Abstract
Background
The risks of bariatric surgical procedures must be balanced against their benefits and require further characterization.
Methods
Longitudinal Assessment of Bariatric Surgery-1 (LABS-1) was a prospective, multi-center observational study of 30-day outcomes in consecutive patients undergoing bariatric surgical procedures at 10 clinical sites in the United States (2005-2007). A composite endpoint of 30-day major adverse outcomes (death; venous thromboembolism; percutaneous, endoscopic, or operative reintervention; no discharge) was evaluated among patients undergoing first-time bariatric surgery.
Results
There were 4776 patients (mean age 44.5 years, 21.1% male, 10.9% non-white, median BMI of 46.5 kg/m2) who had a first-time procedure. Over half had at least two comorbid conditions. Roux-en-y gastric bypass was performed in 3412 (87.2% laparoscopic) and laparoscopic adjustable gastric banding in 1198. The 30-day mortality rate for Roux-en-y gastric bypass or laparoscopic adjustable gastric banding was 0.3%; 4.3% of participants had at least one major adverse outcome. A history of deep vein thrombosis or pulmonary embolus, obstructive sleep apnea, and functional status were each independently associated with increased risk of the composite endpoint. Extreme values of BMI were significantly associated with an increased risk of the composite endpoint, while age, sex, race, ethnicity and other co-morbid conditions were not.
Conclusion
The overall risk of death and adverse outcome after bariatric surgery was low, varying considerably with patient characteristics. In helping patients make appropriate choices, short-term safety should be considered in conjunction with both the longer term effects of bariatric surgery and the risk of living with extreme obesity.
Keywords: bariatric surgery, safety, LABS, predictors, outcomes, risk, obesity
The benefits of bariatric surgery are increasingly reported. A recent, small randomized trial [1] noted remission of diabetes in a majority of patients undergoing bariatric surgery, and the favorable impact of bariatric surgery on cardiovascular disease was demonstrated in a large, matched cohort undergoing surgery or usual care [2]. Recent studies [3, 4] reported approximately 35% lower risk of death over time in extremely obese patients receiving bariatric surgery compared to those who did not. Yet, concerns about the safety of bariatric surgery have grown with its increasing popularity, heightened by periodic high-profile reports in the lay press of deaths after bariatric surgery and the closure or threatened suspension of bariatric programs for safety issues. Malpractice insurers have expressed concerns about their increased risk when providing liability insurance coverage to bariatric surgeons; further, some reports suggest higher-than-expected rates of death in high-risk populations undergoing bariatric surgery [5, 6].
Determining the incidence of infrequent adverse outcomes and the factors associated with them requires large prospective cohorts with standardized, pre-surgical evaluation and complete assessment of outcomes. The Longitudinal Assessment of Bariatric Surgery (LABS) is a prospective, multi-center observational cohort study [7] that uses a standardized assessment in consecutive patients undergoing bariatric surgery (LABS-1). This report presents the incidence of, and factors associated with, 30-day safety outcomes in patients from this cohort undergoing an initial bariatric surgical procedure.
Methods
Patients
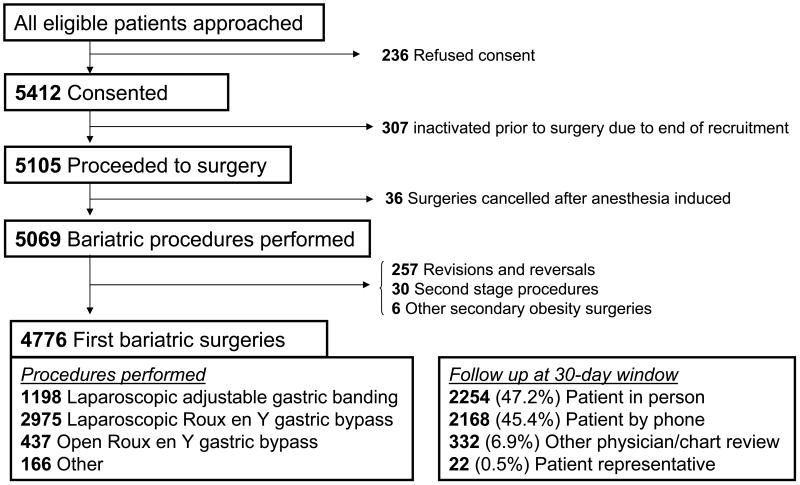
The study included consecutive patients 18 years or older who underwent bariatric surgical procedures between March 11, 2005 and December 31, 2007, by 33 LABS-certified surgeons (see list of centers and data coordinating center). The LABS consortium designed the study, gathered data, assured data quality and analysis, wrote the paper, and decided to publish it. The study protocol and consent form were approved by the Institutional Review Board at each institution [7]. Surgeons were certified to participate in LABS, but bariatric surgical accreditation did not exist when LABS began. By December 31, 2007, 5648 patients were approached for the study and 4776 underwent primary operations. Roux-en-y gastric bypass was performed either laparoscopically (LRYGB) or through an “open” approach (ORYGB); laparoscopic adjustable gastric banding (LAGB) was considered separately. Procedures started laparoscopically and “converted” to open surgery were considered open. Procedures that comprised less than 3% of all procedures (biliopancreatic diversion with or without a duodenal switch, sleeve gastrectomy, vertical banded gastroplasty, and open adjustable gastric banding) were excluded in outcome analyses.
Data Definitions
Details of the pre-operative, operative, and post-operative data collection forms and definitions have been previously reported [7]. The pre-operative evaluation was completed through in-person interview, physical examination, and chart review by LABS-certified data collectors. Standardized protocols and instruments were used to measure weight, height, and blood pressure within 30 days prior to surgery. Comorbid conditions were self-reported, and severity was based on associated healthcare utilization (e.g., patients were asked if they had sleep apnea; if so, whether they used a continuous positive airway pressure machine). The primary outcome was a composite endpoint (CE) of any of the following occurring within 30 days of surgery: death; deep vein thrombosis (DVT) or venothromboembolism (VTE); reintervention using percutaneous, endoscopic, or operative techniques; or failure to discharge from the hospital within 30 days of surgery. We did not consider readmission per se as an adverse event due to the variable severity of problems that lead to readmission. We did not collect information on insurance status and research funds were not used to pay for procedures.
Sample Size Calculation
Enrollment was dictated by the number of procedures performed by participating surgeons. Prior to patient enrollment, the sample sizes needed to have 90% power to detect a doubling in the risk of selected outcome events occurring with various incidences were calculated, given the prevalence of several putative risk factors.
Statistical Analyses
Participant characteristics across procedures were compared using Pearson's chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. The incidence of 30-day adverse outcomes was compared across procedures using the Pearson chi-square test. Generalized linear mixed effect models were used to evaluate predictors of the composite endpoint, modeling the log-odds of events as a linear function of baseline covariates. Correlation among patients of the same surgeon was accounted for by including different random intercepts for sites and for surgeons within site. Linear and quadratic effects of BMI on the composite endpoint were considered, as the unadjusted analysis showed a quadratic relationship. For multivariable models, a stepwise variable selection was performed, starting with all the variables from univariate models with p<0.25 as potential predictors. Variables were eliminated if the p-value was greater than 0.10 and the process was continued. Following inclusion of main effects with p<0.10, variables previously considered were again included in models to determine whether further adjustment changed the p-value of those effects. The final model included only main effects with p<0.10 at which point two-way interactions among all the main effects were evaluated. Predicted probabilities of the composite endpoint were calculated based on the multivariable model. All p-values reported are two-sided.
Results
Of 4776 patients undergoing first time or “primary” bariatric procedures, the mean age (±standard deviation) was 44.5 (±11.5) years. The median BMI was 46.5 kg/m2; 21.2% of participants were men. The most common procedure was roux-en-y gastric bypass in 3412 (71.4%), 87.2% of which were laparoscopic and 12.8% open. 25.1% of the participants underwent laparoscopic adjustable gastric banding and comorbid conditions were common (Supplemental Table 1)-- 82.1% of patients had at least one; 53.9%, two or more; and 26.5%, three or more. Most common were hypertension (55.1%), obstructive sleep apnea (48.9%), diabetes (33.2%), and asthma (23.1%). Other relatively less frequent comorbid conditions included ischemic heart disease (4.4%), venous edema with ulcerations (4.0%), history of DVT/VTE (3.5%), supplemental oxygen dependence (3.5%), congestive heart failure (2.2%), inability to walk 200 ft (1.8%), and pulmonary hypertension (1.2%). Preoperative medication use included antidepressants (39.9%), statins (26.6%), beta-blockers (17.9%) and narcotics (16.1%).
Compared to patients having laparoscopic roux-en-y gastric bypass or laparoscopic adjustable gastric banding, patients undergoing open roux-en-y gastric bypass had higher BMI (median BMI (kg/m2): 50.9 for open roux-en-y gastric bypass, 46.9 for laparoscopic roux-en-y gastric bypass, 44.1 for laparoscopic adjustable gastric banding, p=<0.001) and more comorbid conditions (p=<0.001). Patients undergoing open roux-en-y gastric bypass generally had the most severe comorbid issues (e.g., use of insulin for diabetes, use of assistive devices for inability to walk at least 200 feet); patients undergoing either laparoscopic roux-en-y gastric bypass or open roux-en-y gastric bypass had more comorbid conditions than those undergoing laparoscopic adjustable gastric banding (Supplemental Table 1).
Outcomes analyses excluded the 166 patients who underwent relatively rarely performed operations (Table 2) since the putative risks are quite disparate and the frequencies, too small for meaningful analyses. Within 30 days of surgery, 0.3% of the patients died, ranging from none in 1198 undergoing laparoscopic adjustable gastric banding to 0.2% in 2975 laparoscopic roux-en-y gastric bypass to 2.1% among 437 open roux-en-y gastric bypass. The composite endpoint of death, DVT/VTE, reintervention, or no discharge at 30 days occurred in 4.1% of patients (1.0% in laparoscopic adjustable gastric banding; 4.8% in laparoscopic roux-en-y gastric bypass; 7.8% in open roux-en-y gastric bypass [Table 2]). Open roux-en-y gastric bypass patients whose surgery was converted from laparoscopic to open had a lower incidence of composite endpoint than patients whose surgery began as open (3.9% vs. 8.3%, respectively). The most commonly occurring components of the endpoint were abdominal reoperation (2.6%) and endoscopic intervention (1.1%). While the scheme for reporting adverse outcomes relied on reintervention we collected information on intraoperative events. There was one unplanned splenectomy during a gastric bypass and three patients underwent a concurrent blood vessel repair/ligation secondary to bleeding. Data about postoperative bleeding resolving without reintervention were not collected.
Table 2. Frequency of Adverse Outcomes within 30 days, by Surgical Procedure.
| Characteristic | Total (N=4610*) |
LAGB (N=1198) |
LRYGB (N=2975) |
ORYGB (N=437) |
p value** |
|---|---|---|---|---|---|
| Death, n (%) | 15 (0.3) | 0 (0.0) | 6 (0.2) | 9 (2.1) | <0.001 |
| DVT or VTE, n (%) | 20 (0.4) | 3 (0.3) | 12 (0.4) | 5 (1.1) | 0.05 |
| Tracheal reintubation, n (%) | 20 (0.4) | 2 (0.2) | 12 (0.4) | 6 (1.4) | 0.004 |
| Endoscopy, n (%) | 51 (1.1) | 1 (0.1) | 45 (1.5) | 5 (1.1) | <0.001 |
| Operation | |||||
| Tracheostomy, n (%) | 11 (0.2) | 0 (0.0) | 6 (0.2) | 5 (1.1) | 0.001 |
| Placement of percutaneous drain, n (%) | 16 (0.5) | 0(0.0) | 13 (0.4) | 3 (0.7) | 0.48 |
| Abdominal operation, n (%) | 118 (2.6) | 9 (0.8) | 94 (3.2) | 15 (3.4) | <0.001 |
| Not discharged by day 30, n (%) | 17 (0.4) | 0 (0.0) | 13 (0.4) | 4 (0.9) | 0.02 |
| Composite event***, n (%) | 189 (4.1) | 12 (1.0) | 143 (4.8) | 34 (7.8) | <0.0001 |
Excluding 166 “Other”, i.e., SG (n=117), BPD/BPDS (n=47), VBG (n=1), OAGB (n=1)
Chi-square test
Death, DVT/VTE, reintervention using percutaneous, endoscopic or operative techniques, or failure to discharge from the hospital within 30 days of surgery.
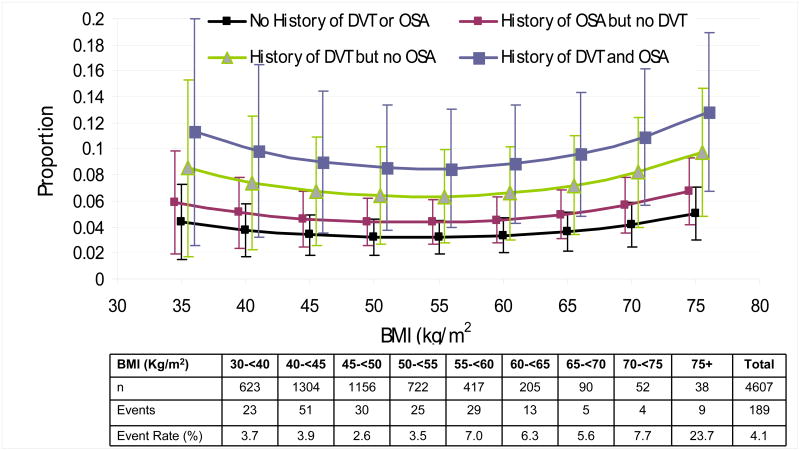
Several patient characteristics and procedure type were associated with the composite endpoint in unadjusted analyses (Supplemental Table 2). Due to interrelationships among several of those variables, after adjustment (Supplemental Table 2), only type of procedure (open and laparoscopic roux-en-y gastric bypass compared to laparoscopic adjustable gastric banding), extremes of BMI, inability to walk 200 feet, history of DVT/VTE, and history of obstructive sleep apnea were significantly associated with the composite endpoint. Adjusting for significant patient characteristics, the risk of the composite endpoint was 4.8- or 5.8-fold higher for laparoscopic roux-en-y gastric bypass or open roux-en-y gastric bypass, respectively compared to laparoscopic adjustable gastric banding. After adjustment, there was no statistically significant difference in the odds of the composite endpoint for open roux-en-y gastric bypass compared to laparoscopic roux-en-y gastric bypass, irrespective of including conversions from laparoscopic to open (odds ratio=1.21, 95% confidence interval 0.71-2.04) or not (odds ratio=1.38, 95% confidence interval 0.77-2.49). Independent of procedure type, the predicted probability of the composite endpoint was lowest for participants without a history of DVT/VTE or obstructive sleep apnea and in the middle range of the spectrum of BMI for the cohort. The estimated percentage of participants with the composite endpoint ranged from approximately 3% for patients without a history of obstructive sleep apnea or VTE and a BMI in the 50s to over 10% for patients with a history of both DVT/VTE and obstructive sleep apnea and a BMI of 70 kg/m2 (Fig. 2). Despite higher point estimates for the model-based predicted probability of the composite endpoint in people with BMIs lower than 53 kg/m2, the value at which the predicted probability of the composite endpoint is lowest, the confidence intervals about those estimates are wide; only 13.5% of procedures and 12.2% of events occurred in patients with a BMI under 40.
Figure 2. Predicted Probabilities of Adverse Outcomes, According to a History of Deep-Vein Thrombosis or Venous Thromboembolism (DVT) or Obstructive Sleep Apnea (OSA).
Probabilities of outcomes are shown as a function of the body-mass index (the weight in kilograms divided by the square of the height in meters) and have been adjusted according to functional status (ability or lack of ability to walk 61 m [approximately 200 ft]) and surgical procedures on the basis of the multivariable model (for details, see Table 2 in the Supplementary Appendix).
Discussion
This study reports major peri-surgical adverse outcomes in a recent cohort of patients undergoing the most common bariatric surgical procedures performed by experienced surgeons in established US centers. Despite multiple co-morbidities in this severely obese population, the overall risk of 30-day mortality (0.3%) and major adverse outcomes was low. Specific presurgical health conditions and more extreme obesity were associated with more adverse outcomes within 30 days of surgery. These higher-risk characteristics were more common among patients undergoing more invasive procedures, who also had a higher risk of events.
Patient (male sex, medical conditions, higher BMI), operative (based on degree of invasiveness), surgeon and site characteristics have been thought to increase the risk of adverse outcomes. LABS-1 provides standardized, prospective data on a sufficiently large cohort from multiple centers to evaluate potential factors associated with safety outcomes. Understanding the factors underlying risk is imperative for developing risk stratification models both for comparing hospital and surgeon outcomes and in better advising patients. The complex interplay of factors associated with adverse outcomes is challenging for determining a predictive model for risk in bariatric surgery. At least one scoring system of risk created from a single-site retrospective cohort including age, BMI, sex, hypertension, and VTE risk has been proposed [8] and validated in an alternative cohort [9]. Of these, only BMI and history of VTE were independently associated with the composite endpoint in LABS.
Although higher BMI is reported to increase patient risk following bypass procedures [5], LABS-1 observed that BMI had a quadratic relationship to the predicted probability of the composite endpoint. The lowest predicted risk was found at BMI of 53 kg/m2. The odds of the composite endpoint was 61% higher for patients with BMI 75 kg/m2, compared to those with BMI of 53 kg/m2 (OR 1.61; 95% CI [1.04,2.48]). The predicted risk of the composite endpoint among patients with lower BMI was also higher compared to those at a BMI of 53, but the OR did not differ significantly (p>0.05) for any of the BMI values below 53 kg/m2.
Although Black/African Americans undergoing bariatric surgery have been reported to lose less weight than White/Caucasians after bariatric surgery [10] [11] [12], disparity in safety outcome has not been rigorously evaluated. Safety outcomes may turn out to be independent from weight loss outcomes. The LABS centers' experience may be unique, and perhaps prior studies showing race-based outcome differences did not adequately control for the relationship between race and other factors. Further, the statistical power to disentangle race from other confounding factors may be inadequate in LABS-1, since only 10.9% of patients was non-white. Interestingly, although older age has been associated with an increased likelihood of adverse outcomes in many studies [6] [13] [14], age was not significantly associated with outcome in LABS. This may be explained in part either by the relatively small number of older patients (over half the participants were in their 30s or 40s) or by preferential selection of healthier participants. In the LABS-1 cohort, older patients had, on average, a lower BMI compared to younger patients [15]. A recent single surgeon report of 1000 roux-en-y gastric bypass procedures observed that obstructive sleep apnea conferred a 3-fold increased risk of perioperative mortality [16]. Whether an established diagnosis of obstructive sleep apnea is a marker of other factors predictive of adverse outcome (perhaps through increased use of obstructive sleep apnea screening in patients at high risk) or whether obstructive sleep apnea itself truly confers an increased risk of the composite endpoint is not determined. A history of DVT/VTE is a well known risk factor for subsequent DVT/VTE events.
Procedure type was clearly associated with different risk of the composite endpoint. Patients having an open roux-en-y gastric bypass after an attempted laparoscopic bypass had a similar rate of the composite endpoint as those with successful laparoscopic bypass, while the rate of the composite endpoint in patients with an intended open bypass was two-fold higher. This effect persisted after adjustment for other patient characteristics and surgical center.
When choosing among different bariatric procedures, short-term safety may not be the only relevant metric. Laparoscopic adjustable gastric banding and laparoscopic roux-en-y gastric bypass affect weight and obesity-related conditions relevant to patients differently. In most patients, roux-en-y gastric bypass impacts glycemic control even before weight loss occurs [17], distinct from the weight loss-dependent effects of laparoscopic adjustable gastric banding [1] [18]. Data concerning effectiveness and durability of effect are critical [19]. While LABS-1 was designed to evaluate short-term safety events, LABS-2, a long-term prospective cohort evaluation which has just finished recruitment (last procedure performed, April, 2009; longer term follow-up available in mid-2010 or later), intends to assess the impact of these operations on health conditions, quality of life, health economic issues, diet and exercise behavior and other psychosocial issues. While LABS-1 patients were recruited from consecutive patients undergoing surgery, LABS-2 patients constitute both a subset of the larger LABS-1 and patients recruited after LABS-1 recruitment ended. Importantly, the LABS-2 protocol delineates hypotheses that require longer than 30 day follow-up and the sample size for LABS-2 was calculated to provide adequate power to test those hypotheses.
Our study has several limitations, noted in a methods paper [17] and summarized here. Whether these findings would be those expected in the general community is unknown. Recent administrative data report improved in-hospital outcome following bypass surgery [20] [21] [22]. Due to Center of Excellence programs and formal training programs, we anticipate that the low rates of perioperative death and adverse outcomes seen in LABS centers will be achievable elsewhere. The present study is a large, multi-centered, prospective study with some subgroups that are large, but the limited size of some patient subsets may have resulted in a Type II error that failed to identify a differential in safety between groups. Although we found expected differences in rates of safety events between procedures based on their degree of invasiveness, between-procedure comparisons based on a common safety metric may not be appropriate. Some adverse outcomes are only expected to occur for certain procedures (e.g., leaks of gastrointestinal anastomoses are a risk in roux-en-y gastric bypass but not in laparoscopic adjustable gastric banding), so a common metric inclusive of reoperation biases against procedures with anastomoses. The present study is further limited by patient self-report to describe the preoperative existence and severity of conditions. Lastly, while a center's or surgeon's case volume is worthwhile in evaluating outcomes, we could not determine center volume, because at some centers not all surgeons participated in the LABS consortium, and surgeons in LABS were certified by the consortium to participate at various times during the year. Thus, the actual surgical volume for that year may not have been captured. Multivariable analysis adjusted for center, but not explicitly for center volume.
Obesity remains a major cause of morbidity and mortality, and bariatric surgery appears to be the only intervention consistently resulting in substantial, sustained weight loss. Safety of such surgery is an important consideration, and our report indicates that the incidence of death and adverse events within 30 days of bariatric surgery is low but varied among different risk groups. These short-term risks should be considered in the context of the longer-term health effects of surgically induced weight loss on co-morbid health conditions, the longer term risks of the bariatric surgery itself, the competing risk of death from extreme obesity, and the relative benefits of the rate and durability of weight loss.
Supplementary Material
Figure 1. Enrollment, Treatment, and Follow-up of the Patients.
Table 1.
Characteristics of LABS-1 Participants with No Previous Bariatric Surgical Procedure
| Characteristic | Total* (N=4776) |
LAGB (N=1198) |
LRYGB (N=2975) |
ORYGB (N=437) |
SG (N=117) |
BPD-DS (N=47) |
p value** |
|---|---|---|---|---|---|---|---|
| Patient age (years), mean ± SD | 44.5±11.5 | 46.0±12.5 | 43.6±11.0 | 45.9±10.7 | 46.3±13.6 | 43.9±10.7 | <0.001 |
| Patient age (years), n (%) | <0.001 | ||||||
| <30 years | 513 (10.7) | 133 (11.1) | 321 (10.8) | 37 (8.5) | 18 (15.4) | 4 (8.5) | |
| 30-<40 years | 1196 (25.0) | 244 (20.4) | 826 (27.8) | 92 (21.1) | 18 (15.4) | 16 (34.0) | |
| 40-<50 years | 1380 (28.9) | 337 (28.1) | 868 (29.2) | 134 (30.7) | 28 (23.9) | 13 (27.7) | |
| 50-<60 years | 1200 (25.1) | 300 (25.0) | 728 (24.5) | 128 (29.3) | 34 (29.1) | 8 (17.0) | |
| 60-<65 years | 318 (6.7) | 93 (7.8) | 172 (5.8) | 36 (8.2) | 12 (10.3) | 5 (10.6) | |
| 65+ years | 169 (3.5) | 91 (7.6) | 60 (2.0) | 10 (2.3) | 7 (6.0) | 1 (2.1) | |
| BMI (kg/m2), median (25th, 75th percentiles) | 46.5 (42.1,52.4) | 44.1 (40.5, 49.0) | 46.9 (42.7, 52.3) | 50.9 (44.7, 60.0) | 56.2 (44.4, 62.5) | 48.9 (43.2, 53.1) | <0.001 |
| BMI (kg/m2), n (%) | <0.001 | ||||||
| <40 kg/m2 | 640 (13.4) | 262 (21.9) | 332 (11.2) | 30 (6.9) | 10 (8.5) | 6 (12.8) | |
| 40-<50 kg/m2 | 2518 (52.7) | 674 (56.3) | 1613 (54.2) | 175 (40.0) | 34 (29.1) | 21 (44.7) | |
| 50-<60 kg/m2 | 1188 (24.9) | 205 (17.1) | 810 (27.2) | 123 (28.1) | 31 (26.5) | 18 (38.3) | |
| 60+ kg/m2 | 430 (9.0) | 57 (4.8) | 220 (7.4) | 109 (24.9) | 42 (35.9) | 2 (4.3) | |
| Male, n (%) | 1009 (21.1) | 277 (23.1) | 534 (17.9) | 140 (32.0) | 42 (35.9) | 16 (34.0) | <0.001 |
| Non-white, n (%) | 516 (10.9) | 130 (11.0) | 338 (11.5) | 31 (7.1) | 14 (12.0) | 3 (6.4) | 0.07 |
| missing, n | 46 | 14 | 32 | 0 | 0 | 0 | |
| Hispanic, n (%) | 293 (6.1) | 66 (5.5) | 196 (6.6) | 16 (3.7) | 13 (11.1) | 2 (4.3) | 0.02 |
| missing, n | 2 | 1 | 1 | 0 | 0 | 0 | |
| Smoker within last year, n (%) | 679 (14.2) | 124 (10.4) | 458 (15.4) | 79 (18.1) | 14 (12.0) | 4 (8.5) | <0.001 |
| missing, n | 1 | 0 | 0 | 1 | 0 | 0 |
Two people underwent either vertical banded gastroplasty or adjustable gastric banding).
Chi-square test for categorical variables, Kruskal-Wallis test for continuous variables
Abbreviations: LRYGB = laparoscopic roux-en-y gastric bypass, ORYGB = open roux-en-y gastric bypass; LAGB= laparoscopic adjustable gastric banding; SG=sleeve gastrectomy; BPD-DS = biliopancreatic diversion with or without a duodenal switch
LABS Study Acknowledgments
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia University - U01-DK66667; University of Washington - U01-DK66568 (in collaboration with GCRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585; Oregon Health & Science University – U01-DK66555.
Footnotes
Disclosure: Dr. Chapman reports receiving grant support from Covidien and has served as an expert witness on legal cases involving MIS and bariatric surgery. Dr. Courcoulas reports receiving grant support from Allergan, Covidien, and Pfizer. Dr. Flum reports receiving grant support from Sanofi-Aventis and Covidien and has served as an expert witness on cases involving adverse events after bariatric surgery. Dr. McCloskey reports receiving grant support from Stryker. Dr. Mitchell reports receiving grant support from GlaxoSmithKline, Lilly Research and Pfizer. Dr. Patterson reports receiving consulting fees from Allergan Health; lecture fees from Allergan Health and Legacy Health; grant support from Allergan Health and Legacy Health; Dr. Patterson also served as an expert witness in a case involving adverse events after bariatric surgery. Dr. Pomp reports receiving consulting fees from Covidien, W.L. Gore & Associates and Ethicon EndoSurgery; lecture fees from Covidien and W.L. Gore & Associates; grant support from Covidien and Ethicon EndoSurgery; he has also served as an expert witness for legal cases involving bariatric and other laparoscopic procedures. Dr. Pories reports receiving consulting fees and grant support from Johnson & Johnson, and lecture fees from Tyco. Dr. Wolfe reports receiving grant support from EnteroMedics and consulting fees from Johnson & Johnson. Dr. Yanovski reports that her spouse has received grant support from Obecure. No other potential conflicts of interest relevant to this article were reported.
LABS personnel contributing to the study include:
Columbia University Medical Center, New York, NY: Paul D. Berk, MD, Marc Bessler, MD, Amna Daud, MD, MPH, Dan Davis, DO, W. Barry Inabnet, MD, Munira Kassam, Beth Schrope, MD, PhD Cornell University Medical Center, New York, NY: Greg Dakin, MD, Faith Ebel, Michel Gagner, MD, Jane Hsieh, Alfons Pomp, MD, Gladys Strain, PhD East Carolina Medical Center, Greenville, NC: Rita Bowden, RN, William Chapman, MD, FACS, Lynis Dohm, PhD, John Pender MD, Walter Pories, MD, FACS Neuropsychiatric Research Institute, Fargo, ND: Michael Howell, MD, Luis Garcia, MD, Michelle Kuznia, BA, Kathy Lancaster, BA, James E. Mitchell, MD, Tim Monson, MD, Jamie Roth, BA Oregon Health & Science University: Clifford Deveney, MD, Katherine Elder, PhD, Stefanie Green, Robyn Lee, Jonathan Purnell, MD, Robert O'Rourke, MD, Chad Sorenson, Bruce M. Wolfe, MD, Zachary Walker Legacy Good Samaritan Hospital, Portland, OR: Valerie Halpin, MD, Jay Jan, MD, Crystal Jones, Emma Patterson, MD, Milena Petrovic, Cameron Rogers Sacramento Bariatric Medical Associates, Sacramento, CA: Iselin Austrheim-Smith, CCRP, Laura Machado, MD University of Pittsburgh Medical Center, Pittsburgh, PA: Anita P. Courcoulas, MD, MPH, FACS, George Eid, MD, William Gourash, MSN, CRNP, Lewis H. Kuller, MD, DrPH, Marsha Marcus, PhD, Carol A. McCloskey MD, Ramesh Ramanathan MD University of Washington, Seattle, WA: Jo Ann Broeckel Elrod, PhD, David E. Cummings, MD, E. Patchen Dellinger, MD, Allison Devlin, MS, David R. Flum, MD, MPH, Sarah Hammond, CCRC, Kris Kowdley, MD, Juanita Law, Kelly Lucas, CCRC, Anne MacDougall, BA, Brant Oelschlager, MD, Andrew Wright, MD Virginia Mason Medical Center, Seattle, WA: Lily Chang, MD, Nickolas Dasher, CCRC, Stephen Geary, RN, Jeffrey Hunter, MD, Ravi Moonka, MD, Olivia A. Seibenick, CCRC, Richard Thirlby, MD Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Steven H. Belle, PhD, MScHyg, Michelle Caporali, BS, Wendy C. King, PhD, Kevin Kip, PhD, Laurie Koozer, BA, Kira Leishear, MPH, Debbie Martin, BA, Rocco Mercurio, MBA, Faith Selzer, PhD, Abdus S. Wahed, PhD National Institute of Diabetes and Digestive and Kidney Diseases: Mary Evans, Ph.D, Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD National Cancer Institute: David E. Kleiner, MD, PhD
References
- 1.Dixon JB, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez AZ, Jr, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239(5):698–702. doi: 10.1097/01.sla.0000124295.41578.ab. discussion 702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flum DR, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294(15):1903–8. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 7.Belle SH, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3(2):116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMaria EJ, Portenier D, Wolfe L. Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg Obes Relat Dis. 2007;3(2):134–40. doi: 10.1016/j.soard.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.DeMaria EJ, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246(4):578–82. doi: 10.1097/SLA.0b013e318157206e. discussion 583-4. [DOI] [PubMed] [Google Scholar]
- 10.Parikh M, et al. Comparison of outcomes after laparoscopic adjustable gastric banding in African-Americans and whites. Surg Obes Relat Dis. 2006;2(6):607–10. doi: 10.1016/j.soard.2006.08.012. discussion 610-2. [DOI] [PubMed] [Google Scholar]
- 11.Lutfi R, et al. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc. 2006;20(6):864–7. doi: 10.1007/s00464-005-0115-8. [DOI] [PubMed] [Google Scholar]
- 12.Buffington CK, Marema RT. Ethnic differences in obesity and surgical weight loss between African-American and Caucasian females. Obes Surg. 2006;16(2):159–65. doi: 10.1381/096089206775565258. [DOI] [PubMed] [Google Scholar]
- 13.Pope GD, Birkmeyer JD, Finlayson SR. National trends in utilization and in-hospital outcomes of bariatric surgery. J Gastrointest Surg. 2002;6(6):855–60. doi: 10.1016/s1091-255x(02)00085-9. discussion 861. [DOI] [PubMed] [Google Scholar]
- 14.Zingmond DS, McGory ML, Ko CY. Hospitalization before and after gastric bypass surgery. JAMA. 2005;294(15):1918–24. doi: 10.1001/jama.294.15.1918. [DOI] [PubMed] [Google Scholar]
- 15.Livingston EH, Langert J. The impact of age and Medicare status on bariatric surgical outcomes. Arch Surg. 2006;141(11):1115–20. doi: 10.1001/archsurg.141.11.1115. discussion 1121. [DOI] [PubMed] [Google Scholar]
- 16.Flancbaum L, Belsley S. Factors affecting morbidity and mortality of Roux-en-Y gastric bypass for clinically severe obesity: an analysis of 1,000 consecutive open cases by a single surgeon. J Gastrointest Surg. 2007;11(4):500–7. doi: 10.1007/s11605-007-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belle SH, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS) Surg Obes Relat Dis. 2008;4(4):474–80. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer PR, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 20.Flum DR, Khan TV, Dellinger EP. Toward the rational and equitable use of bariatric surgery. Jama. 2007;298(12):1442–4. doi: 10.1001/jama.298.12.1442. [DOI] [PubMed] [Google Scholar]
- 21.Perry CD, et al. Survival and changes in comorbidities after bariatric surgery. Ann Surg. 2008;247(1):21–7. doi: 10.1097/SLA.0b013e318142cb4b. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Encinosa W. Bariatric Surgery Utilization and Outcomes in 1998 and 2004, S.B. #2. Agency for Healthcare Research and Quality; Rockville, MD: Jan, 2007. Editor. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.