Abstract
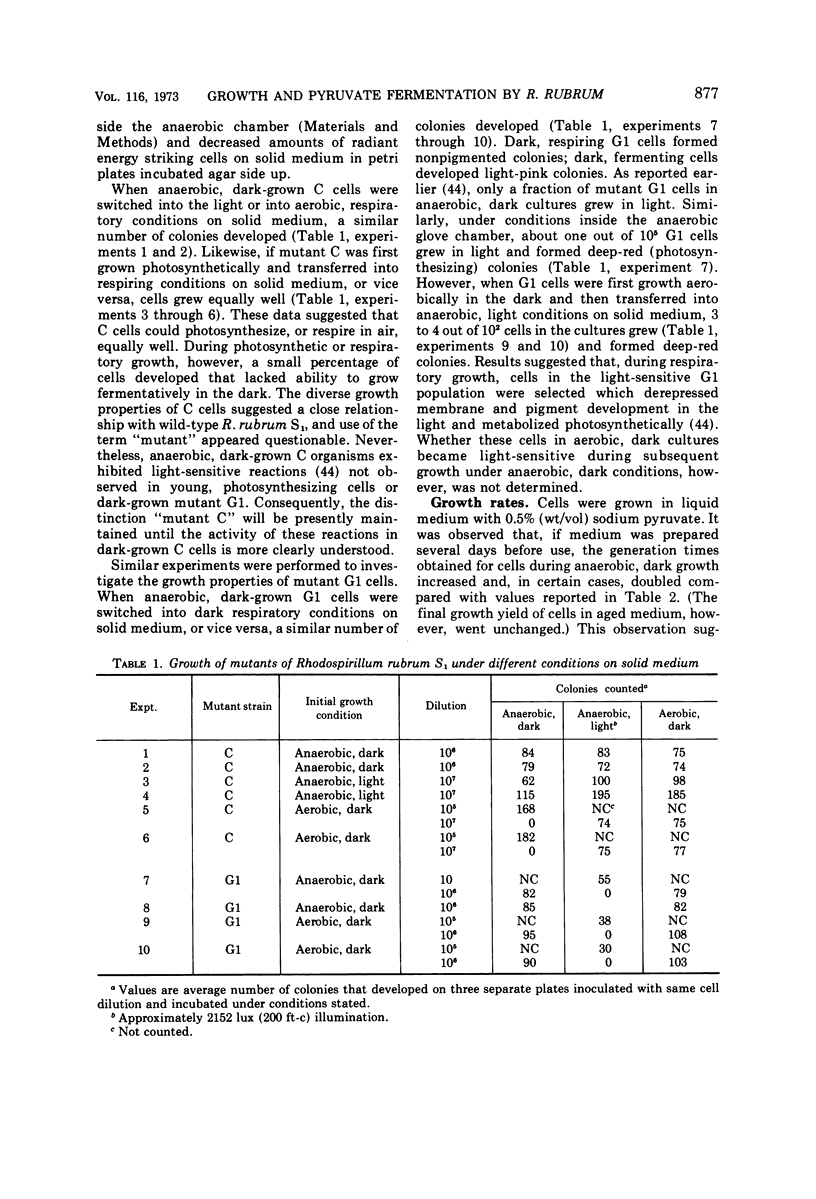
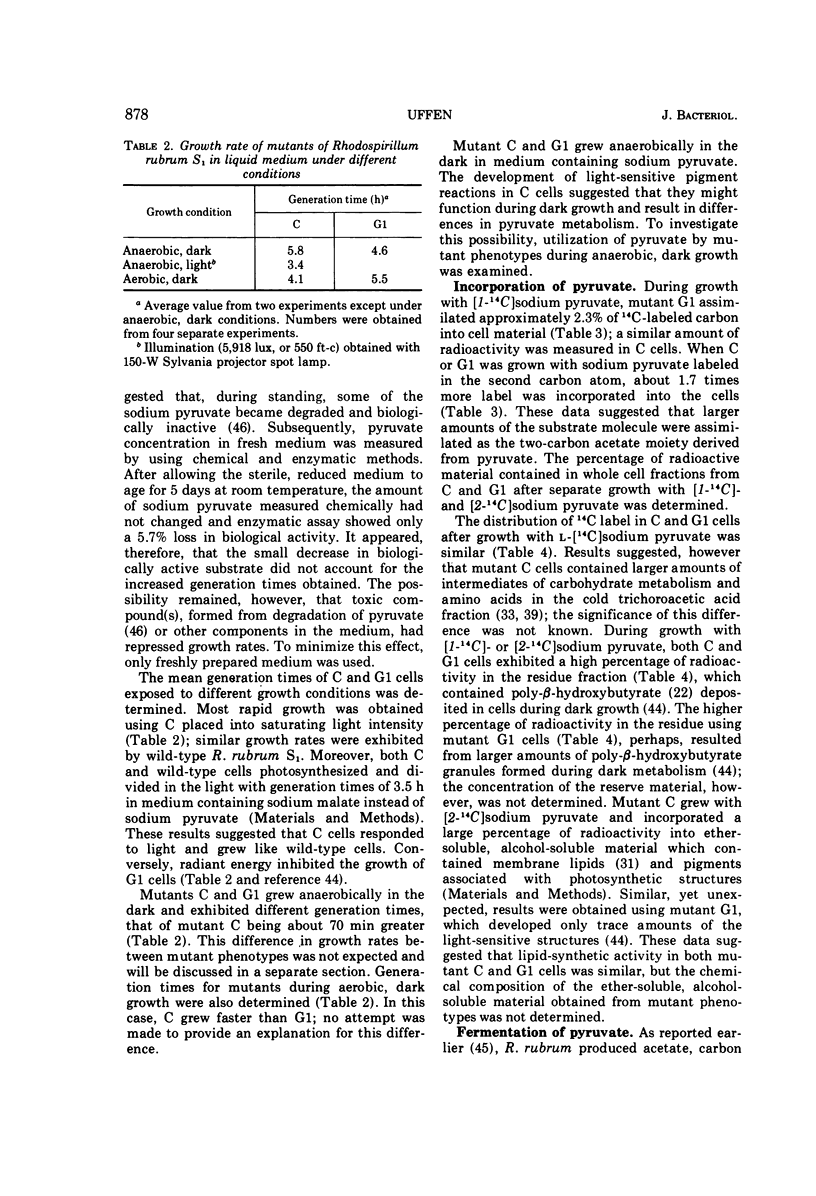
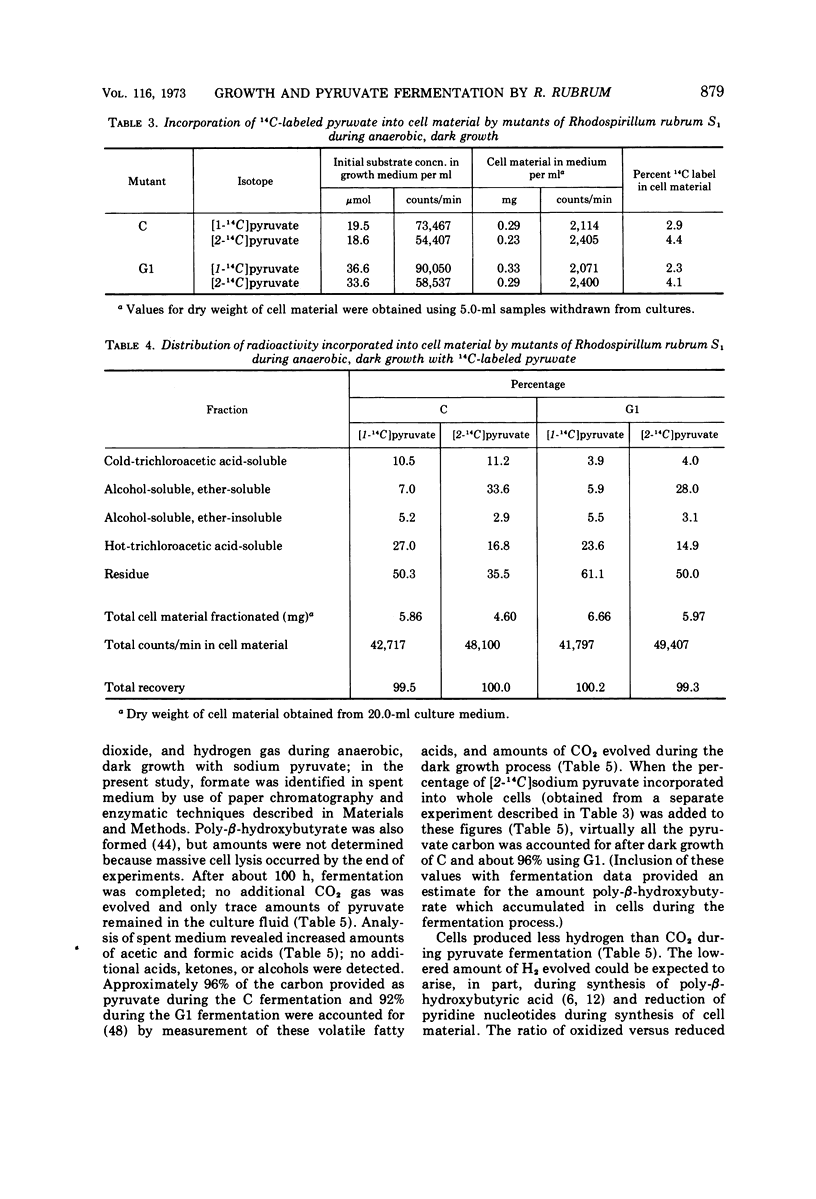
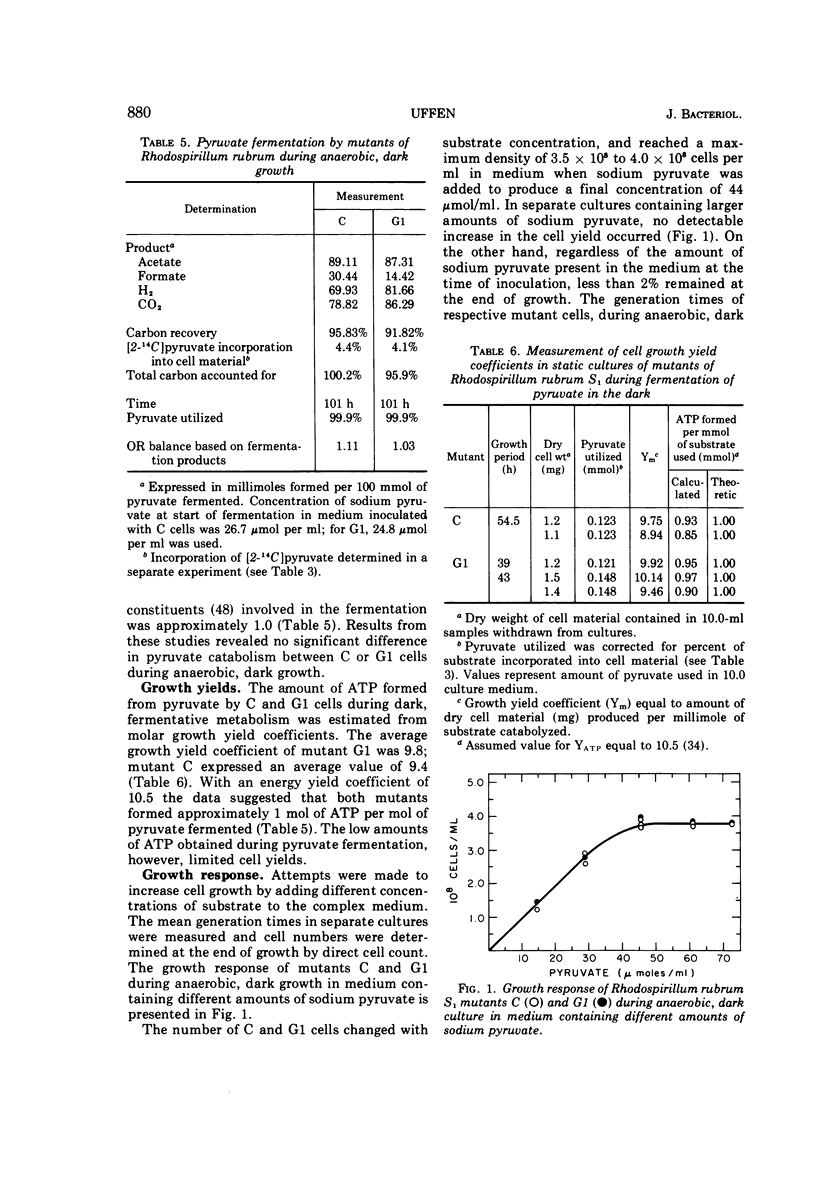
Mutant C and G1 were obtained earlier from Rhodospirillum rubrum S1 during growth in the dark under strict anaerobic conditions in medium containing sodium pyruvate. Mutant C and mutant G1 grew in the dark with generation times of 5.8 h and 4.6 h, respectively. Mutant C cells grew equally well when switched between anaerobic (dark or light) or aerobic, dark conditions. Mutant G1 cells grew only in the dark (anaerobic or aerobic conditions), but a fraction of cells in anaerobic, dark cultures grew when placed in light. This number increased about 3,000-fold when G1 cells were incubated aerobically in the dark. During anaerobic, dark growth, C and G1 organisms incorporated similar amounts of [2-14C]sodium pyruvate. About 34% of the incorporated radioactivity was found in lipid fractions from C cells that developed chromatophores during dark growth. Similar results were obtained using G1 cells, which formed only trace amounts of photosynthetic structures. Both mutants fermented sodium pyruvate and produced acetate, formate, carbon dioxide, and hydrogen gas. Molar growth yield coefficients indicated that the cells obtained about 1 mol of adenosine triphosphate per mol of sodium pyruvate fermented. Results suggested that pyruvate fermentation during dark growth occurred via a pyruvate formate-lyase or the pyruvate ferredoxin-oxidoreductase pathway, or both.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W. NEGATIVE STAINING OF PHOSPHOLIPIDS AND THEIR STRUCTURAL MODIFICATION BY SURFACE-ACTIVE AGENTS AS OBSERVED IN THE ELECTRON MICROSCOPE. J Mol Biol. 1964 May;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- BENNETT R., RIGOPOULOS N., FULLER R. C. THE PYRUVATE PHOSPHOROCLASTIC REACTION AND LIGHT-DEPENDENT NITROGEN FIXATION IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:762–768. doi: 10.1073/pnas.52.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLE-JONES E. W. Occurrence of Indole-3-acetic acid in laminae of Hevea brasiliensis. Nature. 1954 Jan 16;173(4394):127–128. doi: 10.1038/173127b0. [DOI] [PubMed] [Google Scholar]
- Biedermann M., Drews G., Marx R., Schröder J. Der Einfluss des Sauerstoffpartialdruckes und der Antibiotica Actinomycin und Puromycin auf das Wachstum, die Synthese von Bacteriochlorophyll und die Thylakoidmorphogenese in Dunkelkulturen von Rhodospirillum rubrum. Arch Mikrobiol. 1967 Feb 20;56(2):133–147. [PubMed] [Google Scholar]
- Bosshard-Heer E., Bachofen R. Synthese von Specicherstoffen aus Pyruvat durch Rhodospirillum rubrum. Arch Mikrobiol. 1969;65(1):61–75. [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H., ORMEROD J. G., ORMEROD K. S. Photometabolism of Rhodospirillum rubrum: light-dependent dissimilation of organic compounds to carbon dioxide and molecular hydrogen by an anaerobic citric acid cycle. Arch Biochem Biophys. 1962 Apr;97:21–33. doi: 10.1016/0003-9861(62)90039-5. [DOI] [PubMed] [Google Scholar]
- GEST H. Oxidation and evolution of molecular hydrogen by microorganisms. Bacteriol Rev. 1954 Mar;18(1):43–73. doi: 10.1128/br.18.1.43-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Properties of cell-free hydrogenases of Escherichia coli and Rhodospirillum rubrum. J Bacteriol. 1952 Jan;63(1):111–121. doi: 10.1128/jb.63.1.111-121.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert R. E. Effect of oxygen on viability and substrate utilization in Chromatium. J Bacteriol. 1967 Apr;93(4):1346–1352. doi: 10.1128/jb.93.4.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemme J. H., Gest H. Regulatory properties of an inorganic pyrophosphatase from the photosynthic bacterium Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1971 Apr;68(4):721–725. doi: 10.1073/pnas.68.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe J., Schacht J., Möckel W., Höpner T., Vetter H., Jr, Edenharder R. Pyruvate formate-lyase reaction in Escherichia coli. The enzymatic system converting an inactive form of the lyase into the catalytically active enzyme. Eur J Biochem. 1969 Dec;11(2):316–327. doi: 10.1111/j.1432-1033.1969.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Kupfer D. G., Canale-Parola E. Pyruvate metabolism in Sarcina maxima. J Bacteriol. 1967 Oct;94(4):984–990. doi: 10.1128/jb.94.4.984-990.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Midwinter G. G., Krampitz L. O. Properties of the pyruvate formate-lyase reaction. Arch Biochem Biophys. 1971 Apr;143(2):526–534. doi: 10.1016/0003-9861(71)90237-2. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Olsen I., Merrick J. M. Identification of propionate as an endogenous CO2 acceptor in Rhodospirillum rubrum and properties of purified propionyl-coenzyme A carboxylase. J Bacteriol. 1968 May;95(5):1774–1778. doi: 10.1128/jb.95.5.1774-1778.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr An enzymatic method for the determination of formic acid. J Biol Chem. 1957 Nov;229(1):321–328. [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön G., Biedermann M. Bildung flüchtiger Säuren bei der Vergärung von Pyruvat und Fructose in anaerober Dunkelkultur von Rodospirillum rubrum. Arch Mikrobiol. 1972;85(1):77–90. [PubMed] [Google Scholar]
- Uffen R. L. Effect of low-intensity light on growth response and bacteriochlorophyll concentration in Rhodospirillum rubrum mutant C. J Bacteriol. 1973 Nov;116(2):1086–1088. doi: 10.1128/jb.116.2.1086-1088.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Sybesma C., Wolfe R. S. Mutants of Rhodospirrillum rubrum obtained after long-term anaerobic, dark growth. J Bacteriol. 1971 Dec;108(3):1348–1356. doi: 10.1128/jb.108.3.1348-1356.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



