Abstract
Many women of reproductive age are affected by polycystic ovary syndrome (PCOS), a heterogeneous endocrinopathy characterized by androgen excess, chronic oligo-anovulation and/or polycystic ovarian morphology. In addition, PCOS is often associated with insulin resistance, systemic inflammation and oxidative stress which, on one hand, lead to endothelial dysfunction and dyslipidemia with subsequent cardiovascular sequelae and, on the other hand, to hyperplasia of the ovarian theca compartment with resultant hyperandrogenism and anovulation. While traditionally statins have been used to treat dyslipidemia by blocking HMG-CoA reductase, the rate limiting step in cholesterol biosynthesis; in fact, they possess pleiotropic actions, resulting in antioxidant, anti-inflammatory and anti-proliferative effects. Statins offer a novel therapeutic approach to PCOS in that they address the dyslipidemia associated with the syndrome, as well as hyperandrogenism/hyperandrogenemia. These actions may be due to an inhibition of the effects of systemic inflammation and insulin resistance/hyperinsulinemia. Evidence to date, both in vitro and in vivo, suggests that statins have potential in the treatment of PCOS; however, further clinical trials are needed before they can be considered a standard of care in the medical management of this common endocrinopathy.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age with prevalence rates estimated at between 6-10% 1. As PCOS represents a heterogeneous endocrinopathy, its diagnosis is often hampered by controversy regarding its definition. Recent consensus favors the National Institutes of Health (NIH) criteria for PCOS, which includes women with a combination of 1) hyperandrogenism or hyperandrogenemia and 2) oligo- or anovulation in the absence of other etiologies for these symptoms, such as Cushing’s syndrome, thyroid disorders, or congenital adrenal hyperplasia, among others 2. PCOS is, in effect, a diagnosis of exclusion.
While the above definition describes a more severe form of PCOS, the Rotterdam consensus definition coined during the 2003 Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE) adds to the NIH criteria two additional subsets of women, who have a partial PCOS syndrome based on the presence of polycystic ovarian appearance on ultrasound 3. According to the Rotterdam definition, any two of the three criteria (hyperandrogenism, anovulation, and/or polycystic ovarian appearance) are sufficient to make a diagnosis of PCOS. Therefore, this definition broadens the NIH criteria by including 1) women with polycystic ovaries and hyperandrogenism, but no ovulatory dysfunction and 2) women with oligo-anovulation and polycystic ovaries, but no evidence of androgen excess. The inclusion of these two phenotypes as a part of PCOS is debatable, as there is less convincing evidence to show that they lead to the metabolic complications associated with PCOS defined by the NIH criteria 2.
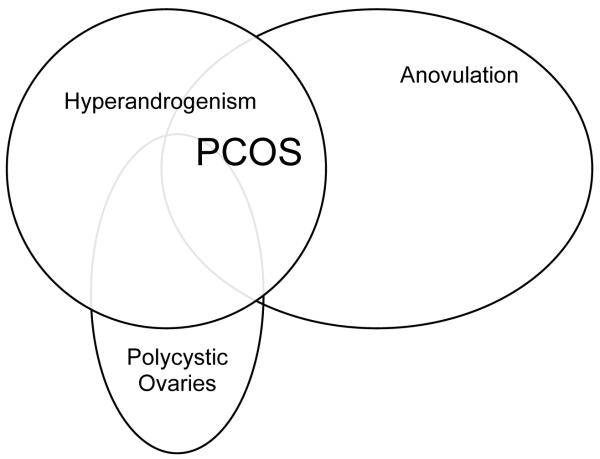
In 2006, the Androgen Excess Society weighed in on the controversy over the diagnostic criteria for PCOS and recommended the presence of clinical and/or biochemical hyperandrogenism and either 1) oligo-anovulation or 2) polycystic ovarian morphology to make the diagnosis 2. As illustrated by the Venn diagram in Figure 1, PCOS may be viewed as a spectrum of disorders including the complete syndrome, but also various partial syndromes. It is unclear whether the so-called partial syndromes are part of a continuum that can lead to full-blown PCOS or whether they are milder, genetically/etiologically distinct forms of PCOS with potentially less significant sequelae. The genetic basis for PCOS is an area of active investigation with more than 70 candidate genes identified thus far and significant familial clustering 4, 5.
Fig. 1.
Diagram illustrating the criteria defining PCOS.
Criteria defining polycystic ovary syndrome (PCOS).
Whether the syndrome is partial or complete, women with PCOS suffer from many consequences, including those related to hyperandrogenism, ovulatory dysfunction, polycystic ovarian appearance, and cardiovascular risks. While not part of the diagnostic criteria, obesity and insulin resistance are also very common among women with PCOS and have long-term sequelae. This review will address the various clinical manifestations of PCOS as well as its pathophysiology. Subsequently, the rationale and evidence for the use of statins for the potential treatment of this syndrome will be introduced and discussed in detail.
Consequences of hyperandrogenism
Hyperandrogenemia or clinical manifestations of hyperandrogenism, such as hirsutism, male-pattern balding, and acne, are common among women with PCOS. In fact, up to 90% of women with PCOS have elevated androgen levels 6. With respect to hirsutism, androgens are involved in the irreversible transformation of fine vellus hairs into coarse terminal hairs 7. Androgens also contribute to the pathogenesis of acne vulgaris in that androgen receptors and 5-alpha reductase, the enzyme that transforms testosterone to the more potent dihydrotestosterone (DHT), are both present within the sebaceous follicle 8, 9. Left untreated, hyperandrogenism can lead to long-term psychological sequelae, for example, related to facial scarring from acne 10.
Androgen excess may also contribute to the cardiovascular risks associated with PCOS, which will be discussed below. For instance, the dyslipidemia of PCOS correlates with hyperandrogenemia 11, and treatment of the latter leads to improvements in lipid profile 12, 13. Hyperandrogenemia also represents an independent risk factor for the development of hypertension among women with PCOS 14. Furthermore, androgen excess may lead to decreased insulin sensitivity as seen in women with congenital adrenal hyperplasia 15and among those treated with exogenous testosterone 16. A recent study of postmenopausal women with current hyperandrogenemia and a history of oligomenorrhea showed an increased rate of Type II diabetes, metabolic syndrome, and angiographic evidence of coronary artery disease with decreased 5 year cardiovascular event-free survival compared to women without clinical features of PCOS 17.
PCOS is a frequent cause of female infertility 18. According to a 31- year follow-up study, almost 18% of women with PCOS were infertile compared to 1.3% among their age-matched counterparts 19. Poor reproductive function among women with PCOS is due to anovulation as well as a high rate of early pregnancy loss 20, 21. In addition to infertility, a consequence of the chronic anovulation associated with PCOS is endometrial hyperplasia, which can progress to endometrial adenocarcinoma. Endometrial hyperplasia has been reported to occur in up to 35% of untreated women with PCOS. While increased mortality risk from endometrial carcinoma among women with PCOS remains controversial 19, 22, 23, an association between the presence of polycystic ovaries and endometrial carcinoma was recently documented among patients less than 50 years old undergoing surgery for the latter 24.
Consequences of polycystic ovarian appearance
The current sonographic definition of polycystic ovaries requires the presence of 12 or more follicles measuring 2-9 mm in diameter per ovary or ovarian volume above 10 cc 3. This finding is seen in 20% of women who do not meet other criteria of PCOS. Conversely, and as alluded to above, not all women with PCOS have polycystic ovarian morphology. Nevertheless, the polycystic appearance of ovaries has important prognostic value not only in regard to the risk of endometrial carcinoma as mentioned above 24, but also with respect to treatment of anovulation. Women with polycystic ovaries have an increased chance of having a multiple gestation after ovulation induction and also are at higher risk for the development of ovulation hyperstimulation syndrome (OHSS). One recent study reported a 36% multiple gestation rate among PCOS patients, who underwent ovulation induction with gonadotropins 25. The risk of moderate to severe OHSS among women with PCOS undergoing in vitro fertilization has been estimated to be 10.5% compared to less than 4% among non-PCOS patients 26. This increased risk can be predicted by the polycystic ovarian appearance on baseline ultrasound; a recent meta-analysis found an almost 7-fold increased risk for the development of OHSS among women with polycystic ovaries compared to controls with sonographically normal appearing ovaries 27.
Consequences of obesity and insulin resistance
Among women diagnosed with PCOS in the United States, 60% are obese 28. Insulin resistance with resulting hyperinsulinemia also occurs frequently among both lean and obese women with PCOS, and glucose intolerance rates of up to 40% have been reported 29-31. Furthermore, type 2 diabetes is diagnosed in approximately 10% of women with PCOS 29-31, and while impaired glucose tolerance and type 2 diabetes are most common among women with PCOS who are in their thirties or forties, a significant percentage of adolescents with PCOS are also affected 32. The implications of obesity and insulin resistance in the setting of women with PCOS are many.
During pregnancy, obesity is associated with various maternal-fetal complications, including gestational hypertension, preeclampsia, gestational diabetes, fetal macrosomia, shoulder dystocia, and failure to progress in labor requiring Cesarean section 33-36. Among obese women, the latter is complicated by an increased risk of intra-operative hemorrhage, postpartum endometritis, and wound infection37. In addition, most anesthesia-related complications leading to maternal morbidity and mortality occur in the obese gravida 38-40; in one study, 75% of anesthesia related maternal deaths occurred in obese women 41. Similarly, preexisting insulin resistance confers an increased risk for gestational diabetes with its associated maternal and fetal consequences, including polyhydramnios, fetal macrosomia, birth trauma, operative delivery, and neonatal metabolic complications and higher perinatal mortality 42, 43.
Outside of pregnancy, the consequences of obesity and insulin resistance are their associations with cardiovascular disease. Specifically, both obesity and insulin resistance predispose women with PCOS to endothelial dysfunction 44-46, and obese, insulin-resistant women with PCOS have metabolic profiles consistent with dyslipidemia 47, 48. Obesity and insulin resistance also represent independent risk factors for the metabolic syndrome, which will be discussed below.
Cardiovascular risks
In the long-term, women with PCOS are at increased risk for dyslipidemia, hypertension, and related cardiovascular morbidity and possibly mortality 49-51. The dyslipidemia of PCOS is characterized by elevated plasma levels of cholesterol, low density lipoproteins (LDL), very low density lipoproteins (VLDL), and triglycerides with concomitantly reduced concentrations of high density lipoproteins (HDL) 52-55. Homocysteine levels are also higher in women with PCOS compared to controls56-59, and hyperhomocysteinemia represents another independent risk factor for the development of cardiovascular disease60. Moderate hyperhomocysteinemia predisposes individuals to endothelial dysfunction via a mechanism involving increased oxidative stress 61.
Both symptomatic and asymptomatic women with PCOS have signs of significant vascular impairment. For example, common carotid artery vascular compliance is decreased, while arterial stiffness is increased 62, and endothelial dysfunction manifests as impaired vasodilation in hyperandrogenic, insulin-resistant women with PCOS when compared with age- and weight-matched controls 63. PCOS is also associated with increased thickness of arterial intima-media and greater prevalence of subclinical significant occlusion in more arterial segments compared to women with normal appearing ovaries 54. A recent study non-invasively assessed coronary artery calcium (CAC) by computed tomography and reported that 33% of young obese women with PCOS had evidence of early coronary atherosclerosis compared to 8% of age and weight matched controls 64. The presence and quantity of CAC is directly related to the risk of subsequent coronary events, namely, myocardial infarction and sudden death, in both asymptomatic and symptomatic patients 65. One study found that women with PCOS had a 7-fold increased risk for myocardial infarction 52.
The Metabolic Syndrome
A major risk factor for the development of cardiovascular disease is the metabolic syndrome, which consists of a combination of factors, including obesity, dyslipidemia, hypertension, and glucose intolerance 66. According to the National Cholesterol Education Program’s Adult Treatment Panel (NCEP ATP III), metabolic syndrome in women is defined as the presence of at least three of the following: waist circumference > 88cm, serum triglycerides > 150 mg/dl, serum HDL < 50 mg/dl, blood pressure greater than 130/85, and serum fasting glucose over 110/mg/dl 67.
Among the 7 to 10 million American women with PCOS, the prevalence of the metabolic syndrome is approximately 43%, which is 2-fold higher than that for age-matched controls 68. A recent study found an 11-fold increase in metabolic syndrome in women with PCOS, and even young women (<30 years old) had a significantly higher risk 69. The most prominent metabolic syndrome features among women with PCOS are, in decreasing order, decreased HDL levels, obesity, and hypertension 68.
Insulin resistance, one of the major causative factors involved in the development of metabolic syndrome 70, is prevalent in both lean and obese women with PCOS and potentiates the dyslipidemia, obesity, and glucose intolerance associated with this disorder. Due to the high prevalence of impaired glucose tolerance among women with PCOS, the Androgen Excess Society recently issued a position statement urging providers to screen all PCOS patients for impaired glucose tolerance using a 2-hour oral glucose tolerance test at least once every two years 71.
Despite the multitude of cardiovascular risk factors associated with PCOS, increased mortality due to cardiovascular disease has not been clearly demonstrated among this population 19, 72. However, given that long-term data are lacking and PCOS affects many women of reproductive age, the increased risk for cardiovascular disease in the long-term is concerning.
Pathophysiology of PCOS
While the root causes of PCOS are still not known, a broad range of interactions between endocrine and metabolic derangements have been described. Thus, for example, the etiology of anovulation in PCOS is multifactorial and includes alterations in ovarian, hypothalamic and pituitary function. Most women with PCOS have elevated plasma concentrations of luteinizing hormone (LH) and normal or decreased serum levels of follicle-stimulating hormone (FSH) 73. The LH-enriched milieu promotes thecal steroidogenesis and thus contributes to the hyperandrogenism typically seen with this disorder. In addition, there is a strong association between insulin resistance and hyperandrogenism, and most evidence indicates that hyperinsulinemia induces hyperandrogenism 74, 75.
Insulin stimulates the production of androgens by ovarian thecal and stromal cells 76. PCOS is also associated with increased levels of bio-available insulin-like growth factor I (IGF-I) 77-80, and both insulin and IGF-I not only induce proliferation of theca-interstitial cells 81-84, but also prevent these cells from undergoing apoptosis 85. Thus, excessive androgen production in PCOS may be due, in part, to stimulation of the proliferation of the theca-interstitial compartment by insulin and IGF-I. In addition to stimulating ovarian steroidogenesis, elevated insulin levels inhibit hepatic production of SHBG and thereby further increase bioavailable androgens 86. Excess insulin has other untoward metabolic effects, including those on muscle, fat, and the vasculature as discussed above.
The molecular basis for insulin resistance, though not yet fully understood, may be related to a post-receptor defect involving oxidative stress-mediated serine phosphorylation of the insulin receptor substrates 1 and 2 (IRS-1, IRS-2), which leads to an abrogation of insulin signaling via its receptor 87, 88. Specifically, serine phosphorylated IRS molecules lose efficacy in binding to the insulin receptor as well as downstream targets, and this leads to impaired insulin action with compensatory hyperinsulinemia 89, 90. In addition, when phosphorylated, IRS molecules are more susceptible to degradation 89, 90. The alterations in IRS function and integrity result in impaired metabolic effects of insulin, particularly with respect to glucose transport, but paradoxically, the mitogenic effects of insulin persist 91. In fact, certain mitogenic pathways, including those involving the mitogen activated protein kinase (MAPK), are activated in PCOS and may potentiate the resistance to insulin since MAPK activity leads to phosphorylation of IRS-1 92.
Oxidative stress, which is an imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses favoring the former, is associated with a variety of pathological conditions including diabetes, cancer, and cardiovascular disease 93-95. PCOS is also associated with increased oxidative stress and systemic inflammation 96-98. Furthermore, antioxidant reserve appears to be compromised in women with PCOS 97. Even lean women with PCOS exhibit increased oxidative stress, as measured by levels of malonyldialdehyde, a marker of lipid peroxidation, and they have decreased serum total antioxidant levels compared to controls 56. Recent evidence suggests that inflammatory cytokines and measures of oxidative stress, such as tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) 99, 100, may play a role in the dysregulation of the theca-interstitial compartment, and both of these factors are elevated in PCOS 97, 98, 101, 102.
ROS have a bimodal effect on theca cells such that modest oxidative stress stimulates proliferation of theca cells in vitro, while both greater oxidative stress and antioxidants, such as vitamin E succinate, inhibit their proliferation 100. TNF-α and insulin also stimulate theca-interstitial cell proliferation 81, 82, 99; furthermore, several in vitro and in vivo studies have shown that insulin and TNF-α also induce oxidative stress 103-105. It is well known that ROS mediate proliferation of various other cell types, including fibroblasts and aortic endothelial cells 106, while antioxidants, such as vitamin E succinate, inhibit proliferation of vascular smooth muscle, fibroblasts, and many cancer cell lines 107-110.
In addition to simulating proliferation of theca-interstitial cells, moderate oxidative stress also induces testosterone and progesterone production by enhancing thecal expression of key steroidogenic enzymes, such as CYP17, CYP11A1, and 3βHSD 111. Thus, the excess androgen production in PCOS is not only due to increased numbers of theca cells, but also to an induction of their steroidogenic capacity. Furthermore, oxidative stress, as discussed above, impairs insulin signaling, resulting in a compensatory hyperinsulinemia, which, in turn, further stimulates thecal steroidogenesis 89, 90. Another significant result of oxidative stress is endothelial dysfunction and the subsequent development of cardiovascular disease 112.
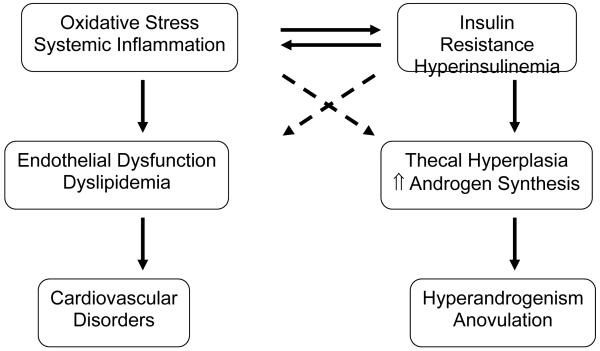
Thus, the pathophysiology of PCOS involves oxidative stress with systemic inflammation and insulin resistance with resulting hyperinsulinemia, which promote dysregulation of the ovarian thecal compartment and dysfunction of endothelial cells, such that hyperandrogenism, anovulation, and cardiovascular disorders ensue (Figure 2). Given the significant sequelae of PCOS, prompt and effective treatment is warranted over the long term. The standard medical management of PCOS was recently reviewed 113 and will not be addressed here. Rather, the remainder of this review will focus on the rationale and evidence for the potential use of statins in the treatment of PCOS.
Fig. 2.
Proposed pathophysiology and sequelae of PCOS.
Proposed pathophysiology and sequelae of polycystic ovary syndrome. Oxidative stress with systemic inflammation and insulin resistance with resulting hyperinsulinaemia promote dysregulation of the ovarian thecal compartment and dysfunction of endothelial cells. Hyperandrogenism, anovulation and cardiovascular disorders ensue. ↑ indicates increased; solid line indicates estabilished cause and effect; dashed line indicates proposed pathway.
Statins
Statins are selective inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the cholesterol biosynthetic pathway. Statins improve the lipid profile primarily by decreasing total cholesterol and LDL levels 114, 115. It has been well established that these medications significantly reduce both non-fatal and fatal cardiovascular disease events in primary and secondary prevention trials and thereby decrease cardiovascular morbidity and mortality 114-117.
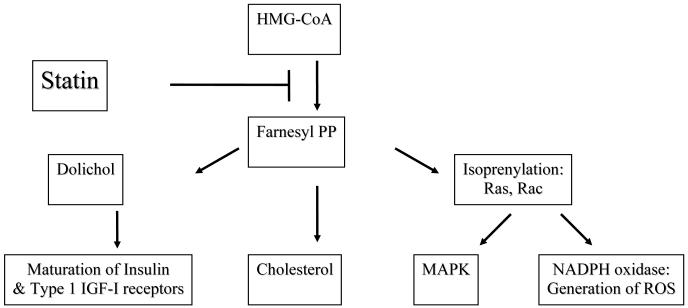
In order to understand the potential role of statins in the treatment of PCOS, it is essential to review how statins affect the mevalonate pathway (Figure 3). This pathway consists of the reactions starting from acetyl-coenzyme A (acetyl-CoA) and leads to the formation of farnesyl pyrophosphate (FPP), which then serves as the substrate for several biologically important agents, including cholesterol, isoprenylated proteins, coenzyme Q, and dolichol 118, 119. Particularly relevant to PCOS are the dolichols, which mediate the maturation of the insulin and Type 1 IGF-1 receptors 120, and cholesterol, which serves as the substrate for steroid hormones.
Fig. 3.
The mevalonate pathway and mechanism of statin action.
The mevalonate pathway and mechanism of HMG-CoA reductase inhibitor (statin) action. IGF-I = insulin-like growth factor I; MAPK = mitogen activated protein kinase; NADPH = nicotinamide adenosine dinucleotide phosphate; PP = pyrophosphate; ROS = reactive oxygen species
In addition to serving as a substrate for downstream products in the mevalonate pathway, FPP mediates the post-translational modification of other proteins, a process known as isoprenylation [#31]. Isoprenylation is required for membrane attachment and function of several families of proteins, including Ras and Ras-related GTP binding proteins (small GTPases) and protein kinases 121. Specifically essential are members of the Ras superfamily, which include Ras, Rho, Rac, and Cdc 42. The functions of these proteins depend on their association with the cytoplasmic leaflet of cellular membranes, a process that requires isoprenylation 121. Since these small GTPases modulate proliferation, apoptosis, and function of cells, any interference with isoprenylation may have profound effects.
Interestingly, the effects of the mevalonate pathway correlate with several sites of insulin action as insulin increases ovarian steroidogenesis, protein isoprenylation, and ovarian theca-interstitial cell proliferation 83, 122-125. Insulin simulates the phosphorylation and activation of farnesyl transferase 122, 123, 126-128, thereby augmenting the isoprenylation of Ras and other small GTPases 122-124, 129. This leads to cellular proliferation by activation of the MAPK pathway, which is mediated by the small GTPases and their downstream effectors, such as Ras-Raf-extracellular signal-regulated kinase 1/2 (Erk 1/2).
Dolichol, a product of the mevalonate pathway, is required for the maturation of insulin and Type 1 IGF-I receptors 120. Specifically, dolichol acts as a carbohydrate donor during N-linked glycosylation of membrane-targeted proteins 130. While this post-translational modification is not critical for most cell surface receptors, the Type 1 IGF and insulin receptors require glycosylation for proper proreceptor cleavage, without which the proreceptor is retained within the endoplasmic reticulum 131, 132; thus dolichol is essential for insulin and IGF-I signaling.
The mevalonate pathway also mediates the effects of oxidative stress, which shares some signal transduction pathways with insulin and IGF-I, specifically the Erk 1/2 and p70s6K pathways 133. A convergence of the actions of insulin/IGF-I and ROS at the Erk1/2 and p70s6K pathways may explain the comparable effects of these agents on proliferation. Furthermore, ROS production by NADPH oxidase depends on isoprenylation, as the assembly of this enzyme requires the presence of isoprenylated Rac at the plasma cell membrane 134. Cytosolic components of NADPH oxidase p47phox and p67phox complex with Rac1 to induce NADPH oxidase activity 135. Thus, disruption of isoprenylation can lead to profound disturbances in cellular function, including decreased generation of intracellular ROS.
Statins reversibly block the conversion of HMG-CoA to mevalonate by HMG-CoA reductase, and the resulting depletion of mevalonate leads to a decrease in FPP with a consequent decrease in isoprenylation. In addition, by decreasing FPP and subsequently dolichol synthesis, statins have an inhibitory effect on N-linked glycosylation 130 and therefore, insulin and Type 1 IGF-1 proreceptor cleavage may be impaired. In addition, statins possess both indirect and direct antioxidant activity 136. The antioxidant actions of statins include inhibition of NADPH oxidase activity, preservation of relative levels of vitamins C and E, as well as inhibition of the uptake and generation of oxidized LDL 134, 137. The intrinsic antioxidant activity of statins involves both anti-hydroxyl and anti-peroxyl radical activity 136. In vivo, statins reduce plasma levels of nitrotyrosine and chlorotyrosine 138, and they also exert anti-inflammatory effects by lowering C-reactive protein levels and suppressing pro-inflammatory agents, such as TNF-α 139.
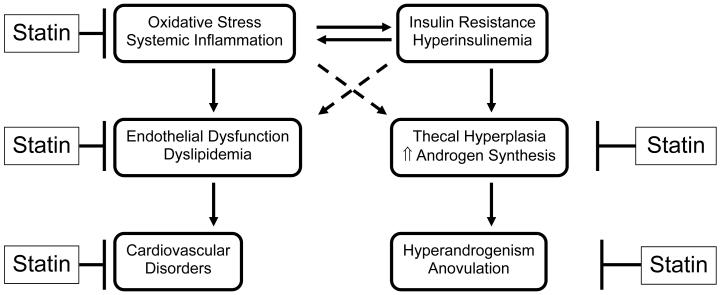
The effects of statins on ovarian function, specifically in women with PCOS, are likely to involve multiple pathways (Figure 4). Firstly, by directly inhibiting production of cholesterol, the substrate for testosterone, statins can improve hyperandrogenemia. Secondly, statins may limit actions of insulin and IGF-I on the ovary not only by decreasing N-linked glycosylation and thus, maturation of insulin and Type I IGF-I receptors, but also by decreasing isoprenylation of small GTPases, such as Ras and Rac, which mediate some pathways of insulin signaling. In this way, blockade of the mevalonate pathway by statins, can lead to an abrogation of the stimulatory effects of hyperinsulinemia on thecal proliferation and steroidogenesis. Similarly, statins can directly and indirectly block the oxidative stress- mediated increases in cellular proliferation, steroidogenesis, and insulin resistance. By inhibiting isoprenylation, ROS generation by NADPH oxidase can be reduced by statins. The decreased oxidative stress along with statin-mediated improvement in lipid profile, can have a beneficial effect on the long-term cardiovascular morbidity and mortality associated with PCOS.
Fig. 4.
Rationale for the use of statins for treatment of PCOS.
Rationale for the use of HMG-CoA reductase inhibitors (statins) for the treatment of polycystic ovary syndrome. ↑ indicates increased; solid line indicates established cause and effect; dashed line indicates proposed pathway.
Evidence for statin actions in vitro
In vitro, the statin mevastatin inhibits the proliferation of theca-interstitial cells and also inhibits LH-stimulated production of both progesterone and testosterone through a mechanism that is independent of its effect on cell number 140. The inhibitory effects of mevastatin on ovarian cell proliferation are consistent with previous reports regarding other mesenchymal cell types, including vascular smooth muscle 141-143, cardiomyocytes 144, and mesangial cells 145.
The effects of statins on ovarian steroidogenesis may be due to several mechanisms. Besides impairing the availability of the substrate cholesterol, statins also decrease the expression of several key enzymes involved in testosterone production including P450scc, P450c17, and 3βHSD as demonstrated in adrenocortical cells 146, 147, and similar findings have been observed in ovarian cells 148. It has been established previously that oxidative stress increases the expression of these same steroidogenic enzymes in the ovary 111. A major source of ROS is the enzyme NADPH oxidase, which is activated by various cytokines 149. Mevastatin and simvastatin, in the presence of LH, inhibit the expression of p22phox, a membrane-bound subunit essential for function of NADPH oxidase in theca-interstitial cells 150. The expression of another NADPH oxidase subunit p47phox, is also decreased by these statins 150.
In addition, mevastatin blocks basal and insulin-dependent activation of the MAP kinase pathway in vitro as measured by phosphorylation of Erk1/2, a downstream kinase 151. A recent study demonstrated that the inhibitory effects of simvastatin on theca-interstitial cell proliferation are only partially explained by blocked isoprenylation, and proliferation of these cells is sensitive to diverse inhibitors of glycosylation 152. Therefore, part of the inhibitory effects of statins on insulin-mediated proliferation may be due to inhibition of N-linked glycosylation secondary to blockade of dolichol production. While decreased insulin and IGF-1 signaling at the level of ovarian thecal and stromal cells may be beneficial, decreased insulin receptor function at the level of other target tissues, such as liver, muscle, and adipose, may have potential negative consequences in terms of glucose metabolism. However, there is no evidence to date that statins cause or exacerbate insulin resistance.
Mevastatin and insulin have opposite effects on two signal transduction pathways that modulate proliferation: insulin alone stimulates the phosphorylation of the MAP kinase Erk1/2, while mevastatin blocks both its basal and insulin-dependent activation 151. In contrast, mevastatin has no effect on either basal or insulin-stimulated Akt/protein kinase B (PKB) phosphorylation in theca-interstitial cells 151. The above findings underscore the potential therapeutic importance of selective inhibition of Erk1/2, but not Akt/PKB by statins, such that insulin-mediated cellular proliferation of the theca-interstitial compartment may be abrogated without significantly affecting insulin-dependent glucose uptake, a process that appears to rely mostly on the Akt/PKB pathway 153, 154.
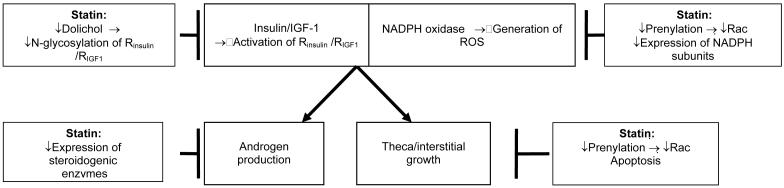
Thus, as summarized in Figure 5, the in vitro studies on ovarian theca-interstitial cells demonstrate that statins decrease cell proliferation and testosterone production, inhibit the expression of steroidogenic enzymes, decrease expression of NADPH oxidase subunits, and block MAPK-dependent phosphorylation. Furthermore, the proliferation of these cells is sensitive to glycosylation, a post-translational modification blocked by statins. Taken together, these finding raise the possibility that the use of statins in women with PCOS could decrease thecal hyperplasia, hyperandrogenism, and oxidative stress.
Figure 5.
Postulated effects of statins on ovarian theca-interstitial cells. Abbreviations: Rinsulin, insulin receptor; RIGF1, insulin-like growth factor 1 receptor.
Evidence for stain actions in clinical trials
Recently, a randomized, prospective clinical trial investigated the effects of simvastatin on women with PCOS 155 and was followed by a cross-over study evaluating the effects of simvastatin and a combined oral contraceptive pill (OCP) on PCOS 156. Women with PCOS, as defined by the ESHRE criteria, were treated with simvastatin plus OCP or OCP alone. Testosterone levels declined more in the presence of statin compared to the OCP alone; furthermore hirsutism was slightly, but significantly, improved in the presence of statin, while there was a non-significant decrease in hirsutism in subjects receiving OCP alone. In contrast to the effects on testosterone, simvastatin had no effect on DHEAS levels, suggesting that the actions of statins are selective and may not alter adrenal steroidogenesis.
However, simvastatin did affect the hypothalamo-pituitary axis, since between the groups, there were distinctly different responses noted with respect to gonadotropin levels. LH levels decreased more in the presence of statin, and as FSH levels did not significantly change, the net effect was a reduction in the LH:FSH ratio. Neither of the treatments had a significant effect on body mass index (BMI). The improvements in testosterone and LH by statin were not mediated by improved insulin sensitivity as determined by fasting and post-glucose challenge levels of insulin and glucose. In fact, fasting insulin and fasting glucose levels as well as oral glucose tolerance test results were not significantly altered by either treatment. This finding points to the different pathways of insulin with respect to its actions on glucose transport and other cellular functions, such as cellular proliferation, as discussed earlier.
As expected, total cholesterol and LDL decreased in the statin group, while there were small increases in these parameters in the OCP group. There was a small, but significant increase in HDL in both groups, and triglyceride levels were not affected by simvastatin treatment, while they increased in the OCP group. The improvement of the lipid profile by simvastatin is of particular value in PCOS, a condition characterized by dyslipidemia and other cardiovascular risk factors. In addition, while CRP levels slightly increased in the OCP group, a significant decrease from baseline was observed in the statin group, and statin treatment produced a more pronounced decrease in soluble vascular cell adhesion molecule-1 (VCAM-1) levels compared to OCP’s alone VCAM-1 is expressed by the vascular endothelium under pro-inflammatory conditions and appears to play a role in the pathogenesis of atherosclerosis by mediating adhesion of activated leukocytes to the vasculature 157. Regulation of VCAM-1 expression represents one on the many pleiotropic effects of statins 158. The effects of OCPs and simvastatin in women with PCOS are summarized in Table 1.
Table 1.
Summary of effects of OCP and simvastatin. Data are presented as geometric means with 95% confidence intervals in parenthesis
| Parameter | Baseline | OCP | Statin + OCP |
Difference (Statin + OCP vs. OCP) |
Effect of simvastatin (P-value) |
|---|---|---|---|---|---|
| Total Testosterone (ng/mL) |
0.77 (0.69, 0.84) |
0.57 * (0.51, 0.64) |
0.48 * (0.43, 0.54) |
−0.09 (−0.14, −0.03) |
0.004 |
| Free Testosterone (pg/ml) |
1.20 (0.98, 1.48) |
0.78 * (0.63, 0.97) |
0.51 * (0.41, 0.64) |
−0.27 (−0.40, −0.09) |
0.006 |
| DHEAS (μg/mL) |
3.15 (2.77, 3.59) |
2.22 * (1.99, 2.47) |
2.27 * (2.03, 2.53) |
0.05 (−0.20, 0.33) |
0.70 |
| SHBG (nmol/L) |
64 (48, 79) |
139 * (123, 155) |
136 * (119, 152) |
−3.4 (−16.9, 10.2) |
0.62 |
| LH (IU/L) |
8.10 (6.74, 9.46) |
6.62 * (5.78, 7.46) |
5.07 * (4.20, 5.93) |
−1.55 (−2.47, −0.63) |
0.002 |
| FSH (IU/L) |
5.68 (5.21, 6.16) |
6.32 * (5.78, 6.87) |
6.01 (5.46, 6.57) |
−0.31 (−0.91, 0.29) |
0.30 |
| LH:FSH ratio | 1.27 (1.10, 1.46) |
0.95 * (0.81, 1.10) |
0.76 * (0.65, 0.89) |
−0.19 (−0.30, −0.05) |
0.01 |
| Prolactin (ng/mL) |
21.9 (19.1, 24.7) |
23.1 (20.6, 25.6) |
20.2 (17.5, 22.8) |
−2.9 (−6.6, 0.7) |
0.11 |
| Hirsutism 1 | 8.6 (7.3, 9.8) |
8.2 * (8.0, 8.4) |
7.9 * (7.6, 8.1) |
−0.3 (−0.6, −0.1) |
0.02 |
| BMI (kg/m2) |
22.3 (21.3, 23.3) |
22.3 (22.1, 22.6) |
22.3 (22.1, 22.6) |
0.0 (−0.2, 0.2) |
0.99 |
| Total Cholesterol (mg/dL) |
190 (180, 201) |
200 * (192, 207) |
176 * (168, 184) |
−24.0 (−34.6, −13.3) |
<0.001 |
| LDL Cholesterol (mg/dL) |
107 (98, 115) |
108 (101, 115) |
85 * (77, 92) |
−22.9 (−32.5, −13.4) |
<0.001 |
| HDL (mg/dL) |
63 (59, 68) |
70 * (67, 73) |
72 * (69, 75) |
1.8 (−1.1, 4.7) |
0.22 |
| Triglycerides (mg/dL) |
86 (76, 96) |
103 * (94, 113) |
86 (78, 95) |
−17.3 (−26.8, −6.7) |
0.003 |
| Fasting Insulin (μU/mL) |
8.5 (7.3, 9.8) |
9.4 (8.1, 10.6) |
9.4 (8.1, 10.7) |
0.04 (−1.40, 1.49) |
0.95 |
| Fasting Glucose (mg/dL) |
85.2 (82.0, 88.4) |
92.0 * (88.5, 95.5) |
90.7 * (87.0, 94.3) |
−1.3 (−5.7, 3.0) |
0.55 |
| Insulin AUC | 47.0 (40.8, 54.0) |
55.3 * (48.9, 62.6) |
61.7 * (54.4, 70.0) |
6.4 (−1.8, 15.9) |
0.13 |
| Glucose AUC | 107 (101, 113) |
113 * (107, 118) |
112 (106,118) |
−1.0 (−7.4, 5.4) |
0.75 |
| Insulin Sensitivity Index 2 | 5.34 (4.62, 6.15) |
4.54 * (3.97, 5.19) |
4.16 * (3.63, 4.77) |
−0.39 (−0.97, 0.30) |
0.25 |
| hs-CRP (mg/L) 3 |
1.61 (1.08, 2.42) |
1.70 (1.24, 2.34) |
0.92 * (0.66, 1.29) |
−0.78 (−1.10, −0.29) |
0.006 |
| sVCAM-1 (μg/L) 4 |
583 (531, 640) |
522 * (487, 559) |
478 * (446, 513) |
−44 (−740, −12) |
0.01 |
Adapted with permission from: Banaszewska, B, Pawelczyk, L, Spaczynski, RZ, Dziura, J, Duleba, AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective randomized cross-over trial. J Clin Endocrinol Metabol 2007 Feb: 92(2):456-461. Copyright 2007, The Endocrine Society.
Difference vs. baseline (P<0.05).
Determined by Ferriman/Gallwey score
Insulin Sensitivity Index determined as described by Matsuda and De Fronzo [1]
hs-CRP: C-reactive protein
sVCAM: soluble vascular cell adhesion molecule-1.
Taken together, these data suggest that the use of statins in women with PCOS is likely to ameliorate the hyperandrogemia and dysregulation of gonadotropins secretion and it will offer significant protection from long-term cardiovascular morbidity. One limitation of the above studies was the concomitant use of OCPs with statins due to the fact that statins are considered pregnancy category X. The potential teratogenicity of statins limits their use in women who are at risk of conceiving. Recently, the risk of statin use during pregnancy has been extensively reviewed, and the available evidence suggests that statins are not major teratogens; the actual risk for an exposed pregnancy is low 159. Still, given the paucity of research on statin use during pregnancy, and the pleiotropic actions of statins on numerous cellular processes, caution must be exercised in using these medications in women of reproductive age.
Conclusions
Statins appear to be a promising new therapeutic option for the medical management of PCOS. Statins target not only the dyslipidemia frequently associated with this endocrinopathy, but also the underlying stimulation of thecal proliferation and steroidogenesis. Given that the mechanisms of statin actions are diverse and address the oxidative stress, systemic inflammation and effects of insulin resistance/hyperinsulinemia associated with the pathophysiology of PCOS, it is likely that they will be useful medications for the treatment of PCOS.
Further clinical studies of statins, particularly in the absence of OCPs should shed additional light on the mechanism of actions of these drugs on PCOS. As the use of statins in the treatment of PCOS is in its infancy, there are still numerous unanswered questions such as: What are the long-term effects of statins on women of reproductive age? Are the effects of statins different among patients with different PCOS phenotypes; for example, those with hyperinsulinemia versus normoinsulinemia? Or those without hyperandrogenemia? Should statins be used in women with PCOS, who have normal lipid profiles? Can statins be used effectively in women with PCOS from different ethnic groups? Can statins restore ovulation?
Given the heterogeneity of PCOS and the fact that it affects women who may or may not be interested in conceiving, the medical management of PCOS requires individualized considerations and patient-specific therapies. Based on the available evidence, statins can be used in women with PCOS, who have dyslipidemia and are not at risk of conceiving. Further studies of this potentially promising therapy are needed before it can be recommended for routine clinical use.
Contributor Information
Pinar H. Kodaman, Yale University School of Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences, Section of Reproductive Endocrinology and Infertility, 333 Cedar Street, New Haven, CT 06520
Antoni J. Duleba, University of California at Davis, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, 4860 Y Street, Suite 2500 ACC, Sacramento, CA 95817
References
- 1.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS) arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrin Metab. 1999;84:1897–9. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dawailly D, et al. Position statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrin Metab. 2006;91(11):4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 3.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:103–11. doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 5.Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28:23–30. doi: 10.1159/000293493. [DOI] [PubMed] [Google Scholar]
- 6.DeVane GW, Czekala NM, Judd HL, Yen SS. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121:496. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endo Rev. 2000;21:347–62. doi: 10.1210/edrv.21.4.0401. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry R, Hodgins MB, Van der Kwast T, Brinkman AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair grown, sebacious glands, and sweat glands. J Endocrinol. 1992;133:467–75. doi: 10.1677/joe.0.1330467. [DOI] [PubMed] [Google Scholar]
- 9.Baille A, Calman K, Milne J. Histochemical distribution of hydroxysteroid dehydrogenases in human skin. Br J Derm. 1965;7:610–6. doi: 10.1111/j.1365-2133.1965.tb14591.x. [DOI] [PubMed] [Google Scholar]
- 10.Moghetti P, Toscano V. Treatment of hirsutism and acne in hyperandrogenism. Best Pract Res Clin Endocrinol Metab. 2006;20(2):221–34. doi: 10.1016/j.beem.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Amowitz LL, Sobel BE. Cardiovascular consequences of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:439–58. doi: 10.1016/s0889-8529(05)70079-7. [DOI] [PubMed] [Google Scholar]
- 12.Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol. 2004;60:241–9. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure anti-androgen receptor blocker flutamide on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2699–705. doi: 10.1210/jcem.83.8.5041. [DOI] [PubMed] [Google Scholar]
- 14.Chen M-J, Yang W-S, Yang J-H, Chen C-L, Ho H-N, Yang Y-S. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–7. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 15.Speiser PW, Serrat J, New MI, Gertner JM. Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992;75(6):1421–4. doi: 10.1210/jcem.75.6.1464643. [DOI] [PubMed] [Google Scholar]
- 16.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83(12):4420–5. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Bairey Merz CN, Azziz R, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93(4):1276–84. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol. 1987;1:235–45. doi: 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- 19.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome:results of a 31 year follow-up study. Hum Fert. 2000;3(2):101–5. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 20.Homburg R, Armar NA, Eshel A, Adams J, Jacobs HS. Influence of serum luteinising hormone concentrations on ovulation, conception and early pregnancy loss in polycystic ovary syndrome. Br Med J. 1988;297:1024–6. doi: 10.1136/bmj.297.6655.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagle M, Bishop K, Ridley N, et al. Recurrent early miscarriage and polycystic ovaries. Br Med J. 1988;297:1027–8. doi: 10.1136/bmj.297.6655.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunde O, Tanbo T. Polycystic ovary syndrome: A follow-up study on diabetes mellitus, cardiovascular disease, and malignancy 15-25 years after ovarian wedge resection. Gynecol Endocrinol. 2007;23(12):704–9. doi: 10.1080/09513590701705189. [DOI] [PubMed] [Google Scholar]
- 23.Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–2. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 24.Pillay OC, Te Fong LF, Crow JC, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod. 2006;21:924–9. doi: 10.1093/humrep/dei420. [DOI] [PubMed] [Google Scholar]
- 25.Ratts VS, Pauls RN, Pinto AB, Kraja A, Williams DB, Odem RR. Risk of multiple gestation after ovulation induction in polycystic ovary syndrome. J Reprod Med. 2007;52(10):896–900. [PubMed] [Google Scholar]
- 26.Lin K, Coutifaris C. In vitro fertilization in the polycystic ovary syndrome patient: An update. Clin Obstet Gynecol. 2007;50(1):268–76. doi: 10.1097/GRF.0b013e3180305fe4. [DOI] [PubMed] [Google Scholar]
- 27.Tummon I, Gavrilova-Jordan L, Allemand MC, Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstet Gynecol Scand. 2005;84:611–6. doi: 10.1111/j.0001-6349.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 28.Dokras A. Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):39–44. doi: 10.1055/s-2007-992923. [DOI] [PubMed] [Google Scholar]
- 29.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence and predictors of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–6. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 30.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 31.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effect of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:66–71. doi: 10.1210/jc.2004-0229. [DOI] [PubMed] [Google Scholar]
- 32.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–23. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 33.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436–40. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 35.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol. 2004;190(4):1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Robinson HE, O’Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106(6):1357–64. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 37.Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet Gynecol. 2002;100(5 Pt 1):959–64. doi: 10.1016/s0029-7844(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 38.D’Angelo R. Anesthesia-related maternal mortality: a pat on the back or a call to arms? Anesthesiology. 2007;106(6):1082–4. doi: 10.1097/01.anes.0000267587.42250.29. [DOI] [PubMed] [Google Scholar]
- 39.Wali A, Suresh MS. Maternal morbidity, mortality, and risk assessment. Anesthesiol Clin. 2008;26(1):197–230. ix. doi: 10.1016/j.anclin.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Kabiru W, Raynor BD. Obstetric outcomes associated with increase in BMI category during pregnancy. Am J Obstet Gynecol. 2004;191(3):928–32. doi: 10.1016/j.ajog.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 41.Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106(6):1096–104. doi: 10.1097/01.anes.0000267592.34626.6b. [DOI] [PubMed] [Google Scholar]
- 42.Hollander MH, Paarlberg KM, Huisjes AJ. Gestational diabetes: a review of the current literature and guidelines. Obstet Gynecol Surv. 2007;62(2):125–36. doi: 10.1097/01.ogx.0000253303.92229.59. [DOI] [PubMed] [Google Scholar]
- 43.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dokras A, Jagasia DH, Maifeld M, Sinkey CA, VanVoorhis BJ, Haynes WG. Obesity and insulin resistance but not hyperandrogenism mediates vascular dysfunction in women with polycystic ovary syndrome. Fertil Steril. 2006;86(6):1702–9. doi: 10.1016/j.fertnstert.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 46.Paradisi G, Steinberg HO, Hempfling A, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103(10):1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 47.Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem. 2004;50(12):2316–22. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]
- 48.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 49.Wild RA, Applebaum-Bowden D, Demers LM, et al. Lipoprotein lipids in women with androgen excess: independent associations with increased insulin and androgens. Clin Chem. 1990;36:283–9. [PubMed] [Google Scholar]
- 50.Dahlgren E, Johansson S, Lindstedt G, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505–13. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 51.Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction. Acta Obstet Gynecol Scand. 1992;71:599–604. doi: 10.3109/00016349209006227. [DOI] [PubMed] [Google Scholar]
- 52.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–51. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 53.Mahabeer S, Naidoo C, Norman RJ, Jialal I, Reddi K, Joubert SM. Metabolic profiles and lipoprotein lipid concentrations in non-obese and obese patients with polycystic ovarian disease. Horm Metab Res. 1990;22:537–40. doi: 10.1055/s-2007-1004966. [DOI] [PubMed] [Google Scholar]
- 54.Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997;126:32–5. doi: 10.7326/0003-4819-126-1-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 55.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol. 1992;37:119–25. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz M, Bukan N, Ayvaz G, et al. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum Reprod. 2005;20(12):3333–40. doi: 10.1093/humrep/dei258. [DOI] [PubMed] [Google Scholar]
- 57.Loverro G, Lorusso F, Mei L, Depalo R, Cormio G, Selvaggi L. The plasma homocysteine levels are increased in polycystic ovary syndrome. Gynecol Obstet Invest. 2002;53:157–62. doi: 10.1159/000058367. [DOI] [PubMed] [Google Scholar]
- 58.Randeva H, Lewandowski KC, Drzewoski J, et al. Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:4496–501. doi: 10.1210/jc.2001-012056. [DOI] [PubMed] [Google Scholar]
- 59.Vrbikova J, Bicikova M, Tallova J, Hill M, Starka L. Homocysteine and steroid levels in metformin-treated women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2002;110:74–7. doi: 10.1055/s-2002-23489. [DOI] [PubMed] [Google Scholar]
- 60.Clark R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 61.Kanani P, Sinkey C, Browning R, Allaman M, Knapp H, Haynes W. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocysteinemia in humans. Circulation. 1999;100:1161–8. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 62.Lakhani K, Seifalian AM, Hardiman P. Impaired carotid viscoelastic properties in women with polycystic ovaries. Circulation. 2002;106:81–5. doi: 10.1161/01.cir.0000020681.19400.8a. [DOI] [PubMed] [Google Scholar]
- 63.Paradisi G, Steinberg HO, Hempfling A, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 64.Shroff R, Kerchner A, Maifeld M, Van Beek EJR, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrin Metab. 2007;92(12):4609–14. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 65.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardol. 2000;36:1253–60. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 66.Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev. 2006;14(6):286–91. doi: 10.1097/01.crd.0000233757.15181.67. [DOI] [PubMed] [Google Scholar]
- 67.Third report of the National Cholesterol Education Program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) NIH Publication; 2001. Health NIo. 01-3670. [Google Scholar]
- 68.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 69.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106(1):131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 70.Reaven GM. Banting lecture: Role of insulin resistance in human disease. Diabetes. 1988;37:1597–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 71.Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome. A position statement of the Androgen Excess Society. J Clin Endocrin Metab. 2007;92(12):4546–56. doi: 10.1210/jc.2007-1549. [DOI] [PubMed] [Google Scholar]
- 72.Pierpoint T, McKeigue P, Isaacs AJ, Wild SH, Jacobs H. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51:581–6. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 73.Yen SS, Vela P, Rankin J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J Clin Endocrinol Metab. 1970;30:435–42. doi: 10.1210/jcem-30-4-435. [DOI] [PubMed] [Google Scholar]
- 74.Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990;70:699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- 75.Nestler JE, Barlascini CO, Matt DW, et al. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989;68:1027–32. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- 76.Barbieri RL, Makris A, Ryan KJ. Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet Gynecol. 1984;64:74S–80S. doi: 10.1097/00006250-198409001-00019. [DOI] [PubMed] [Google Scholar]
- 77.Iwashita M, Mimuro T, Watanabe M, et al. Plasma levels of insulin-like growth factor-I and its binding protein in polycystic ovary syndrome. Horm Res. 1990;33(Suppl 2):21–6. doi: 10.1159/000181561. [DOI] [PubMed] [Google Scholar]
- 78.Homburg R, Pariente C, Lunenfeld B, Jacobs HS. The role of insulin-like growth factor-I (IGF-I) and IGF binding protein in patients with polycystic ovarian disease. Hum Reprod. 1992;7:1379–83. doi: 10.1093/oxfordjournals.humrep.a137577. [DOI] [PubMed] [Google Scholar]
- 79.Suikkari AM, Ruutiainen K, Erkkola R, Seppala M. Low levels of low molecular weight insulin like growth factor binding protein in patients with polycystic ovarian disease. Hum Reprod. 1989;4:136–9. doi: 10.1093/oxfordjournals.humrep.a136858. [DOI] [PubMed] [Google Scholar]
- 80.Thierry van Dessel HJ, Lee PD, Faessen G, Fauser BC, Giudice L. Elevated serum levels of free insulin-like growth factor I in polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:3030–5. doi: 10.1210/jcem.84.9.5941. [DOI] [PubMed] [Google Scholar]
- 81.Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997;56:891–7. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- 82.Duleba AJ, Spaczynski RZ, Arici A, Carbone R, Behrman HR. Proliferation and differentiation of rat theca-interstitial cells: comparison of effects induced by platelet-derived growth factor and insulin-like growth factor-I. Biol Reprod. 1999;60:546–50. doi: 10.1095/biolreprod60.3.546. [DOI] [PubMed] [Google Scholar]
- 83.Duleba AJ, Spaczynski RZ, Olive DL. Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril. 1998;69:335–40. doi: 10.1016/s0015-0282(97)00473-1. [DOI] [PubMed] [Google Scholar]
- 84.Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Divergent mechanism regulate proliferation/survival and steroidogenesis of theca-interstitial cells. Mol Hum Reprod. 1999;5:193–8. doi: 10.1093/molehr/5.3.193. [DOI] [PubMed] [Google Scholar]
- 85.Duleba AJ, Spaczynski RZ, Tilly JL, Olive DL. Insulin and insulin-like growth factors protect ovarian theca-interstitial cells from apoptosis; 45th Ann Meeting of Soc Gynecol Investig; Atlanta, GA. 1998. [Google Scholar]
- 86.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–9. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 87.Potashnik R, Bloch-Damti A, Bashan N, Rudich A. IRS-1 degradation and increased serine phosphorylation cannot predict the degree of metabolic insulin resistance induced by oxidative stress. Diabetologia. 2003;46:639–48. doi: 10.1007/s00125-003-1097-5. [DOI] [PubMed] [Google Scholar]
- 88.Evans J, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7):1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 89.Paz K, Voliovitch H, Hadari YR, Roberts CT, LeRoith D, Zick Y. Interaction between the insulin receptor and its downstream effectors-use of individually expressed receptor domains for structure function analysis. J Biol Chem. 1996;271:6998–7003. doi: 10.1074/jbc.271.12.6998. [DOI] [PubMed] [Google Scholar]
- 90.Paz K, Hemi R, LeRoith D, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin- induced tryosine phosphoryalation. J Biol Chem. 1997;272:29911–8. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 91.Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:3110–6. doi: 10.1210/jcem.84.9.6010. [DOI] [PubMed] [Google Scholar]
- 92.Corbould A, Zhao H, Mirzoeva S, Aird F, Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes. 2006;55:751–9. doi: 10.2337/diabetes.55.03.06.db05-0453. [DOI] [PubMed] [Google Scholar]
- 93.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Clarendon Press; Oxford: 1999. [Google Scholar]
- 94.Paolisso G, Esposito R, D’Alessio MA, Barbieri M. Primary and secondary prevention of atherosclerosis: is there a role for antioxidants? Diabetes Metab. 2002;25:298–306. [PubMed] [Google Scholar]
- 95.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association, and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 96.Orio FJ, Palomba S, Cascela T, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;90:2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 97.Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34:407–13. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 98.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–5. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 99.Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–8. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- 100.Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19(7):1519–24. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- 101.Naz RK, Thurston D, Santoro N. Circulating tumor necrosis factor (TNF)-alpha in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am J Reprod Immunol. 1995;34:170–5. doi: 10.1111/j.1600-0897.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism: Clin Experim. 1999;48:437–41. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 103.Adamson GM, Billings RE. Tumor necrosis factor induced oxidative stress in isolated mouse hepatocytes. Arch Biochem Biophys. 1992;294:223–9. doi: 10.1016/0003-9861(92)90161-o. [DOI] [PubMed] [Google Scholar]
- 104.Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2 generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–13. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rifici VA, Schneider SH, Khachadurian AK. Stimulation of low-density lipoprotein oxidation by insulin and insulin like growth factor I. Atherosclerosis. 1994;107:99–108. doi: 10.1016/0021-9150(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 106.Ruiz-Gines JA, Lopez-Ongil S, Gonzalez-Rubio M, Gonzalez -S L, Rodriguez-Puyol M, Rodriguez-Puyol D. Reactive oxygen species induce proliferation of bovine aortic endothelial cells. J Cardiovasc Pharmacol. 2000;35:109–13. doi: 10.1097/00005344-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 107.Azzi A, Aratri E, Boscoboinik D, et al. Molecular basis of alpha-tocopherol control of smooth muscle cell proliferation. Biofactors. 1998;7:3–14. doi: 10.1002/biof.5520070102. [DOI] [PubMed] [Google Scholar]
- 108.Ivanov VO, Ivanova SV, Niedzwiecki A. Ascorbate affects proliferation of guinea pig vascular smooth muscle cells by direct and extracellular matrix-mediated effects. J Mol Cell Cardiol. 1997;29:3293–303. doi: 10.1006/jmcc.1997.0555. [DOI] [PubMed] [Google Scholar]
- 109.Nesaretnam K, Stephen R, Dils R, Darbre P. Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids. 1998;33:461–9. doi: 10.1007/s11745-998-0229-3. [DOI] [PubMed] [Google Scholar]
- 110.Onat D, Boscoboinik D, Azzi A, Basaga H. Effects of alpha-tocopherol and silibin dihemisuccinate on the proliferation of human skin fibroblasts. Biotechnol Appl Biochem. 1999;29:213–5. [PubMed] [Google Scholar]
- 111.Piotrowski P, Rzepczynska I, Kwintkiewicz J, Duleba AJ. Oxidative stress induces expression of CYP11A, CYP17, StAR and 3bHSD in rat theca-interstitial cells; 52nd Annual Meeting of the Society for Gynecologic Investigation; Los Angeles, CA. 2005.Mar 23-26, [Google Scholar]
- 112.Anderson RN, Smith BL. Deaths: Leading Causes for 2002. National Vital Statistics. 2005;53(17) [PubMed] [Google Scholar]
- 113.Kodaman PH, Duleba AJ. Medical management of polycystic ovarian syndrome and its sequelae. In: Seli E, Arici A, editors. Noninvasive Management of Gynecological Disorders. 2007. [Google Scholar]
- 114.Anonymous Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 115.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 116.Shepard J, Cobbe SM, Ford I, et al. West of Scotland Coronary Prevention Study Group Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 117.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;(279) doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 118.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 119.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochem Biophys Acta. 2004;1660:171–99. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 120.Carlberg M, Dricu A, Blegen H, et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-I receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl coenzyme A reductase and cell growth. J Biol Chem. 1996;271:17453–62. doi: 10.1074/jbc.271.29.17453. [DOI] [PubMed] [Google Scholar]
- 121.Zhang FL, Casey PJ. Protein prenylation: molecular mechanism and functional consequences. Ann Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 122.Goalstone ML, Leitner JW, Wall K, et al. Effect of insulin on farnesyltransferase. Specificity of insulin action and potentiation of nuclear effects of insulin-like growth factor-1, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1998;273:23892–6. doi: 10.1074/jbc.273.37.23892. [DOI] [PubMed] [Google Scholar]
- 123.Goalstone ML, Draznin B. Effect of insulin on farnesyltransferase activity in 3T3-L1 adipocytes. J Biol Chem. 1996;271:27585–9. doi: 10.1074/jbc.271.44.27585. [DOI] [PubMed] [Google Scholar]
- 124.Goalstone ML, Leitner JW, Golovchenko I, et al. Insulin promotes phosphorylation and activation of geranylgeranyltransferase II. Studies with geranylgeraylation of rab-3 and rab-4. J Biol Chem. 1999;274:2880–4. doi: 10.1074/jbc.274.5.2880. [DOI] [PubMed] [Google Scholar]
- 125.Barbieri RL, Makris A, Ryan KJ. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil Steril. 1983;40:237–41. [PubMed] [Google Scholar]
- 126.McCarty MF. Insulin’s stimulation of endothelial superoxide generation may reflect up-regulation of isoprenyl transferase activity that promotes rac translocation. Med Hypotheses. 2002;58:472–5. doi: 10.1054/mehy.2001.1455. [DOI] [PubMed] [Google Scholar]
- 127.Draznin B, Miles P, Kruszynska Y, et al. Effects of insulin on prenylation as a mechanism of potentially detrimental influence of hyperinsulinemia. Endocrinol. 2000;141:1310–6. doi: 10.1210/endo.141.4.7411. [DOI] [PubMed] [Google Scholar]
- 128.Goalstone ML, Draznin B. What does insulin do to Ras? Cell Signal. 1998;10:297–301. doi: 10.1016/s0898-6568(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 129.Solomon CS, Leitner JW, Goalstone ML. Dominant negative alpha-subunit of farnesyl- and geranylgeranyl-transferase I inhibits insulin-induced differentiation of 3T3-L1 pre-adipocytes. Int J Obes Relat Metab Disord. 2003;27:40–7. doi: 10.1038/sj.ijo.0802189. [DOI] [PubMed] [Google Scholar]
- 130.Siddals KW, Marshman E, Westwood M, Gibson JM. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion; synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279:38353–9. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- 131.Collier E, Carpentier JL, Beitz L, Carol H, Taylor SI, Gorden P. Specific glycosylation site mutations of the insulin receptor alpha subunit impair intracellular transport. Biochem. 1993;32:7818–23. doi: 10.1021/bi00081a029. [DOI] [PubMed] [Google Scholar]
- 132.Hwang JB, Frost SC. Effect of alternative glycosylation on insulin receptor processing. J Biol Chem. 1999;274:22813–20. doi: 10.1074/jbc.274.32.22813. [DOI] [PubMed] [Google Scholar]
- 133.Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005;30(2):263–70. doi: 10.1007/s11064-005-2449-y. [DOI] [PubMed] [Google Scholar]
- 134.Wassmann S, Laufs U, Muller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–5. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 135.Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Cell Physiol. 2003;285:C723–34. doi: 10.1152/ajpcell.00230.2003. [DOI] [PubMed] [Google Scholar]
- 136.Franzoni F, Quinones-Galvan A, Regoli F, Ferrannini E, Galetta F. A comparative study of the in vitro antioxidant activity of statins. Int J Cardiol. 2003;90:317–21. doi: 10.1016/s0167-5273(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 137.Avram M, Dankner G, Cogan U, Hochgraf E, Brook JGW. Lovastatin inhibits low-density lipoprotein oxidation and alters its fluidity and uptake by macrophages: in vitro and in vivo studies. Metabolism. 1992;41:229–35. doi: 10.1016/0026-0495(92)90263-a. [DOI] [PubMed] [Google Scholar]
- 138.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 139.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–6. [PubMed] [Google Scholar]
- 140.Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ. Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. Fertil Steril. 2004;82:1193–7. doi: 10.1016/j.fertnstert.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 141.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–31. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 142.Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000;35:619–29. doi: 10.1097/00005344-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 143.Buemi M, Allegra A, Senatore M, et al. Pro-apoptotic effect of fluvastatin on human smooth muscle cells. Eur J Pharmacol. 1999;370:201–3. doi: 10.1016/s0014-2999(99)00122-3. [DOI] [PubMed] [Google Scholar]
- 144.El-Ani D, Zimlichman R. Simvastatin induces apoptosis of cultured rat cardiomyocytes. J Basic Clinical Physiol Pharmacol. 2001;12(4):325–38. doi: 10.1515/jbcpp.2001.12.4.325. [DOI] [PubMed] [Google Scholar]
- 145.Danesh FR, Sadeghi MM, Amro N, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/p21 signaling pathway: implications for diabetic nephropathy. Proc Natl Acad Sci USA. 2002;99:8301–5. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu CH, Lee SC, Chiu HH, et al. Morphologic change and elevation of cortisol secretion in cultured human normal adrenocortical cells caused by mutant p21K-ras protein. DNA Cell Biol. 2002;21:21–9. doi: 10.1089/10445490252810285. [DOI] [PubMed] [Google Scholar]
- 147.Dobs AS, Schrott H, Davidson MH, et al. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism: Clinical & Experimental. 2000;49:1234–8. doi: 10.1053/meta.2000.7716a. [DOI] [PubMed] [Google Scholar]
- 148.Rzepczynska I, Piotrowski P, Kwintkiewicz J, Duleba AJ. Effect of mevastatin on expression of CYP17, 3bHSD, CYP11A and StAR in rat theca-interstitial cells; 52nd Annual Meeting of the Society for Gynecologic Investigation; Los Angeles, CA. 2005.Mar 23-26, [Google Scholar]
- 149.Felemban A, Tulandi T. Laparoscopic treatment of polycystic ovarian syndrome-related infertility. Infertil Reprod Med Clin North Am. 2000;11:49–60. [Google Scholar]
- 150.Piotrowski P, Kwintkiewicz J, Rzepczynska I, Duleba AJ. Simvastatin and mevastatin inhibit expression of NADPH oxidase subunits: p22phox and p47phox in rat theca-interstitial cells; 52nd Annual Meeting of the Society for Gynecologic Investigation; Los Angeles, CA. 2005.Mar 23-26, [Google Scholar]
- 151.Kwintkiewicz J, Foyouzi N, Piotrowski P, Rzepczynska I, Duleba AJ. Mevastatin inhibits proliferation of rat ovarian theca-interstitial cells by blocking the mitogen activated protein kinase pathway. Fertil Steril. 2006;86:1053–8. doi: 10.1016/j.fertnstert.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 152.Duleba AJ, Piotrowski P, Kwintkiewicz J, Rzepczynska I. Proliferation of theca-interstitial cells is sensitive to inhibition of glycosylation. Abstract American Society for Reproductive Medicine. 2007 [Google Scholar]
- 153.Hernandez R, Teruel T, Lorenzo M. Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett. 2001;494:225–31. doi: 10.1016/s0014-5793(01)02353-5. [DOI] [PubMed] [Google Scholar]
- 154.Welsh GI, Hers I, Berwick DC, et al. Role of protein kinase B in insulin-regulated glucose uptake. Biochem Soc Trans. 2005;33:346–9. doi: 10.1042/BST0330346. [DOI] [PubMed] [Google Scholar]
- 155.Duleba AJ, Banaszweska B, Spaczynski RZ, Pawelczyk L. Simvastatin improves biochemical parameters of polycystic ovary syndrome: results of a prospective randomized trial. Fertil Steril. 2006;85(4):996–1001. doi: 10.1016/j.fertnstert.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 156.Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective randomized cross-over trial. J Clin Endocrin Metab. 2007;92(2):456–61. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- 157.Preiss J, Sattar N. Vascular cell adhesion molecule-1: a viable therapeutic target for atherosclerosis? Int J Clinical Practice. 2007;61(4):697–701. doi: 10.1111/j.1742-1241.2007.01330.x. [DOI] [PubMed] [Google Scholar]
- 158.Rasmussen LM, Hansen PR, Nabipour MT, Olesen P, Kristiansen MT, Ledet T. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem J. 2001;360:363–70. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906–8. doi: 10.1016/S1701-2163(16)32656-1. [DOI] [PubMed] [Google Scholar]
- [1].Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]