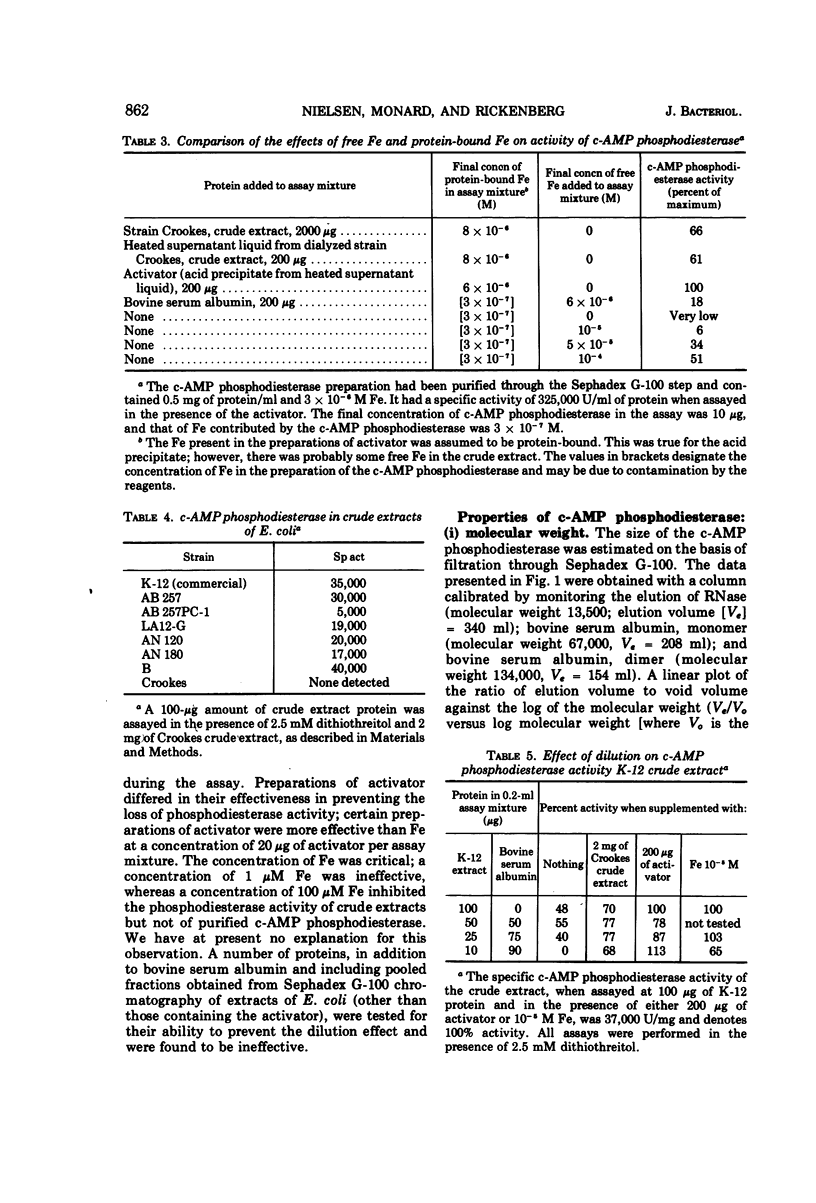
The cyclic 3′,5′-adenosine monophosphate (c-AMP) phosphodiesterase from Escherichia coli has been partially purified. The enzyme has an apparent molecular weight of 30,000, a Michaelis constant of 0.5 mM c-AMP, and a pH optimum of 7. The partially purified enzyme requires for activity the presence of a reducing compound and of either iron or a protein which seemingly acts as iron carrier.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Burger M. Cyclic 3';5' adenosine monophosphate-phosphodiesterase and the release of catabolite repression of beta-galactosidase by exogenous cyclic 3';5' adenosine monophosphate in Escherichia coli. Biochem Biophys Res Commun. 1971 Apr 2;43(1):174–182. doi: 10.1016/s0006-291x(71)80103-1. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braná H., Chytil F. Splitting of the cyclic 3',5'-adenosine monophosphate in cell--free system of Escherichia coli. Folia Microbiol (Praha) 1966;11(1):43–46. doi: 10.1007/BF02877154. [DOI] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Rickenberg H. V. Catabolite repression in Escherichia coli: the role of glucose 6-phosphate. Biochem Biophys Res Commun. 1967 Nov 17;29(3):303–310. doi: 10.1016/0006-291x(67)90453-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Genetic control of catabolite repression of the lac operon in Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 12;20(2):230–234. doi: 10.1016/0006-291x(65)90351-7. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidases and cyclic phosphodiesterases (3'-nucleotidases) of the Enterobacteriaceae. J Bacteriol. 1968 May;95(5):1732–1737. doi: 10.1128/jb.95.5.1732-1737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T., Ide M. Cyclic 3',5'-nucleotide phosphodiesterase of Serratia marcescens. Biochim Biophys Acta. 1970 Oct 14;220(1):116–123. doi: 10.1016/0005-2744(70)90235-4. [DOI] [PubMed] [Google Scholar]
- Okabayashi T., Ide M. Effect of dipicolinic acid on bacterial cyclic 3,5'-nucleotide phosphodiesterase. Biochim Biophys Acta. 1970 Oct 14;220(1):124–126. doi: 10.1016/0005-2744(70)90236-6. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. II. Magnetic resonance studies of the complexes of enzyme, manganese(II), iron(II), and substrates. J Biol Chem. 1971 Sep 25;246(18):5791–5798. [PubMed] [Google Scholar]



