Abstract
A colorimetric detection method using amine functionalized polymer films doped with a pH indicator has been developed for the rapid, sensitive and quantitative detection of gaseous formaldehyde at concentrations well below the IDLH (immediately dangerous to life or health). In one minute, visible color changes are easily observed even down to the PEL (permissible exposure limit) at 750 ppb. The limit of detection is below 50 ppb (7% of PEL) after 10 min exposure. This sensor is essentially unaffected by changes in humidity or temperature (4 to 50 °C) and is not sensitive to common interferents.
Formaldehyde is widely used in the manufacture of many resins, polymers, and construction materials. The thermal or chemical decomposition of such materials, however, occurs surprisingly readily from urea-formaldehyde foam insulation, particle board and formaldehyde-based resins.1 Formaldehyde is a probable human carcinogen, allergenic, and an intense irritant of eyes and mucous membranes and is therefore highly problematic as an indoor pollutant.1–3 The ability to detect formaldehyde at very low concentrations is critical: the World Health Organization has set a standard for safe exposure of 80 ppb averaged over 30 minutes;2 for comparison, OSHA has set the permissible exposure level (PEL) to 750 ppb and the immediately dangerous to life or health (IDLH) level at 20 ppm.3
While there are numerous methods for detecting and measuring gaseous formaldehyde, there remains a need for an inexpensive, sensitive and rapid analytical technology. Past analytical approaches include relatively expensive instrumentation4 (e.g., electrochemical, gas chromatography, optical, chemiluminescence, etc), but also inexpensive colorimetric methods, which see some field use.5 A number of colorimetric or fluorometric methods for the detection of formaldehyde have been proposed,5 e.g., hydrazones, triazoles, pararosaniline, etc. These prior methods are generally relatively slow (typically >30 min), often cumbersome and multistep, and frequently lack sensitivity. Thus, there remains a pressing need for the development of a rapid, sensitive and highly convenient formaldehyde detection method. Herein, we report a simple and highly sensitive colorimetric method for fast formaldehyde detection.
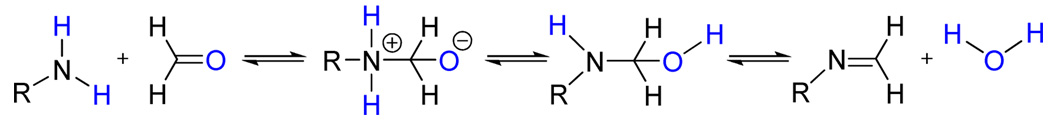
Most colorimetric formaldehyde detection methods are based on nucleophilic addition of an amine to the aldehyde forming an imine via a carbinolamine intermediate, as shown in Scheme 1. For an appropriate choice of the primary amine (e.g., delocalized conjugated substituents), the imine formation generates a color change, and so great effort has gone into the synthesis of sophisticated self-indicating primary amines.5
Scheme 1.
Nucleophilic addition of a primary amine to formaldehyde forming an imine.
In contrast, we find that it is better and easier to use a simple pH indicator to detect the change in basicity upon the reaction of a non-volatile primary amine with formaldehyde. An amine-terminated polymer was used to create a reactive matrix for formaldehyde detection. In order to screen for the optimal choices of indicator and polymeric amine, an array of six different pH indicators in five different polymer formulations (cf. Supporting Information (SI) Table S1) were printed onto PVDF membranes.
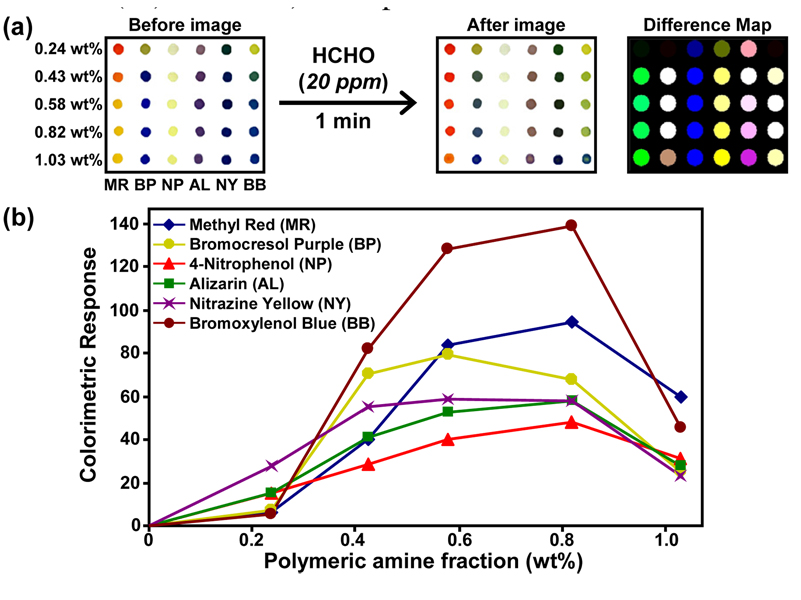
The inexpensive and disposable array was imaged using an ordinary flatbed scanner (cf. SI Figure S1), as described elsewhere for other colorimetric sensor arrays.6 For each spot in the array, the red, green, and blue values were measured before and after exposure to gaseous formaldehyde and color difference maps generated. As shown in Figure 1a, after one minute exposure of 20 ppm (IDLH) formaldehyde, obvious color changes are readily observed even by eye. Simply subtracting the original control image from the sample image provides a color change profile. Figure 1b shows the titration curve of six different pH indicators doped into a PEG polymer blended with a small amount of the same PEG with amine termination after exposure to 20 ppm formaldehyde for one minute. Amine-PEG concentrations of ~0.7 wt% gave the largest overall color changes. The response of these polymer films to formaldehyde is very rapid: as shown in SI Figure S2, the response to 20 ppm formaldehyde is generally >90% complete in less than one minute.
Figure 1.
(a) Image of the array before and after one minute exposure (500 sccm) to formaldehyde at 20 ppm at 298K and 50% RH and the color-change profile. Different polymeric amine weight percentages are listed for six pH indicators. (b) Colorimetric response (i.e.,(ΔR2+ΔG2+ΔB2)½) of each pH indicator versus polymeric amine wt% after one min exposure to 20 ppm formaldehyde; note indicator abbreviations.
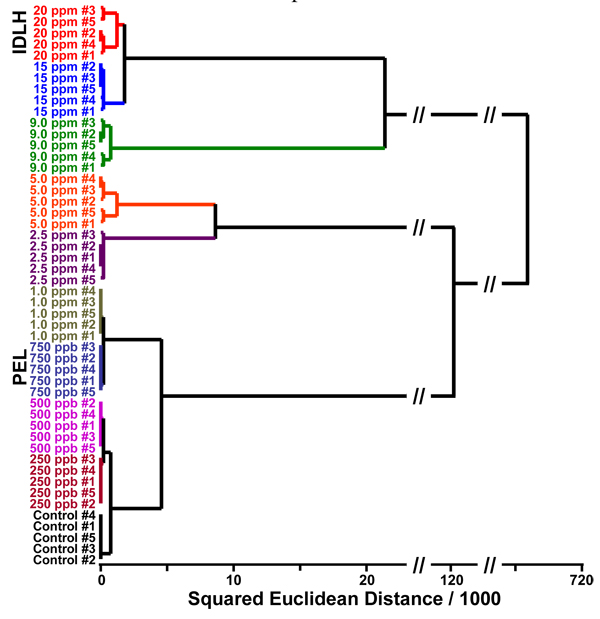
The colorimetric response of these polymeric films can be used for quantitative analysis of formaldehyde concentrations. After a one minute exposure to formaldehyde at different concentrations, the array shows different color change patterns which are easily distinguished by eye even without statistical analysis (cf. SI Figure S3). For quantitative comparisons, a simple statistical approach, hierarchical cluster analysis (HCA),7 was used. HCA is a model-free classification scheme based on the grouping of the analyte vectors (i.e., changes in RGB values) by Euclidean distances in their full dimensionality. Figure 2 shows the HCA dendrogram for one min exposures to gaseous formaldehyde concentrations ranging from 20 ppm (IDLH) down to 250 ppb (i.e., 1/3 of PEL). Remarkably, in quintuplicate trials, all concentrations showed tight clustering with no errors or misclassifications out of 50 cases. One may also base a quantitative analysis simply on the total response of the array (i.e., total Euclidean distance of changes in RGB), as shown in SI Figure S4. By increasing the exposure time of the array, we are able to quantify formaldehyde concentrations to as low as 50 ppb. Color difference maps and the HCA dendrogram (cf. SI Figure S5) shows that 50 ppb, 75 ppb, 100 ppb and a control were accurately identified after 10 minutes of exposure.
Figure 2.
Hierarchical cluster analysis (HCA) for formaldehyde at different concentrations. All experiments were run in quintuplicate; no confusions or errors in classification were observed in 50 trials, as shown.
For many prior formaldehyde detection techniques, errors stemming from changes in relative humidity and/or temperature are highly problematic in real world applications. For this reason, we have selected hydrophobic colorants and an extremely hydrophobic PVDF membrane to minimize the effects of changing humidity. The array itself is unaffected by changes in relative humidity from 10% to 90% compared to 50% relative humidity, as shown in SI Figure S6. Humidity effects during formaldehyde detection were also evaluated, and only minor effects were observed at high humidity (as expected for the dehydration reaction between the amine group and formaldehyde, cf. SI Figure S7.). Temperature changes (from 4 to 50 °C) did not significantly affect influence the overall response (SI Figure S8).
Acetaldehyde, butyraldehyde, and benzaldehyde were also tested to evaluate the selectivity of our method. None of these aldehydes showed any significant response at ~15 ppm even after five min exposure (SI Figure S9). The high selectivity stems from decreased charge (and hence diminished electrophilicity) of the carbonyl carbon in substituted aldehydes compared to formaldehyde. Even acetaldehyde showed only a very weak response at high concentrations, above 200 ppm.
To discriminate formaldehyde from potential acidic or basic interferent vapors, we added a row of several pH indicators in neutral or base-treated forms (SI Table S2). These added indicators permit discrimination of formaldehyde from other acidic or basic interferents, e.g., SO2, NO2, NH3, etc. While this does not permit facile identification of formaldehyde in the presence of such interferents, it does prevent false positives, since formaldehyde itself does not trigger a response from the non-amine polymer films of pH indicators. This array has been extensively tested against 10 common interferents: second-hand smoke, diesel fuel exhaust, gasoline exhaust, floor stripper, Windex, Fantastik, and Clorox bleach at 2% of their saturation vapor concentrations; SO2 and NO2 at their respective PEL concentrations; CO2 at ~375 ppm (i.e., ambient conc.) (SI Figure S10). Only Windex and SO2 provided significant interference, but the additional indicators prevented false positives. The shelf-life of the printed arrays is excellent, with constant response for more than one month after printing. Using these arrays as a screening tool, we have generated a minimalist colorimetric sensor for formaldehyde detection consisting of three spots: BB in a 0.6 wt.% amine-PEG film for formaldehyde detection, and BB and basic NY in a non-amine PEG film as false-positive discriminators against acidic and basic interferents, respectively.
In summary, we have developed a quite simple and highly sensitive colorimetric detection method for gaseous formaldehyde. Using ordinary pH indicators in an amine-functionalized polymer film, one can discriminate formaldehyde concentrations over a wide range: from 20 ppm (IDLH) down to 250 ppb within one minute and down to 50 ppb (7% of PEL) within 10 min. These color changes are easily observed even by eye (SI Figure S11).
We have recently constructed and are in the process of testing a fully functional prototype handheld device that makes use of an inexpensive white LED and an ordinary CMOS camera.6 Combined with a low dead volume cartridge, this handheld device provides a rapid, highly sensitive and quantitative method for the portable monitoring of formaldehyde at various concentrations.
Supplementary Material
Acknowledgment
This work was supported through the NIH Genes, Environment and Health Initiative (U01ES016011).
Footnotes
Supporting Information Available: Experimental procedures, additional figures, and database. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Leikauf GD. In: Environmental Toxicants: Human Exposures and Their Health Effects. 3th ed. Lippmann M, editor. New Jersey: John Wiley & Sons, Inc.; 2009. pp. 257–316. [Google Scholar]
- 2.Copenhagen: World Health Organization; WHO Air Quality Guidelines for Europe. (2nd ed.) 2000
- 3.(a) Armour SJ. Washington: GPO; International Task Force 40: Toxic Industrial Chemicals (TICs)-Operational and Medical Concerns. 2001; (b) http://www.cdc.gov/niosh/npg/npgd0293.html.
- 4.(a) Jaffrezic-Renault N, Dzyadevych SV. Sensors. 2008;8:2569. doi: 10.3390/s8042569. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ferus M, Cihelka J, Civis S. Chemicke Listy. 2008;102:417. [Google Scholar]; (c) Pal R, Kim KH. J. Sep. Sci. 2007;30:2708. doi: 10.1002/jssc.200700206. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mohr GJ. Anal. Bioanal. Chem. 2006;386:1201. doi: 10.1007/s00216-006-0647-3. [DOI] [PubMed] [Google Scholar]; (b) Toda K, Yoshioka KI, Mori K, Hirata S. Anal. Chim. Acta. 2005;531:41. [Google Scholar]; (c) Kawamura K, Kerman K, Fujihara M, Nagatani N, Hashiba T, Tamiya E. Sens. Actuators B: Chem. 2005;105:495. [Google Scholar]; (d) Gibson LT, Kerr WJ, Nordon A, Reglinski J, Robertson C, Turnbull L, Watt CM, Cheung A, Johnstone W. Anal. Chim. Acta. 2008;623:109. doi: 10.1016/j.aca.2008.06.002. [DOI] [PubMed] [Google Scholar]; (e) Maruo YY, Nakamura J, Uchiyama M. Talanta. 2008;74:1141. doi: 10.1016/j.talanta.2007.08.017. [DOI] [PubMed] [Google Scholar]; (f) Suzuki Y, Nakano N, Suzuki K. Environ. Sci. Technol. 2003;37:5695. doi: 10.1021/es0305050. [DOI] [PubMed] [Google Scholar]; (g) Mohr GJ, Spichiger UE, Jona W, Langhals H. Anal. Chem. 2000;72:1084. doi: 10.1021/ac991171t. [DOI] [PubMed] [Google Scholar]
- 6.(a) Rakow NA, Suslick KS. Nature. 2000;406:710. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]; (b) Suslick KS. MRS Bulletin. 2004;29:720. doi: 10.1557/mrs2004.209. [DOI] [PubMed] [Google Scholar]; (c) Suslick KS, Bailey DP, Ingison CK, Janzen M, Kosal MA, McNamara WB, III, Rakow NA, Sen A, Weaver JJ, Wilson JB, Zhang C, Nakagaki S. Quim. Nova. 2007;30:677. [Google Scholar]; (d) Musto CJ, Lim SH, Suslick KS. Anal.Chem. 2009;81:6526. doi: 10.1021/ac901019g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. Nat. Chem. 2009;1:562. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Scott SM, James D, Ali Z. Microchim. Acta. 2007;156:183. [Google Scholar]; (b) Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.