Abstract
Background
The goal of this study was to test whether myocardial triglyceride (TG) turnover including oxidation of TG-derived fatty acids could be assessed with PET and 11C-palmitate.
Methods and Results
26 dogs were studied fasted (FAST), during Intralipid infusion (IL), during a hyperinsulinemic-euglycemic clamp without (HIEG) or with Intralipid infusion (HIEG+IL). 11C-palmitate was injected, and 45 min were allowed for labeling of myocardial TG pool. 3-D PET data were then acquired for 60 min, with first 15 min at baseline followed by 45 min during cardiac work stimulated with constant infusion of either phenylephrine (FAST, n=6; IL, n=6; HIEG+IL, n=6) or dobutamine (FAST, n=4; HIEG, n=4). Myocardial 11C washout during adrenergic stimulation (AS) was fitted to a mono-exponential function (Km(PET)). To determine the source of this 11C clearance, Km(PET) was compared to direct coronary sinus-arterial measurements of total 11C activity, 11C-palmitate, and 11CO2. Before AS, PET curves in all groups were flat indicating absence of net clearance of 11C activity from heart. In both FAST groups, AS resulted in negligible net 11C activity and 11CO2 production higher than net 11C-palmitate uptake. AS with phenylephrine resulted in net myocardial uptake of total 11C activity and 11C-palmitate in IL and HIEG+IL, and 11CO2 production lower than 11C-palmitate uptake. In contrast, AS with dobutamine in HIEG resulted in net clearance of all 11C metabolites (total 11C activity, 11C-palmitate and 11CO2) with 11CO2 contributing 66% to endogenous FA oxidation. AS resulted in significant Km(PET) in all groups, except HIEG+IL. However, positive correlation between Km(PET) and 11CO2 was observed only in HIEG (R2=0.83, P=0.09).
Conclusions
This is the first study to demonstrate that using PET and pre-labeling of intracardiac TG pool with 11C-palmitate, noninvasive assessment of myocardial TG use is feasible under metabolic conditions that favor endogenous TG use such as increased metabolic demand (β-adrenergic stimulation of cardiac work) with limited availability of exogenous substrate (HIEG).
Keywords: myocardial triglyceride turnover, 3-D PET, 11C-palmitate, β-adrenergic stimulation, monoexponential clearance
Introduction
Long-chain fatty acids (FA) are the primary energy source for the heart 1,2. In the healthy heart, 70–90% of the FA entering the cell are immediately oxidized, with the remaining 10–30% stored in the intracardiac triglyceride (TG) pool 3-5. These stores serve as a buffer that compensates for short-term differences in FA supply and lipid oxidative demand, and provide FA when plasma FA concentrations reach low levels 6. Due to its small mass, the turnover of myocardial TG is quick 7, and is inversely related to the concentration of exogenous FA 4,8-10. In normal perfused rat hearts, 11% of mitochondrial ATP production is attributed to oxidation of the myocardial TG-derived FA 4,11, increasing to 50% when exogenous energy sources are limited 4. Myocardial TG turnover can be rapidly accelerated by adrenergic stimulation 12-18.
The contribution of endogenous TG-derived FA to overall energy metabolism appears to be altered in various animal models of cardiac disease. For example, in failing rat hearts endogenous TG, measured with 13C-magnetic resonance spectroscopy, is not oxidized even though exogenous palmitate oxidation is unchanged 19. In contrast, TG turnover provides up to 70% of the ATP production in uncontrolled diabetes 10,20 and during reperfusion of ischemic hearts 5. High levels of TG in the heart cause diminished contractile function, hypertrophy, and myocyte death 21-23. Therefore, lipolysis of intracardiac TG might also play an important physiologic role by protecting the heart from deleterious effects of surplus lipid accumulation.
Relatively little is known about regulation of myocardial TG metabolism in humans in either health or disease. This is primarily due to the lack of adequate methods for its measurement 7,24-26. At least in theory, myocardial lipolysis can be quantified based on the rate of glycerol release from the heart measured with arterio - coronary sinus balance 27. This method, however, requires the tenuous assumptions that all lipolysis is complete (i.e., hydrolysis of one TG molecule yields one glycerol molecule) and that the heart cannot oxidize glycerol. In any case, it is too invasive for routine use. Recently, 1H-MRS was used to detect myocardial TG content and net lipolysis 28-32. However, this approach does not allow for estimation of the rate of the TG turnover. On the other hand, to date the most common method for noninvasive measurement of myocardial FA metabolism is compartmental modeling of PET kinetics of 11C-palmitate 33-37. However, this approach measures only metabolism of exogenous FA and does not account for the contribution from the intracardiac TG pool.
Accordingly, the goal of this study was to investigate the feasibility of assessing myocardial TG turnover non-invasively using PET and 1-11C-palmitate. To do so, we pre-labeled the myocardial TG pool with 11C-palmitate and, taking advantage of the higher sensitivity of 3-D (or septaless) PET 38, measured the rate of 11C washout from the myocardium during adrenergic stimulation in a well-controlled canine model studied over a wide range of substrate and hormonal conditions. To determine the source of the PET 11C clearance, direct coronary sinus – arterial measurements of the total 11C, 11C-palmitate and 11CO2 counts were analyzed and compared with clearance rate of PET 11C activity.
Materials and Methods
Animal Preparation
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals established by Washington University's Animal Studies Committee. Purpose bred 6.2–9.8 kg (7.65 ±0.88 kg) male beagle dogs were fasted, anesthetized and instrumented as reported previously 39,40. One femoral vein was cannulated to administer drugs. Catheters were placed in the thoracic aorta via the femoral arteries for arterial sampling and monitoring of arterial blood pressure. To obtain venous blood samples, a coronary sinus catheter was placed via the right external jugular vein under fluoroscopic guidance as previously described 37. The ECG, arterial blood pressure and heart rate were monitored throughout the study.
To achieve a wide range in myocardial use and oxidation of endogenous TG, twenty six dogs were studied under various conditions established 60 min prior to tracer injection. Ten dogs were studied after overnight fasting (FAST). To increase myocardial FA uptake, a continuous infusion of 20% Intralipid (IL) (Fresenius Kabi Clayton, LP; 1 mL/min) was administered in six dogs. To switch myocardial substrate metabolism from primarily FA to glucose use, a hyperinsulinemic-euglycemic clamp (HIEG) was performed in ten dogs, using a continuous infusion of insulin (70 mU/kg/h) along with an adjustable infusion of 20% dextrose 35. To assess the effect of competing exogenous substrates on the accuracy of the measurement of TG turnover, six of the ten HIEG dogs received concomitant administration of IL (HIEG+IL). Since we wanted to investigate whether an acute increase in cardiac work alone was sufficient to stimulate myocardial TG mobilization, or whether direct activation of lipolysis was required as well, cardiac work was stimulated with either phenylephrine or dobutamine. Accordingly, in six FAST, and all IL and HIEG+IL dogs, cardiac work was increased with phenylephrine (0.84 - 1.6 μg/kg/min). In four FAST and all HIEG dogs, cardiac work was increased with dobutamine (20 μg/kg/min). The difference in these interventions was based on the fact that unlike phenylephrine, dobutamine stimulates lipolysis. Therefore to be able to compare these two groups, IL infusion was added to HIEG to mimic peripheral lipolysis during adrenergic stimulation with phenylephrine.
Experimental Protocol
All PET studies were performed on a microPET 220 (Concorde Microsystems Inc.) which operates solely in 3-D mode. After a position scan, a bolus of 1110 – 1480 MBq of 11C-palmitate was injected, and 45 min were allowed 41 to minimize uptake and oxidation of 11C-palmitate and maximize 11C labeling of myocardial TG. Continuous dynamic PET data acquisition was started after this waiting period. To determine the basal rate of 11C clearance from the myocardium, data were collected for 15 min while the heart was at rest. Cardiac work was then increased with a constant infusion of either phenylephrine (0.84 and 1.6 μg/kg/min) or dobutamine (20 μg/kg/min), which was continued throughout the rest of the study. Simultaneous arterial and coronary sinus blood samples for 11C total activity and 11C-palmitate metabolites (11C-palmitate and 11CO2) (in counts/mL/min) were obtained after 11C-palmitate administration during baseline (at 2, 10, and 45 min) and adrenergic stimulation (at 60, 62, 65, 70, 75, 85, 95 and 105 min). Another set of arterial and coronary sinus blood samples were collected at 0, 10, 45 and 85 min for plasma substrates (glucose, free FA, and lactate) and insulin levels, blood gases, pH, hematocrit and hemoglobin.
Measurements of Unlabeled Plasma Substrates
Plasma glucose and lactate levels were assayed enzymatically with a 2300 STAT Plus Analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma free FA levels were measured using an enzymatic colorimetric method (Wako NEFA C kit, Wako Chemicals USA, Richmond, VA). Plasma insulin was measured by radioimmunoassay (Linco Research Co., St. Charles, MO).
Measurements of Plasma 11C-Palmitate and its Metabolites
Plasma 11C total activity was measured on a gamma counter in counts/mL/min. All directly measured arterial and coronary sinus 11C data were normalized to injected dose as well as decay corrected by time lapsed from tracer injection to blood sampling time. The contribution of 11CO2 and 11C-palmitate to total 11C radioactivity in each of the arterial and coronary sinus samples was determined quantitatively as previously described 35. As we were interested only in clearance of pre-labeled intramyocardial TG, total myocardial 11C activity, 11C-palmitate and 11CO2 were calculated as their coronary sinus minus arterial counts. The positive differences were considered as myocardial clearance of the corresponding measurements, while negative values indicated myocardial uptake of tracer.
PET Image Analysis
Myocardial 11C-palmitate transaxial composite PET images (10-60 min) were summed to place myocardial regions of interest. To minimize contamination from septum and liver, eight regions of interest for each study were placed on the anterior (n=4) and lateral (n=4) walls and traced into each dynamic frame to generate myocardial 11C time activity curves. To investigate whether myocardial clearance of PET 11C-activity during adrenergic stimulation reflected oxidation of endogenous TG, time activity curves for each region corresponding to the first 30 min of adrenergic stimulation were fitted to a mono-exponential function. Due to low 11C activity after 45 minutes of PET data acquisition which led to increased data noise, the last 10 to 15 min of data collection were excluded from analysis.
For each intervention, the estimated rate constants, Km(PET) (min-1), were averaged to generate a mean rate constant per study and compared to myocardial net 11C activity, 11C-palmitate and 11CO2 direct measurements obtained from arterial and coronary sinus sampling.
Statistical Analysis
All data are presented as mean ± SD. Differences in myocardial total 11C activity, 11C-palmitate or 11CO2 measurements between pre- and post adrenergic stimulation for a given intervention were compared by means of two-way analysis of variance (ANOVA) for repeated measurements, where the post-hoc Scheffé test was used to localize differences among measurements. Correlations between Km(PET) and 11C-palmitate or 11CO2 measurements were done by linear regression. A P value of < 0.05 was considered statistically significant.
Results
Hemodynamics
Hemodynamic data are shown in Table 1. At baseline, heart rate and diastolic blood pressure were comparable across groups except in HIEG+IL, where both parameters were highest (P < 0.05 vs. dobutamine FAST). Systolic blood pressure was the highest in HIEG group (P < 0.05 vs. IL). There were no significant differences in rate pressure product among the study groups.
Table 1. Hemodynamics.
| AS: Phenylephrine | AS: Dobutamine | 2-way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FAST (n=6) | IL (n=6) | HIEG+IL (n=6) | FAST (n=4) | HIEG (n=4) | |||||
| BL vs. AS | Intervention | Interaction | |||||||
| HR bpm | BL | 96 ± 28 | 103 ± 17 | 125 ± 20* | 77 ± 15 | 92 ± 13 | P < 0.0001 | P = 0.06 | P < 0.001 |
| AS | 92 ± 18* | 112 ± 18 | 134 ± 19 | 149 ± 29 | 134 ± 55 | ||||
| SBP mm Hg | BL | 90 ± 13 | 79 ± 12 | 83 ± 11 | 101 ± 14 | 103 ± 21** | P < 0.0001 | P < 0.05 | NS |
| AS | 136 ± 31 | 102 ± 24 | 112 ± 24 | 117 ± 6 | 142 ± 11** | ||||
| DBP mm Hg | BL | 55 ± 8 | 49 ± 10 | 44 ± 10* | 62 ± 9 | 60 ± 15 | P < 0.0001 | NS | P < 0.05 |
| AS | 85 ± 24 | 61 ± 17 | 63 ± 22 | 58 ± 9 | 77 ± 11 | ||||
| RPP SBP*HR | BL | 8,601 ± 2,759 | 7,593 ± 1,744 | 10,461 ± 2,764 | 7,837 ± 2,669 | 9,201 ± 498 | P < 0.0001 | NS | NS |
| AS | 11,798 ± 5,101 | 11,128 ± 2,052 | 14,590 ± 4,081 | 17,462 ± 4,299 | 18,685 ± 7,382 | ||||
AS – adrenergic stimulation; BL – baseline; FAST – fasted; IL – Intralipid; HIEG – hyperinsulinemic-euglycemic clamp; HR - heart rate; SBP – systolic blood pressure; DBP – diastolic blood pressure; RPP - rate pressure product.
P < 0.05 vs. FAST in dobutamine study;
P < 0.05 vs. IL;
NS – not significant.
Adrenergic stimulation resulted in an overall increase in all hemodynamic measurements (Table 1, BL vs. AS P < 0.0001). Post-hoc analysis showed that compared to baseline, adrenergic stimulation resulted in (1) increased heart rate in dobutamine FAST only; (2) increased systolic blood pressure in all groups; (3) increased diastolic blood pressure in phenylephrine but not dobutamine groups, and (4) increased rate pressure product in all except HIEG group. Phenylephrine increased the rate pressure product via increases in blood pressure, while dobutamine-induced increases in rate pressure product were due to increases in both heart rate and systolic blood pressure. Rate pressure product was higher during dobutamine than during phenylephrine infusion (ANOVA P < 0.0001, 18,073 ± 5,840 vs. 12,503 ± 3,744, P < 0.01).
Plasma Substrate and Insulin Levels
Arterial plasma levels of substrate and insulin for all interventions are presented in Table 2. Adrenergic stimulation resulted in an overall increase in plasma glucose levels, with baseline levels lowest in the FAST groups (P < 0.05 vs. IL) and no differences among interventions during adrenergic stimulation. At baseline, plasma lactate concentrations were highest in the IL group (P < 0.005 vs. HIEG and both FAST groups). During adrenergic stimulation, while lactate levels remained significantly higher in the IL group, overall lactate levels were not different from baseline. There was a weak but significant increase in plasma FA during adrenergic stimulation (P=0.04). Plasma FA levels by design were highest in IL and HIEG+IL (P < 0.05 vs. HIEG and both FAST groups) during both baseline and adrenergic stimulation. Plasma insulin levels by design were highest in HIEG and HIEG+IL groups (P < 0.05 vs. IL and both FAST groups) during both baseline and adrenergic stimulation. However, adrenergic stimulation did not alter baseline plasma insulin levels.
Table 2. Arterial Plasma Substrate and Insulin Concentrations.
| AS: Phenylephrine | AS: Dobutamine | 2-way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FAST (n=6) | IL (n=6) | HIEG+IL (n=6) | FAST (n=4) | HIEG (n=4) | |||||
| BL vs. AS | Intervention | Interaction | |||||||
| GLUCOSE μmol/mL | BL | 3.01 ± 1.28 # | 3.88 ± 0.68 | 6.51 ± 3.15 | 2.82 ± 0.28 # | 5.83 ± 1.27 | P < 0.0005 | P < 0.05 | NS |
| AS | 4.22 ± 1.06 # | 5.00 ± 0.04 | 8.55 ± 4.97 | 5.11 ± 1.23 | 5.43 ± 0.90 | ||||
| LACTATE nmol/mL | BL | 1,013 ± 321 * | 1,572 ± 289 | 1,303 ± 199 | 805 ± 51* | 717 ± 81* | NS | P < 0.0005 | NS |
| AS | 1,215 ± 673 * | 1,633 ± 432 | 1,218 ± 403 | 568 ± 125 * | 785 ± 142 * | ||||
| FFA nmol/mL | BL | 586 ± 368 * # | 4,027 ± 2,117 | 4,767 ± 3,792 | 1,126 ± 814 * # | 381 ± 244 * # | P < 0.05 | P < 0.05 | NS |
| AS | 508 ± 197 * # | 6,102 ± 807 | 6,605 ± 3,900 | 1,922 ± 714 * # | 765 ± 330 * # | ||||
| INSULIN μU/mL | BL | 2 ± 0 # ** | 8 ± 10 | 177 ± 111* | 2 ± 0 ** | 375 ± 144 * # | NS | P < 0.0001 | NS |
| AS | 2 ± 0 # ** | 5 ± 3 | 199 ± 139* | 88 ± 72 ** | 373 ± 121 * # | ||||
AS – adrenergic stimulation; BL – baseline; FAST – fasted; IL – Intralipid; HIEG – hyperinsulinemic-euglycemic clamp; FFA – free fatty acids.
P < 0.05 vs. IL;
P < 0.05 vs. HIEG+IL;
P < 0.05 vs. HIEG;
NS – not significant.
Direct Coronary Sinus - Arterial Measurements of Total 11C Activity, 11C-Palmitate and 11CO2
Figure 1 shows directly measured differences between coronary sinus and arterial plasma for total 11C activity (black bars), 11C-palmitate (open bars) and 11CO2 production (gray bars) as an average of values obtained at baseline (2, 10, and 45 min) pre-phenylephrine (Fig.1A) and pre-dobutamine (Fig.1C), and during adrenergic stimulation (60, 62, 65, 70, 75, 85, 95 and 105 min) with phenylephrine (Fig.1B) and dobutamine (Fig.1D).
Figure 1.
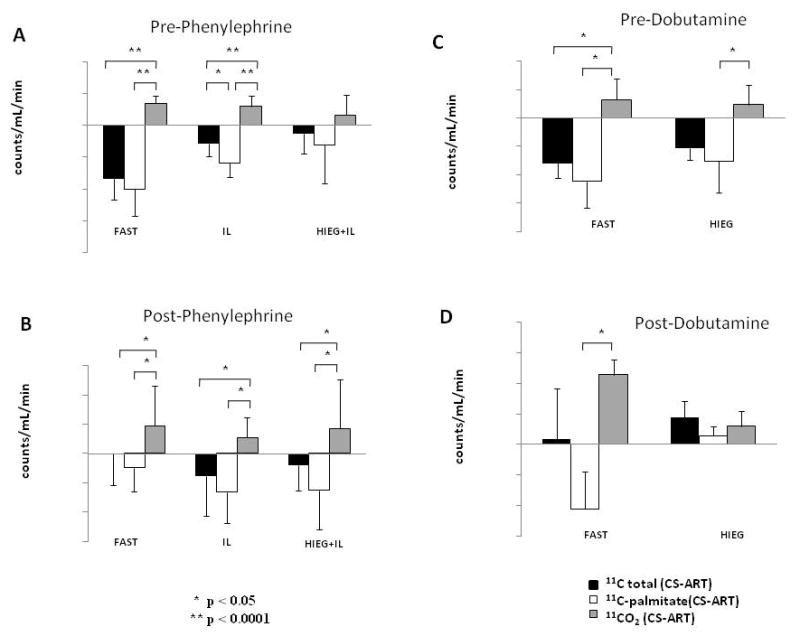
Directly measured averaged coronary sinus - arterial difference (CS-ART) for total 11C activity (black bars), 11C-palmitate (white bars) and 11CO2 (grey bars) before (A and C) and during adrenergic stimulation of cardiac work (B and D) with phenylephrine (A and B) and dobutamine (C and D). Data is presented as group mean ± SD.
Net uptake of total 11C activity and 11C-palmitate, shown as negative values, as well as 11CO2 production, shown as positive values, were observed in all five groups at rest, before adrenergic stimulation of cardiac work (Fig.1A and 1C), as well as in IL and HIEG+IL during phenylephrine stimulation (Fig. 1B). Interestingly, 11CO2 production in these groups never exceeded 11C-palmitate uptake, suggesting that most if not all of the 11CO2 production was likely due to oxidation of exogenous FA. However, in both FAST groups, stimulation with either phenylephrine or dobutamine resulted in negligible total 11C-activity exchange while 11C-palmitate uptake was lower than 11CO2 production in both groups, suggesting oxidation of both exogenous 11C-palmitate and endogenous myocardial TG-derived 11C-palmitate. In contrast, in HIEG group (Fig.1D) we observed net release for all three 11C measurements (total 11C activity, 11C-palmitate and 11CO2), with 2/3 of 11C release accounted for by 11CO2 apparently produced from oxidation of myocardial 11C labeled TG, and 1/3 by the washout of non-11CO2 metabolites attributable to myocardial TG-derived 11C labeled FA.
PET Measurements during Adrenergic Stimulation
Figure 2 shows representative PET myocardial time activity curves (dots) before and during adrenergic stimulation with phenylephrine (Fig.2A-C) and dobutamine, mono-exponential fitting to PET clearance of myocardial 11C activity during adrenergic stimulation (solid lines), and the corresponding clearance rates (Km, min-1) (Fig. 2D-E). Before adrenergic stimulation, PET curves in all groups were nearly flat demonstrating a lack of net clearance of 11C activity. During adrenergic stimulation with phenylephrine, PET Km(min-1) rates were comparable in FAST and IL (0.002 ± 0.001 and 0.003 ± 0.003, respectively, P = NS), and negligible in HIEG+IL (0.0004 ± 0.0005, P = NS from zero).
Figure 2.
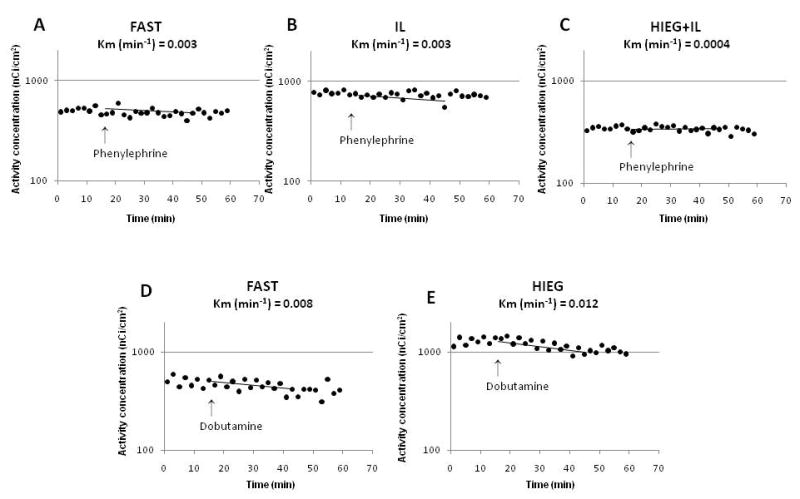
Representative myocardial PET time activity curves (dots) and mono-exponential fitting (lines) of total PET 11C activity (y axes) during adrenergic stimulation of cardiac work with phenylephrine (A-C) and dobutamine (D-E). Km - rate of 11C clearance from myocardium.
In contrast, during dobutamine stimulation, PET Km(min-1) rates were significantly higher than during phenylephrine stimulation of cardiac work (0.006 ± 0.003 vs. 0.002 ± 0.002, P < 0.001) with comparable rates in both FAST and HIEG groups (0.006 ± 0.003 and 0.006 ± 0.003, respectively, P = NS).
During phenylephrine stimulation, there was no significant positive correlation between Km(min-1) and either 11C activity, 11C-palmitate or 11CO2 exchange (not shown). However, when individual dogs were examined, three in FAST, two in IL and two in HIEG+IL studies showed net clearance of 11C activity with 11CO2 release greater than 11C-palmitate uptake. Thus, in these studies 11CO2 production must have been due, at least in part, to oxidation of endogenous FA.
Figure 3 shows correlation between PET Km(min-1) (y-axis) and the difference in directly measured coronary sinus and arterial plasma net myocardial 11C activity (Fig. 3A, 3D), 11C-palmitate (Fig. 3B, 3E) and 11CO2 (Fig. 3C, 3F) during cardiac work stimulated with dobutamine in FAST (Fig. 3A-C) and HIEG (Fig. 3D-F) groups.
Figure 3.
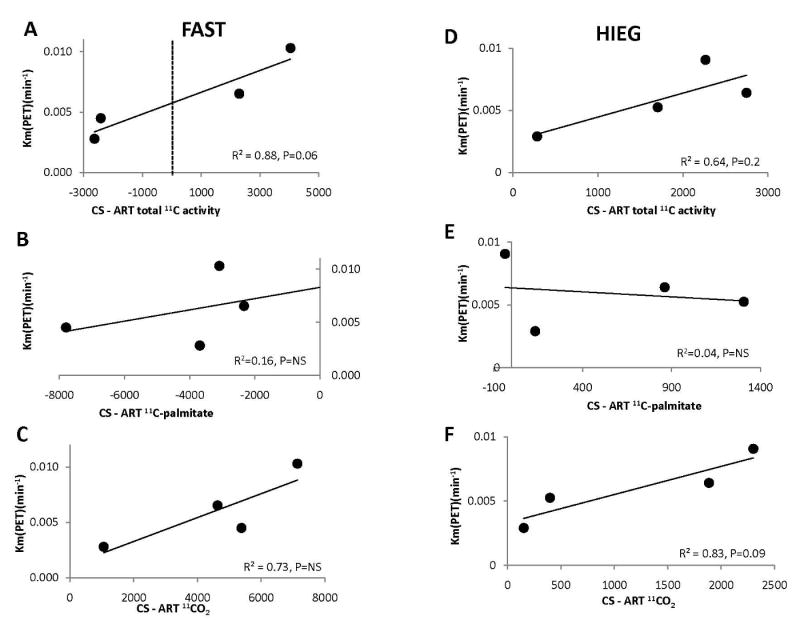
Correlation between total 11C clearance measured by PET (Km(PET) (y axes) (A-F) and averaged coronary sinus – arterial difference (x axes) of total 11C activity (A and D), 11C-palmitate (B and E), and 11CO2 (C and F) during adrenergic stimulation of cardiac work with dobutamine in FAST (A-C) and HIEG (D-F) studies. Negative values on x-axes indicate tracer uptake and positive values tracer clearance.
In FAST studies, there was a strong trend toward significant correlation between Km(min-1) and directly measured net total 11C activity (Fig. 3A, P = 0.06), with no correlation between Km(min-1) and 11C-palmitate (Fig. 3B) or 11CO2 (Fig. 3C). While FAST studies averaged net uptake of 11C-palmitate (Fig. 3B) and production of 11CO2 (Fig. 3C), 11C-total net uptake was observed in two of the animals and net release in the other two (Fig. 3A). In the two studies with 11C-activity release, uptake of net 11CO2 was also greater than 11C-palmitate uptake, indicating that there was oxidation of both exogenous and endogenous sources of FA.
In contrast, in HIEG group in all four dogs, there was 11C clearance resulting in correlative trend between PET Km(min-1) and total 11C activity (Fig. 3D, R2 = 0.64, P = 0.2), but not with 11C-palmitate (Fig. 3E). However, there was strong but not significant correlation between PET Km(min-1) and 11CO2 (Fig. 3F: R2 = 0.83, P = 0.09). These data in conjunction with the observations obtained from directly measured differences in coronary sinus and arterial plasma corroborate that PET clearance of 11C activity during dobutamine-induced cardiac work in HIEG group can be attributed to myocardial 11CO2 production from oxidation of intracardiac 11C labeled TG.
Discussion
To the best of our knowledge, to date non-invasive measurements of myocardial FA metabolism using PET and radionuclide tracers have been limited to measurements of exogenous FA uptake and metabolism, including oxidation and esterification of FA. In this study, we investigated for the first time whether TG turnover including TG degradation and oxidation could be assessed non-invasively with PET and 11C-palmitate.
To do so, we used 11C-palmitate to pre-label the myocardial TG pool of dogs studied under a wide range of substrate and hormonal conditions, and using 3-D PET, measured the rate of 11C washout from the myocardium at baseline and during adrenergic stimulation. To determine whether the latter in fact represented mobilization of the intracardiac TG pool, we correlated the PET rate of 11C clearance (Km(min-1)) with directly measured arterial and coronary sinus total 11C, 11C-palmitate, and 11CO2 counts.
We found that before adrenergic stimulation, there was net uptake of total 11C activity and 11C-palmitate that exceeded 11CO2 production in all groups (Fig. 1A and 1C). In addition, flat PET curves were also observed in all groups indicating absence of net clearance of 11C activity from heart (Fig. 2). Adrenergic stimulation with phenylephrine resulted in net myocardial uptake of total 11C activity and 11C-palmitate in IL and HIEG+IL while 11CO2 production was less than 11C-palmitate uptake. This pattern suggested exogenous FA as the main source of FA oxidation. Adrenergic stimulation with either phenylephrine or dobutamine in FAST groups resulted in negligible net 11C activity with net 11C-palmitate uptake lower than 11CO2 production, suggesting oxidation from both exogenous and endogenous sources independent of the adrenergic stimulation used. In contrast, adrenergic stimulation with dobutamine in HIEG resulted in net clearance of all 11C metabolites (total 11C activity, 11C-palmitate and 11CO2), with 11CO2 contributing 2/3 to total 11C washout suggesting that in this case 11CO2 production could be attributed exclusively to oxidation of endogenous TG.
To our knowledge, this is the first attempt to label the myocardial TG pool with 11C by injection of 11C-palmitate for subsequent imaging with PET. This approach differs significantly from how early kinetics of PET 11C clearance have historically been used to assess myocardial oxidation of exogenous FA, in that we sought to quantify the very late kinetics of the tracer representing the metabolism of myocardial TG. This presented a challenge due to the short half-life of the tracer (20.2 min) compared to the time required to label the TG pool as well as to clear the heart of 11CO2 derived from direct oxidation of the injected 11C-palmitate. However, the 45-min waiting period between the injection of 11C-palmitate and the beginning of the 3-D PET data acquisition was sufficient, as seen from the practically flat time activity curves before adrenergic stimulation during the first 15 min of each scan (Fig. 2A-E). This was true in all animals regardless of the conditions under which they were studied, e.g., in the fasted state vs. infused with glucose and insulin and/or Intralipid. This allowed us to determine whether adrenergic stimulation increased the rate of 11C clearance from the myocardium. Since we wanted to investigate whether an acute increase in cardiac work alone was sufficient to stimulate myocardial TG mobilization, or whether direct activation of lipolysis was required as well, cardiac work was stimulated with either phenylephrine or dobutamine. Dobutamine, as a β2-agonist, has a direct lipolytic effect42,43 while the α1-agonist phenylephrine is an inhibitor of lipolysis 44.
Although both agents increased cardiac work and stimulated PET clearance of 11C from myocardium, phenylephrine infusion resulted in very low Km(PET) in FAST and IL groups (Fig.2A and 2B), with no significant PET clearance in HIEG+IL group (Fig.2C). In contrast, PET Km(min-1) was 3 - 4 folds higher during dobutamine infusion (Fig.2D and 2E). At least in theory, this difference could be due to the fact that phenylephrine increased the rate pressure product by only ∼40%, with this being mostly due to an increase in blood pressure, whereas dobutamine more than doubled the rate pressure product, as a result of increases in both heart rate and systolic blood pressure. These findings are expected given the higher inotropic effect of β-adrenergic agents45. However, this differential response cannot explain the observed differences in PET clearance, because PET Km(min-1) was still significantly higher (P < 0.05) in dobutamine-infused animals (0.006 ± 0.003 min-1) than in phenylephrine-infused animals (0.002 ± 0.002 min-1) even when the analysis was restricted to those with comparable rate-pressure products (16,526 ± 3,826 and 14,059 ± 3,915 beats/min/mmHg for seven dobutamine- and 13 phenylephrine-treated animals, respectively). Thus, these differences could be attributed to lipolytic effect of dobutamine that results in higher TG turnover.
Since the PET clearance of 11C during adrenergic stimulation of cardiac work could have resulted from utilization of either exogenous 11C-palmitate taken up from plasma or from endogenous intracardiac TG pre-labeled with 11C-palmitate, we calculated the directly measured coronary sinus – arterial difference in total 11C activity to determine whether there was a net uptake or net release of tracer. To determine the extent to which any net release of 11C could be attributed to oxidation vs. egress of non-metabolized 11C-palmitate released from endogenous TG, we also calculated coronary sinus - arterial differences in 11CO2 and 11C-palmitate.
The lack of net 11C release from the myocardium following phenylephrine administration in either of study group (Fig. 1B) did not allow for definitive identification of the source of the PET clearance under these conditions. However, analyzing the individual studies we found that in almost 1/3 of the studies there was 11C clearance from myocardium with 11CO2 production greater than 11C-palmitate uptake, suggesting that at least in part myocardium utilized endogenous intracardiac TG. Moreover, in two out of these seven dogs, we observed release from myocardium of not only total 11C and 11CO2 but also intracardiac TG-derived 11C-palmitate. Further attempts to determine under which metabolic conditions or level of cardiac work phenylephrine stimulated myocardial TG turnover fell short due to significant variability of metabolic and hemodynamic parameters among these experiments.
On the other hand, either negligible (FAST) or net release (HIEG) of total 11C activity from the heart was observed during dobutamine stimulation (Fig. 1D), in keeping with the higher values for Km(PET) observed in these experiments (Fig. 2D and 2E). Further analysis of individual FAST dogs showed that in two animals with the highest levels of insulin (102 and 180 μU) there was total 11C net uptake, and in the other two with significantly lower insulin levels (10.3 and 60 μU) there was net 11C release (Fig. 3A) 46. 11CO2 production in FAST dogs was also greater than 11C-palmitate uptake, indicating that there was oxidation of both exogenous and endogenous sources of FA. In contrast, release of all 11C-metabolites in HIEG combined with correlation between Km and 11CO2 production, but not with either total 11C or 11C-palmitate, demonstrate that myocardial TG turnover could be successfully measured under conditions of enhanced cardiac work in the presence of low levels exogenous FA available for oxidation. Of note, the assumption that if there is net uptake, there could not be net clearance of tracer is valid only for averaged net measurements. However, it does not apply to PET dynamic data presented here, where myocardial tracer uptake is mostly represented in the early kinetics of the PET 11C time-activity curves, while oxidation, as measured by the production of 11CO2, and potentially TG turnover, are mostly represented in the late kinetics of the PET curves. The fitting of a mono-exponential function to the late kinetics of PET 11C activity should then be representative of these clearances, even in the presence of net tracer uptake.
There are, however, certain limitations to this method. This approach measures only clearance of the labeled TG and does not allow for the upstream estimation of myocardial TG pool. Therefore, this method provides only an index of myocardial TG turnover. However, we showed that PET clearance curves do reflect the myocardial TG turnover, and combined with the conventional PET protocol for measurement of myocardial exogenous FA metabolism, it may provide additional valuable information regarding the contribution of the endogenous myocardial TG to overall lipid metabolism in heart. Another limitation is the fact that due to confounding effects of continued myocardial uptake of exogenous 11C-palmitate, the current method is unable to measure back-diffusion of TG-derived 11C-palmitate originating from lipolysis. As a consequence, the assessment of total TG lipolysis (as opposed to the component that undergoes oxidation) is underestimated. The third limitation is the high dose/kg of 11C-palmitate used in this study that greatly exceeds the dose allowed for humans. In our study, we had to use high dose of short half-life tracer 11C-palmitate (i.e., 20 min) because of the rather long (105 min = over 5 half-lives) protocol of this study. However, by the end of 45 min waiting period the 11C curve reached the plateau, and it is possible that a shorter period could be sufficient for pre-labeling of myocardial TG pool. We also observed that the tracer washout in response to adrenergic stimulation occurred very fast, within approximately first 15 min. Therefore, it is possible to shorten the current protocol time to 60 min (35-40 min of pre-labeling of the TG pool, and data acquisition for 10 min at baseline and 20 min during adrenergic stimulation), and hence, the dose sufficient for this study could be decreased to a more acceptable 20-25 mCi. Further studies are required to determine whether these measurements can be obtained using a lower dose and imaging with newer and more sensitive human 3-D PET devices. Proven successful, this method can become useful as part of noninvasive basic research and human clinical studies designed to understand the role of myocardial TG turnover in the healthy and diseased heart, using metabolic interventions that result (1) in the storage and (2) in subsequent use of endogenous TG, such as HIEG + dobutamine. Thus, in order to detect alterations in myocardial TG turnover among different populations, clinical (or basic) metabolic studies, such as diabetic or obese patients vs. healthy controls, young vs. aging, or male vs. female subjects, could be implemented after pre-labeling of the TG pool and under the same metabolic intervention (i.e. HIEG clamp and β-adrenergic stimulation of cardiac work). The approach implemented in this study is a first and critical step for the future development of more sophisticated imaging and modeling approaches. These approaches may include multi-imaging/multi-tracer approaches that would combine established imaging methods to measure the TG pool, such as 1H-MRS, and more advance PET kinetic approaches to assess the fate of the myocardial TG pool including back-diffusion of 11C-palmitate derive from TG degradation and TG oxidation.
Conclusion
This is the first study to demonstrate that using PET and pre-labeling of intracardiac TG pool with 11C-palmitate, noninvasive assessment of myocardial TG use is feasible under metabolic conditions that increase demand for substrate (β-adrenergic stimulation of cardiac work) while limit availability of exogenous FA (HIEG).
Acknowledgments
This study was supported by NIH grants HL-69100. We thank Margaret Morris and Paul Eisenbeis for their technical assistance, and anonymous reviewer #1 whose thorough comments resulted in more interesting article.
This work was supported by NIH grant RO1 HL-69100
References
- 1.Bjorkman O. Fuel metabolism during exercise in normal and diabetic man. Diabetes Metab Rev. 1986;1:319–57. doi: 10.1002/dmr.5610010402. [DOI] [PubMed] [Google Scholar]
- 2.Gold M, Spitzer JJ. Metabolism of free fatty acids by myocardium and kidney. Am J Physiol. 1964;206:153–8. doi: 10.1152/ajplegacy.1964.206.1.153. [DOI] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–76. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 4.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266:8162–70. [PubMed] [Google Scholar]
- 5.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover during reperfusion of isolated rat hearts subjected to a transient period of global ischemia. J Biol Chem. 1992;267:3825–31. [PubMed] [Google Scholar]
- 6.Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, Frayn KN. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci. 1990;79:339–48. doi: 10.1042/cs0790339. [DOI] [PubMed] [Google Scholar]
- 7.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–66. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crass MF., III Exogenous substrate effects on endogenous lipid metabolism in the working rat heart. Biochim Biophys Acta. 1972;280:71–81. doi: 10.1016/0005-2760(72)90213-5. [DOI] [PubMed] [Google Scholar]
- 9.Crass MF, III, Shipp JC. Metabolism of exogenous and endogenous fatty acids in heart muscle. Rec Adv Stud Cardiac Struct Metab. 1972;1:115–26. [PubMed] [Google Scholar]
- 10.Paulson DJ, Crass MF., III Endogenous triacylglycerol metabolism in diabetic heart. Am J Physiol Heart Circ Physiol. 1982;242:H1084–94. doi: 10.1152/ajpheart.1982.242.6.H1084. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab. 2006;290:E448–55. doi: 10.1152/ajpendo.00139.2005. [DOI] [PubMed] [Google Scholar]
- 12.Crass MF., III Heart triglyceride and glycogen metabolism: effects of catecholamines, dibutyryl cyclic AMP, theophylline, and fatty acids. Rec Adv Stud Cardiac Struct Metab. 1973;33:275–90. [PubMed] [Google Scholar]
- 13.Crass MF., III Regulation of triglyceride metabolism in the isotopically prelabeled perfused heart. Federation Proc. 1977;36:1995–9. [PubMed] [Google Scholar]
- 14.Crass MF, III, Shipp JC, Pieper GM. Effects of catecholamines on myocardial endogenous substrates and contractility. Am J Physiol. 1975;228:618–27. doi: 10.1152/ajplegacy.1975.228.2.618. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1490–501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–9. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 17.Kerner J, Zaluzec E, Gage D, Bieber LL. Characterization of the malonyl-CoA-sensitive carnitine palmitoyltransferase (CPTo) of a rat heart mitochondrial particle. Evidence that the catalytic unit is CPTi. J Biol Chem. 1994;269:8209–19. [PubMed] [Google Scholar]
- 18.Swanton EM, Saggerson ED. Effects of adrenaline on triacylglycerol synthesis and turnover in ventricular myocytes from adult rats. Biochem J. 1997;328:913–22. doi: 10.1042/bj3280913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–22. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saddik M, Lopaschuk GD. Triacylglycerol turnover in isolated working hearts of acutely diabetic rats. Can J Physiol Pharmacol. 1994;72:1110–9. doi: 10.1139/y94-157. [DOI] [PubMed] [Google Scholar]
- 21.van der Vusse GJ, Glatz JFC, Stam HCG, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev. 1992;72:881–940. doi: 10.1152/physrev.1992.72.4.881. [DOI] [PubMed] [Google Scholar]
- 22.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976;58:421–31. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurley BF, Nemeth PM, Martin WH, III, Hagberg JP, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–7. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- 26.Wicklmayr M, Rett K, Dietze G, Mehnert H. Inhibition of muscular triglyceride lipolysis by ketone bodies: a mechanism for energy-preservation in starvation. Horm Metab Res. 1986;18:476–8. doi: 10.1055/s-2007-1012350. [DOI] [PubMed] [Google Scholar]
- 27.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177–93. [PubMed] [Google Scholar]
- 28.Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005;289(5):E935–9. doi: 10.1152/ajpendo.00095.2005. [DOI] [PubMed] [Google Scholar]
- 29.Kankaanpää M, Lehto HR, Pärkkä JP, Komu M, Viljanen A, Ferrannini E, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91(11):4689–95. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer RW, Hammer S, Smit JW, Frölich M, Bax JJ, Diamant M, et al. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes. 2007;56(12):2849–53. doi: 10.2337/db07-0768. [DOI] [PubMed] [Google Scholar]
- 31.van der Meer RW, Doornbos J, Kozerke S, Schär M, Bax JJ, Hammer S, et al. Metabolic imaging of myocardial triglyceride content: reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology. 2007;245(1):251–7. doi: 10.1148/radiol.2451061904. [DOI] [PubMed] [Google Scholar]
- 32.Hammer S, van der Meer RW, Lamb HJ, Schär M, de Roos A, Smit JW, Romijn JA. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab. 2008;93(2):497–503. doi: 10.1210/jc.2007-2015. [DOI] [PubMed] [Google Scholar]
- 33.Schon HR, Schelbert HR, Robinson G, et al. C-11 labeled palmitic acid for the noninvasive evaluation of regional myocardial fatty acid metabolism with positron-computed tomography I. Kinetics of C-11 palmitic acid in normal myocardium. Am Heart J. 1982;103:532–47. doi: 10.1016/0002-8703(82)90341-6. [DOI] [PubMed] [Google Scholar]
- 34.Lerch RA, Bergmann SR, Ambos HD, et al. Effect of flow-independent reduction of metabolism on regional myocardial clearance of 11C-palmitate. Circulation. 1982;65(4):731–8. doi: 10.1161/01.cir.65.4.731. [DOI] [PubMed] [Google Scholar]
- 35.Fox KAA, Abendschein DR, Ambos HD, Sobel BE, Bergmann SR. Eflux of metabolized and nonmetabolized fatty acids from canine myocardium: implication for quantifying myocardial metabolism tomographically. Circ Res. 1985;57:232–43. doi: 10.1161/01.res.57.2.232. [DOI] [PubMed] [Google Scholar]
- 36.Lerch RA, Ambos HD, Bergmann SR, et al. Localization of viable, ischemic myocardium by positron-emission tomography with 11C-palmitate. Circulation. 1981;64:689–99. doi: 10.1161/01.cir.64.4.689. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using positron emission tomography. J Nucl Med. 1996;37:1723–30. [PubMed] [Google Scholar]
- 38.Pajevic S, Daube-Withersoppon ME, Bacharach SL, Carson RE. Noise characteristics of 3-D and 2-D PET images. IEEE Transactions on Med Imaging. 1998;17:9–23. doi: 10.1109/42.668691. [DOI] [PubMed] [Google Scholar]
- 39.Jesmok GJ, Warltier DC, Gross GJ, et al. Transmural triglycerides in acute myocardial ischaemia. Cardiovasc Re. 1978;12:659–65. doi: 10.1093/cvr/12.11.659. [DOI] [PubMed] [Google Scholar]
- 40.Evans JR. Importance of fatty acids in myocardial metabolism. Circ Res. 1964;14&15 II:96–106. [PubMed] [Google Scholar]
- 41.Nellis SH, Liedtke AJ, Renstrom B. Fatty acid kinetics in aerobic myocardium: characteristics of tracer carbon entry and washout and influence of metabolic demand. J Nucl Med. 1992;33(10):1864–74. [PubMed] [Google Scholar]
- 42.Palmer WK, Caruso RA, Oscai LB. Possible role of lipoprotein lipase in the regulation of endogenous triacylglycerols in the rat heart. Biochem J. 1981;198(1):159–66. doi: 10.1042/bj1980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreisberg RA. Effect of epinephrine on myocardial triglyceride and free fatty acid utilization. Am J Physiol. 1966;210:385–9. doi: 10.1152/ajplegacy.1966.210.2.385. [DOI] [PubMed] [Google Scholar]
- 44.Lafontan M, Dang-Tran L, Berlan M. Alpha-adrenergic antilipolytic effect of adrenaline in human fat cells of the thigh: comparison with adrenaline responsiveness of different fat deposits. Eur J Clin Invest. 1979;9(4):261–6. doi: 10.1111/j.1365-2362.1979.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 45.Borthne K, Langslet A, Lindberg H, Skomedal T, Osnes JB. Differential recruitment of alpha 1- and beta-adrenoceptors in inotropic control of atrial child myocardium by endogenous noradrenalin. Acta Physiol Scand. 2000;170(1):21–31. doi: 10.1046/j.1365-201x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- 46.Soto PF, Herrero P, Kates AM, Dence CS, Ehsani AA, Dávila-Román V, Schechtman KB, Gropler RJ. Impact of aging on myocardial metabolic response to dobutamine. Am J Physiol Heart Circ Physiol. 2003;285(5):H2158–64. doi: 10.1152/ajpheart.00086.2003. [DOI] [PubMed] [Google Scholar]


