Abstract
The parasite Trypanosoma cruzi, which causes Chagas’ disease, differentiates in the cytosol of its host cell and then replicates and spreads infection, processes that require the long-term survival of the infected cells. Here, we show that in the cytosol, parasite-derived neurotrophic factor (PDNF), a trans-sialidase that is located on the surface of T. cruzi, is both a substrate and an activator of the serine-threonine kinase Akt, an antiapoptotic molecule. PDNF increases the expression of the gene that encodes Akt while suppressing the transcription of genes that encode proapoptotic factors. Consequently, PDNF elicits a sustained functional response that protects host cells from apoptosis induced by oxidative stress and the proinflammatory cytokines tumor necrosis factor–α and transforming growth factor–β. Given that PDNF also activates Akt by binding to the neurotrophic surface receptor TrkA, we propose that this protein activates survival signaling both at the cell surface, by acting as a receptor-binding ligand, and inside cells, by acting as a scaffolding adaptor protein downstream of the receptor.
INTRODUCTION
Chagas’ disease can afflict patients for many years or even decades and commonly starts when the obligate intracellular parasite Trypanosoma cruzi gains access to cells in the skin or in the mucosa after release from reduviid insect excreta. T. cruzi binds to receptors on the surface of host cells, which leads to its internalization in phagolysomes. It then escapes to the cytosol where it differentiates, replicates, grows, and spreads the infection to neighboring cells through the extracellular matrix and to distant cells through the circulation (1, 2). T. cruzi also uses the cell cytosol as reservoir, as exemplified by the infection of adipose tissue in the murine model of Chagas’ disease (3). The crosstalk between T. cruzi and components of the host cytosol is critical for survival of the parasite and the dissemination and maintenance of infection in mammalian hosts; however, the molecular basis underlying the interaction of the parasite with the intracellular milieu remains largely unexplored. For example, little, if anything, is known about why cells stay alive for so long while harboring a large number of trypanosomes that require space, nutrients, and other host-cell factors for proper intracellular parasitism.
We have shown that the glycosylphosphatidylinositol (GPI)–anchored parasite-derived neurotrophic factor (PDNF) of T. cruzi, known mostly for its neuraminidase (4) and sialyltransferase (5) activities, binds to the receptor tyrosine kinases TrkA and TrkC (6, 7). These receptors are typically activated after engagement with the neurotrophins nerve growth factor (NGF) and neurotrophin-3 (NT-3) during development and the repair of the nervous system (8). Neurotrophin–Trk receptor interactions activate downstream signaling cascades, including the phosphatidylinositol 3-kinase (PI3K)–Akt kinase pathway, which enhances cell survival, proliferation, and size, as well as protein synthesis, response to nutrient availability, and other activities that are important for cellular survival and homeostasis (9, 10). Underscoring its mimicry of neurotrophins, the binding of PDNF to TrkA and TrkC induces the survival and differentiation of neurons and Schwann cells (6, 7, 11). Uniquely, the recognition of TrkA by T. cruzi promotes cellular invasion (12). These actions require the activation of downstream signaling pathways, including the PI3K-Akt kinase pathway (6, 7). It is thought that the activation of Trk-dependent PI3K-Akt signaling by T. cruzi is important for the survival of infected cells (6, 7, 12). The interactions between T. cruzi and Trks and other cell surface receptors last for only minutes and, thus, cannot solely account for the protection against the damaging events that result from long-lasting intracellular parasitism. However, host cell defense must be an important factor that enables T. cruzi to establish chronic infection despite a strong immune response to the parasite (13).
PDNF is anchored to the surface of T. cruzi by a GPI linkage (14) and shed into the environment, including the cell cytosol (14–17), so that cytoplasmic PDNF is readily available to interact with Akt and other cytoplasmic signaling factors. Here, we show that Akt phosphorylates PDNF, which in turn activates Akt, increases the expression of the gene that encodes Akt, and inhibits the expression of genes that encode proapoptotic proteins. Consequently, T. cruzi–infected and PDNF-transfected cells strongly resist the potent proapoptotic stimuli tumor necrosis factor–α (TNF-α) and transforming growth factor–β (TGF-β) and oxidative stress induced by hydrogen peroxide (H2O2). PDNF and activated Akt are most abundant late in the T. cruzi intracellular cycle, when the parasite burden is maximal. Thus, the targeting of Akt by T. cruzi could be an important mechanism that underlies the long-term survival of infected cells.
RESULTS
PDNF is a substrate of the Ser-Thr kinase Akt
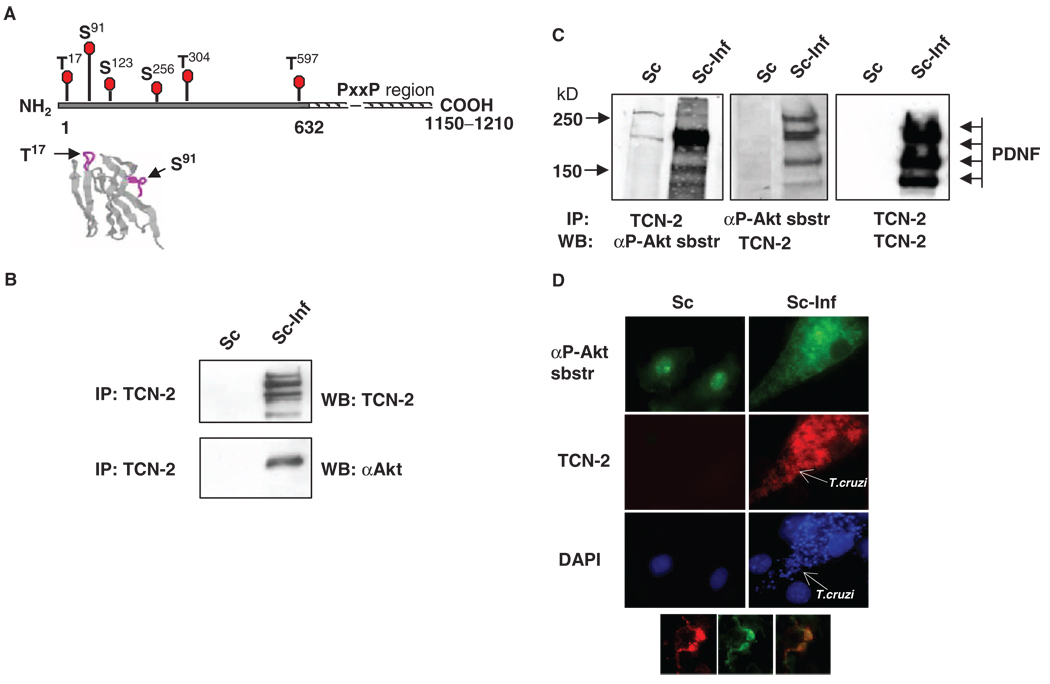
We used a combination of bioinformatics, immunochemistry, intracellular colocalization microscopy, and in vitro enzymatic approaches to address the question of whether PDNF is a substrate of the Ser-Thr kinase Akt [also known as protein kinase B (PKB)]. The optimum Akt phosphorylation motif is R-X-R-X-X-S/T-B, where X and B represent any amino acid residue and bulky hydrophobic residues, respectively, and S or T represent the phosphorylation targets serine and threonine, respectively (18). Scanning the PDNF clone 19Y, which consists of an N-terminal region of 632 amino acid residues that contains the trans-sialidase catalytic domain and a C-terminal region composed of a tandem repeat unit of 12 amino acid residues (Asp-Ser-Ser-Ala-Asn-Gly-Thr-Pro-Ser-Thr-Pro-Ala) (19, 20), the motif-searching program Scansite (http://scansite.mit.edu) (21) predicted the presence of five sites that could be phosphorylated by Akt (Thr17, Ser91, Ser123, Thr304, andThr597) (Fig. 1A and table S1) (20). The Thr17- and Ser91-containing motifs have a β-turn and are located on the surface of PDNF (Fig. 1A, right) (22). Thus, the phosphorylation motifs of PDNF should be readily accessible to Akt if it were to interact with T. cruzi.
Fig. 1.
PDNF interacts with Akt and with an antibody against Akt-phosphorylated substrates in T. cruzi–infected Schwann cells. (A) Putative S and T within PDNF that are targets for phosphorylation by Akt. The N-terminal (solid line) and the C-terminal proline-rich region of tandem 12–amino acid residue repeats (hatched line) (19, 20). Below: Location of Thr17 and Ser91 in β-turns (purple lines) in the three-dimensional structure of PDNF (amino acid residues 1 to 124) (59). (B) PDNF coimmunoprecipitates with Akt. Lysates of uninfected (Sc) or T. cruzi–infected (Sc-Inf) Schwann cells (4 days PI) were immunoprecipitated (IP) with the PDNF-specific mAb TCN-2, followed by Western blotting analysis (WB) with TCN-2 or an antibody against Akt (αAkt). (C) PDNF coimmunoprecipitates with Akt-phosphorylated substrates. Lysates of Sc cells and Sc-Inf cells immunoprecipitated with TCN-2 were incubated with an antibody against Akt-phosphorylated substrates (left, αP-Akt sbstr) or with TCN-2 (right). The same lysates immunoprecipitated with an antibody against Akt-phosphorylated substrates were incubated with TCN-2 (middle). (D) PDNF colocalizes with an Akt-phosphorylated substrate in T. cruzi–infected Schwann cells. Uninfected and T. cruzi–infected Schwann cells were incubated with an antibody against Akt-phosphorylated substrates (green), the anti-PDNF mAb TCN-2 (red) and DAPI (blue). Inset shows two intracellular trypomastigotes that contain Akt-phosphorylated substrate (left) and PDNF (middle); (right) represents merged (left) and (middle). Original magnification, × 1000.
A physical interaction between PDNF and Akt was demonstrated by coimmunoprecipitation (IP) assays. We infected Schwann cells with T. cruzi for 2 hours, removed parasites from the medium overlay by washing, and cultured the cells for 4 days to allow intracellular parasites to differentiate, grow, and multiply. Lysates of uninfected and infected Schwann cells were immunoprecipitated with the monoclonal antibody (mAb) TCN-2, which is specific for the C-terminal tandem repeat unit of PDNF (Fig. 1A) (23), and Western blots of these immunoprecipitates were incubated with PDNF-and Akt-specific antibodies. The results showed that TCN-2 coimmunoprecipitated PDNF and Akt from the lysates of T. cruzi–infected human Schwann cells, but not from uninfected Schwann cells (Fig. 1B).
In addition, immunoprecipitated PDNF was detected with an antibody that was specific for degenerate phosphopeptides of the sequence R-X-R-X-X-pT/S and thus could detect phosphorylated substrates of Akt (21, 24) (Fig. 1C). Converse immunoprecipitation assays showed that proteins coimmunoprecipitated by the antibody specific for phosphorylated substrates of Akt were readily detected with the PDNF-specific antibody TCN-2 (Fig. 1C, middle) and migrated in SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels similarly to PDNF(Fig. 1C, right). Thus, it seemed that Akt phosphorylated PDNF in T. cruzi–infected Schwann cells, a conclusion consistent with fluorescence microscopy analysis that showed a profound increase in the amount of Akt-phosphorylated substrate in Schwann cells that bore cytosolic parasites (Fig. 1D), which colocalized with PDNF on the surface of intracellular T. cruzi trypomastigotes (Fig. 1D, inset, right panel).
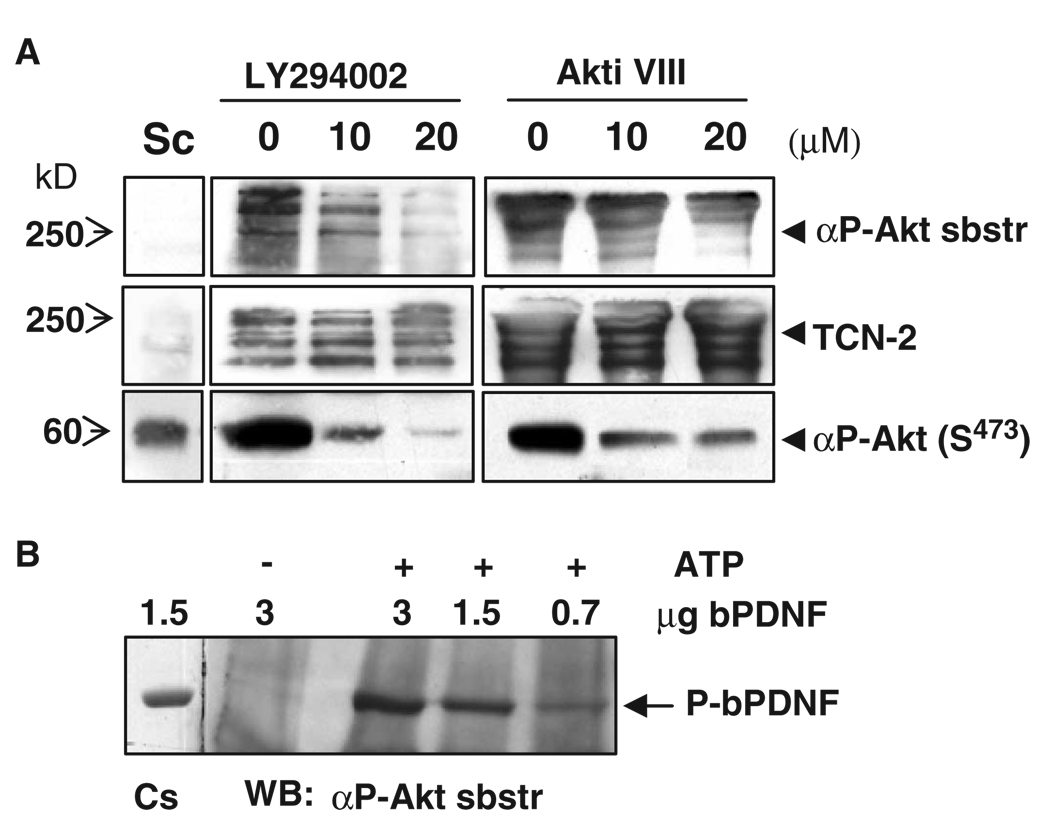
To provide further evidence that the phosphorylation of PDNF depended on host Akt, T. cruzi–infected Schwann cells were treated with LY294002, a pharmacological inhibitor of PI3K, which phosphorylates and activates Akt (10), and with Akti VIII, a specific allosteric inhibitor of Akt that prevents the phosphorylation of Akt at Ser473 and Thr308, which are critical for the activation of Akt (25). Lysates of treated cells were assayed for phosphorylated Akt (pAkt) and, after immunoprecipitation with a PDNF-specific antibody, for the presence of Akt-phosphorylated substrates. The inhibition of the phosphorylation of PDNF corresponded with a reduction in abundance of activated Akt (Fig. 2A). The block in the formation of phospho-PDNF was specific, because inhibitors of the activation of Akt did not affect the expression of unphosphorylated PDNF(Fig. 2A). The conclusion that PDNF was a substrate of Akt was further reinforced by in vitro kinase assays. Mixing unphosphorylated recombinant PDNF isolated from Escherichia coli [bacterial PDNF (bPDNF)] (26) with purified, activated Akt generated phosphorylated PDNF in vitro in a dose-dependent and ATP-dependent manner (Fig. 2B).
Fig. 2.
Akt phosphorylates PDNF in T. cruzi–infected cells and in vitro. (A) Akt-dependent phosphorylation of PDNF in infected cells. Uninfected (Sc) or T. cruzi–infected (Sc-Inf) Schwann cells were treated with vehicle (0), with the PI3K inhibitor LY294002, or with the Akt inhibitor Akti VIII for 24 hours before harvesting. Cells were lysed and lysates were incubated with antibodies against pAkt (Ser473) or, after immunoprecipitation with an antibody against PDNF (TCN-2), with antibodies specific for Akt-phosphorylated substrates (αP-Akt sbstr). (B) Akt phosphorylates PDNF in vitro. Bacterially expressed PDNF (bPDNF) was used as a substrate for immunopurified Akt in kinase reactions with (+) or without (−) ATP. Phosphorylation of PDNF was analyzed with an antibody specific for Akt-phosphorylated substrates. Cs, Coomassie blue–stained bPDNF. Identical results were obtained in at least three experiments.
The activation of Akt in T. cruzi–infected Schwann cells is developmentally regulated
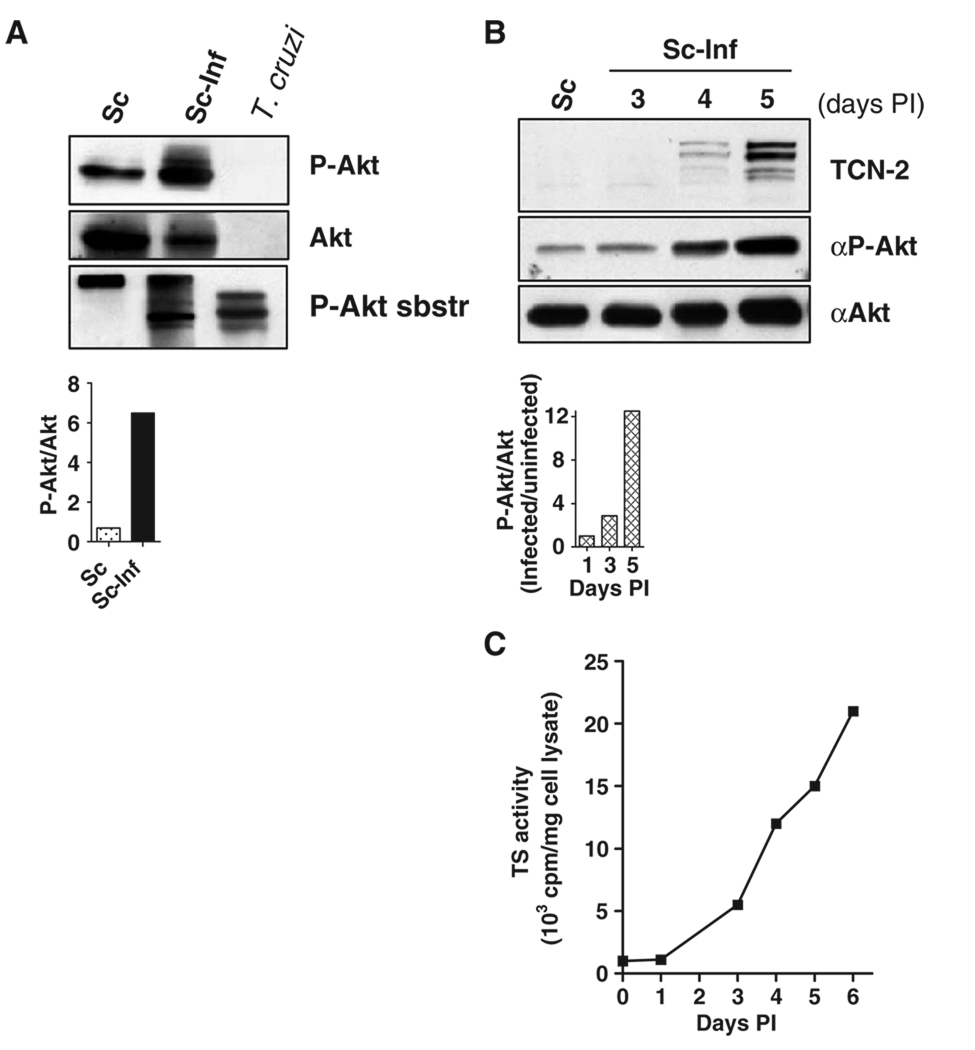
To determine whether intracellular T. cruzi activated Akt, we examined lysates of uninfected and 4- to 5-day–infected Schwann cells by Western blotting with antibodies specific for Akt phosphorylated at Ser473, a marker of the activation of Akt (10), in parallel with antibodies specific for Akt and phosphorylated substrates of Akt. These experiments showed that pAkt increased by sixfold in T. cruzi–infected cells compared to that in uninfected cells (Fig. 3A), which indicated the robust activation of Akt in infected cells. This was accompanied by a substantial increase in the formation of phosphorylated substrates of Akt (Fig. 3A, lower panel), which indicated that the activated Akt was functional.
Fig. 3.
Developmental regulation of the activation of Akt in T. cruzi–infected Schwann cells. (A) Lysates of Schwann cells, uninfected (Sc) or infected with T. cruzi (Sc-Inf), were incubated with antibodies specific for pAkt (Ser473), total Akt, or Akt-phosphorylated substrates (P-Akt-sbstr). Lower graph shows the ratio of pAkt to total Akt in the blot. (B) Activation of Akt increases with the progress of the infection. Uninfected Schwann cells (Sc) or Schwann cells infected with T. cruzi (Sc-Inf) for 3, 4, or 5 days, were lysed and analyzed by Western blotting with antibodies specific for PDNF (TCN-2), pAkt (Ser473) (αP-Akt), and total Akt (αAkt). The lower bar graph shows the ratio of pAkt to total Akt for this blot. At 5 days, Schwann cells were filled with a mixture of amastigotes and mobile trypomastigotes. (C) The trans-sialidase activity of PDNF increases with the progression of infection. Specific trans-sialidase activity was determined in lysates of infected Schwann cells [same samples as in (B)].
Because Schwann cells were filled with T. cruzi 4 to 5 days postinfection (PI), the observed increase in the abundance of pAkt could be an artifact caused by a cross-reaction of the Akt-specific antibodies with an Akt-like enzyme from T. cruzi. However, this possibility could be excluded because the antibodies specific for Akt and for pAkt did not react with purified T. cruzi trypomastigotes (Fig. 3A). In contrast, the antibody specific for phosphorylated substrates of Akt bound to the purified parasites (Fig. 3A). This finding further confirmed that Akt phosphorylated T. cruzi and that the parasites bore Akt-phosphorylated substrates such as PDNF–trans-sialidase.
To determine whether the activation of Akt by T. cruzi was developmentally regulated, we incubated lysates of Schwann cells infected with T. cruzi for 3, 4, or 5 days with antibodies specific for PDNF, activated Akt, and total Akt and assayed them for trans-sialidase enzymatic activity, a widely-studied property of PDNF (5). Confirming earlier results (4, 16), we found that the production of PDNF, as determined by Western blotting and trans-sialidase activity assays, increased with the progression of infection (Fig. 3, B and C). This increase correlated with the formation of pAkt, which was 10-fold more abundant in cells 4 days PI than in uninfected cells (Fig. 3B).
Cytosolic PDNF activates Akt, increases expression of the gene encoding Akt, and inhibits the expression of genes encoding proapoptotic factors
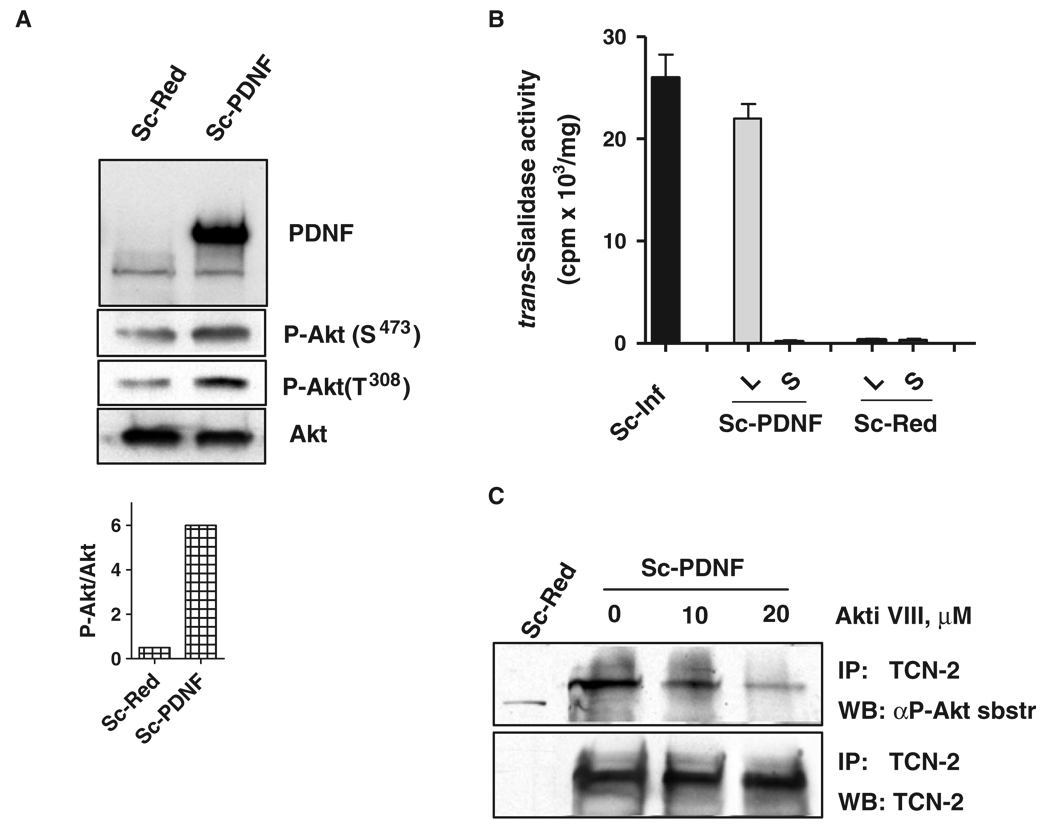
To validate the Akt-dependent phosphorylation of PDNF, we subcloned the full-length complementary DNA (cDNA) of the gene encoding PDNF into the mammalian pIRES2-DsRed-Express bicistronic expression vector. Schwann cells were then transfected either with empty vector or with the plasmid containing the PDNF gene. Schwann cell–expressed PDNF caused a five-fold increase in the abundance of pAkt (Ser473) and a six-fold increase in the abundance of pAkt (Thr308) compared to that in control transfected cells (Fig. 4A and inset), confirming that the increased activation of Akt in T. cruzi–infected cells was, at least in part, triggered by PDNF. The increase in the activation of Akt was not caused by leakage of PDNF into the culture supernatants (Fig. 4B), which otherwise might activate Akt through its binding to TrkC receptors on the surface of Schwann cells. The expression of PDNF in transfected Schwann cells was comparable to that in T. cruzi–infected cells (4 days PI), as determined by measurement of the intrinsic trans-sialidase activity of PDNF (Fig. 4B). Furthermore, precipitation of PDNF from the lysates of PDNF-expressing Schwann cells with the mAb TCN-2 and examination of the immunoprecipitates for the presence of phosphorylated substrates of Akt corroborated the results from the T. cruzi–infected Schwann cells, namely, that the detection of phosphorylated PDNF with the antibody specific for phosphorylated substrates of Akt was dependent on Akt, because the specific Akt inhibitor VIII substantially decreased the extent of the phosphorylation of PDNF (Fig. 4C).
Fig. 4.
PDNF in the cytosol of Schwann cells activates Akt. (A) Lysates of Schwann cells transfected with empty vector (Sc-Red) or with a plasmid encoding PDNF (Sc-PDNF) were analyzed by Western blotting with antibodies against PDNF, pAkt (Ser473), pAkt (Thr308), and Akt. The lower graph shows the ratio of pAkt to Akt in the lysates of Sc-Red and Sc-PDNF cells determined by scanning densitometry of the blot. (B) Specific trans-sialidase activity of infected and transfected Schwann cells. Specific enzyme activity in lysates of Schwann cells infected with T. cruzi (Sc-Inf) and in lysates (L) and culture supernatants (S) of Schwann cells transfected with plasmid encoding PDNF (Sc-PDNF) or with empty vector (Sc-Red). (C) Akt phosphorylates PDNF in transfected cells. Schwann cells transfected with empty vector (Sc-Red) or with a plasmid encoding PDNF (Sc-PDNF) were treated with the Akt inhibitor Akti VIII for 24 hours before harvesting. Cell lysates were immunoprecipitated with the PDNF-specific antibody TCN-2 and samples were then incubated with antibodies against Akt-phosphorylated substrates (αP-Akt sbstr). All the experiments were repeated at least three times with similar results.
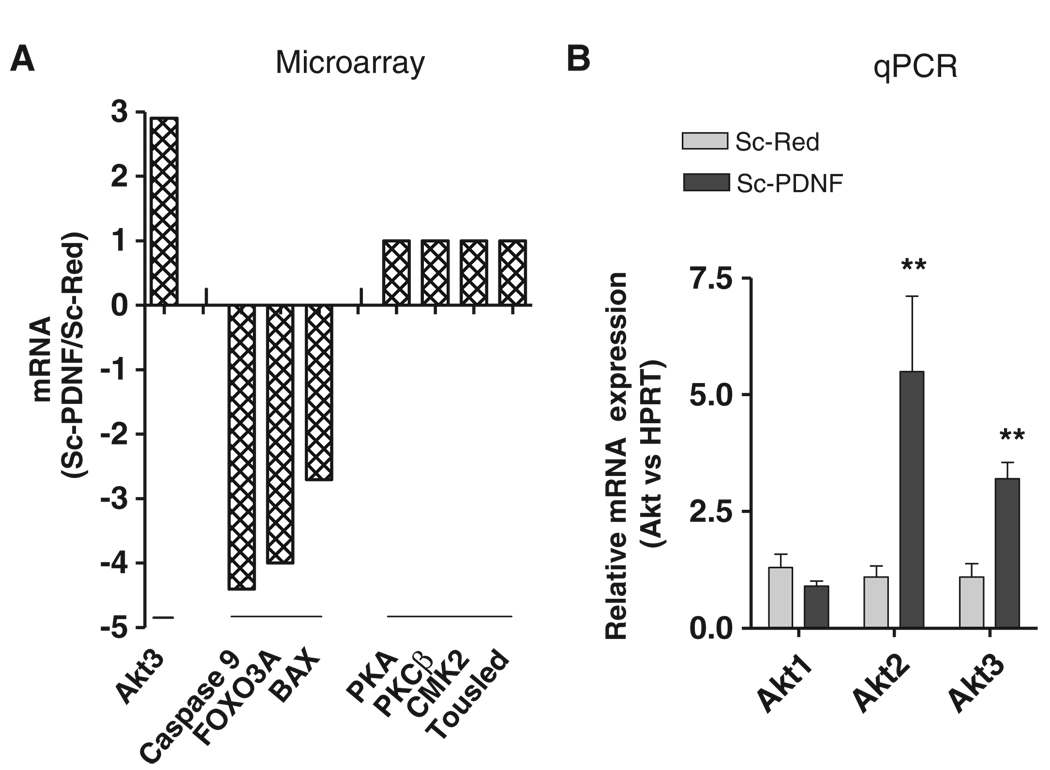
In addition to the enhanced activation of Akt, we found that cytosolic PDNF increased the expression of the gene encoding Akt in Schwann cells as determined by cDNA microarray and real-time polymerase chain reaction (PCR) assays. The results showed that the messenger RNA (mRNA) for isoform 3 of Akt (Akt3) was ~3-fold more abundant in Schwann cells expressing PDNF than in control cells, whereas the expression of the mRNAs for other protein kinases were unaltered (Fig. 5A). In contrast, mRNAs for the proapoptotic proteins caspase-9, the transcription factor FOXO, and the mitochondrial protein BAX were reduced in abundance by ~5.0-, ~4.0- and ~2.6-fold, respectively, in PDNF-expressing Schwann cells compared to those in control cells (Fig. 5A). Real-time PCR confirmed the ~3-fold increased expression of the gene encoding Akt3 in the PDNF-transfected Schwann cells compared to that in control cells. In addition, these experiments showed an increase in the mRNA for Akt2 by ~5.0-fold (P < 0.05) in PDNF-expressing cells compared to that in control cells, but not in the Akt1 mRNA (Fig. 5B).
Fig. 5.
Expression of PDNF in the cytosol of Schwann cells augments transcription of the gene encoding Akt and reduces the transcription of genes encoding proapoptotic factors. (A) Results from microarray analyses are expressed as the ratio of signals from mRNA isolated from Sc-PDNF cells to those of Sc-Red cells. This experiment was repeated twice with similar results. PKA, protein kinase A; PKCβ, protein kinase Cβ; CMK2, cytidine mono-kinase 2; Tousled, tousled kinase. (B) qPCR analysis of the expression of the genes encoding the three isoforms of Akt in Sc-Red cells and Sc-PDNF cells. Results represent the ratio of Akt mRNA relative to that of hypoxanthine-guanine phosphoribosyltransferase (HPRT). Results are presented as the mean ± SD of four independent experiments. **P < 0.05.
Schwann cells bearing cytosolic T. cruzi or PDNF strongly resist exogenous apoptotic stimuli
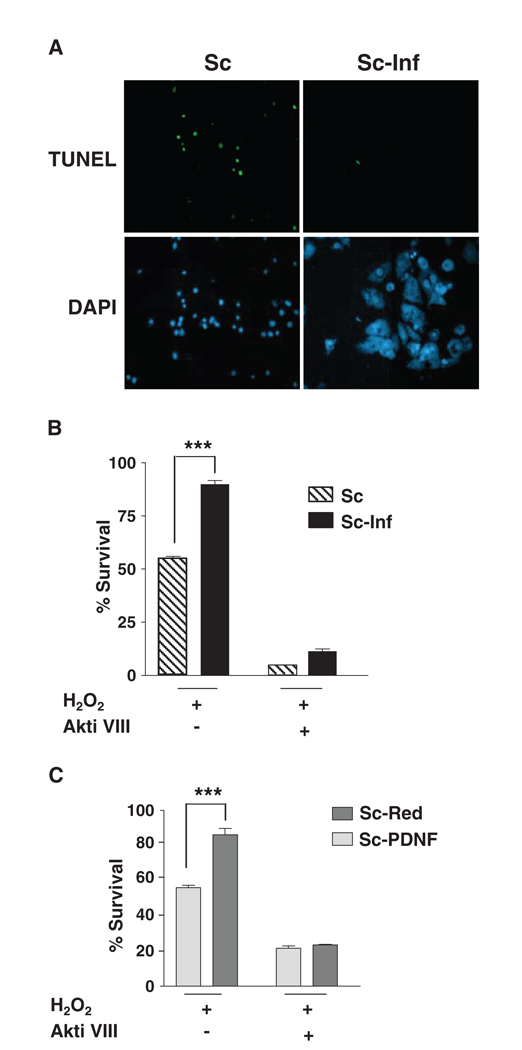
The combination of the activation of Akt, the increased expression of the gene encoding Akt, and the decreased expression of genes encoding proapoptotic factors could represent a strategy by T. cruzi to counter host cell damage. We tested this prediction by determining whether infected Schwann cells or Schwann cells transfected with the PDNF-encoding plasmid resisted the toxic action of H2O2, which causes oxidative stress and caspase-mediated apoptosis in various cell types, including neurons (27, 28). Based on the terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay, we found that, after 6 hours, 100 µM H2O2 induced apoptosis in ~25% of uninfected Schwann cells (Fig. 6A), and, after 24 hours, only half of the cells survived (Fig. 6B). In contrast to uninfected cells, T. cruzi–bearing cells were protected against 100 µM H2O2–induced cellular degeneration (Fig. 6, A and B). Even at 500 µM H2O2, when almost 90% of uninfected cells displayed apoptosis-related DNA damage, few of the T. cruzi–bearing cells showed signs of apoptosis (fig. S1), although parasites seemed to be destroyed, possibly because they are catalase-deficient and unable to neutralize H2O2 (29). Resistance of infected cells to oxidative stress depended on the kinase activity of Akt because it was lost in cells treated with Akti VIII (Fig. 6B). Similar to the infected cells, Schwann cells transfected with a plasmid encoding PDNF were protected from H2O2-induced oxidative stress, and this survival-promoting activity was abrogated after treatment with Akti VIII (Fig. 6C).
Fig. 6.
Schwann cells transfected with a plasmid encoding PDNF or infected with T. cruzi exhibit Akt-dependent resistance to oxidative stress. (A) Uninfected (Sc) or T. cruzi–infected (Sc-Inf) cells were treated with 100 µM H2O2 for 6 hours, fixed, and assessed for apoptosis by the TUNEL assay. Cells were counterstained with DAPI to reveal cell density. Original magnification, × 1000. (B) Infected cells were pretreated with the Akt inhibitor Akti VIII (10 µM) before treatment with 100 µM H2O2 for 24 hours. Cell survival was assessed with an MTT-based assay; (C) Schwann cells transfected with empty vector (Sc-Red) or with a plasmid encoding PDNF (Sc-PDNF) were treated and analyzed for cell survival as described for (B). ***P < 0.001 from three experiments.
To determine whether the oxidative stress–resistant phenotype extended to another apoptotic stimulus relevant to T. cruzi infection in vivo, we assessed whether intracellular PDNF protected human Schwann cells against an immune-based cell death mechanism. The proinflammatory cytokine TNF-α kills Schwann cells synergistically with TGF-β, an event likely related to peripheral nervous system disorders (30). In addition, TNF-α appears to mediate the killing of human Schwann cells by cytotoxic T cells (31). We therefore ascertained whether the introduction of PDNF into the Schwann cell cytosol neutralized the toxicity caused by TNF-α and TGF-β. Based on the TUNEL assay, apoptosis in control Schwann cells increased ~14-fold in medium containing TNF-α (20 ng/ml) and TGF-β (40 ng/ml) (Fig. 7). Introduction of PDNF into the Schwann cell cytosol rescued cells from death caused by TNF-α and TGF-β, because only 2.5 ± 0.7% of the cells underwent apoptosis in the presence of the two cytokines (Fig. 7). The antiapoptotic effect of intracellular PDNF was abolished by the Akt inhibitor Akti VIII (Fig. 7), suggesting that it depended on the activation of Akt by PDNF.
Fig. 7.
Schwann cells transfected with a plasmid encoding PDNF are protected from apoptosis induced by TNF-α and TGF-β. Sc-Red and Sc-PDNF cells were left untreated or were pretreated with the Akt inhibitor Akti VIII (10 µM), cultured for 4 days in DMEM, 1% BSA containing TNF-α (20 ng/ml) and TGF-β1 (40 ng/ml), and analyzed for apoptosis by the TUNEL assay (as described for Fig. 6). (A) Micrographs: original magnification, × 1000. (B) Apoptosis was quantified as percent of the total number of cells that were TUNEL-positive as revealed by DAPI staining. ***P < 0.001 from three experiments.
DISCUSSION
Akt1, Akt2, and Akt3, originally identified as the transforming oncogenes of a murine retrovirus (32), are critical mediators of signal transduction pathways downstream of activated receptor tyrosine kinases (RTKs) and PI3K. Activated Akt promotes cell survival by inhibiting the function of proapoptotic proteins, particularly Bcl-2 homology domain 3 (BH3)–only proteins such as BAD. Once phosphorylated by Akt, BAD binds to the scaffolding adaptor protein 14-3-3, which prevents the release of cytochrome c from mitochondria (33). In addition, Akt inhibits the expression of genes encoding BH3-only proteins, such as the proapoptotic cytokine Fas ligand, by phosphorylating and inactivating transcription factors such as FOXO (34, 35). Akt directly interferes with the caspase cascade by phosphorylating procaspase-9 and rendering it inactive, thereby inhibiting the activation of effector caspases (36). Activated Akt also promotes cell survival through crosstalk with other signaling cascades such as those involving nuclear factor κB (NF-κB) (37) and the mitogen-activated protein kinases (MAPKs) c-Jun N-terminal kinase (JNK) and p38 (38). Finally, the activation of Akt indirectly supports cell survival by increasing uptake of nutrients, metabolism, and maintenance of mitochondrial membrane potential (39).
Here, we demonstrated that Akt interacted with T. cruzi PDNF, a neuraminidase and trans-sialidase, when either the parasite or recombinant PDNF was in the cytosol. The clue for identifying the interaction between T. cruzi and Akt was the bioinformatics Scansite program (21), which predicted five target sites for phosphorylation by Akt in the N-terminal region of PDNF (Fig. 1A). These sites are located in β-hairpin loops on the surface of PDNF (Fig. 1A) and, thus, are readily accessible for phosphorylation by Akt. Hairpin loops or reverse turns commonly mediate specific molecular interactions such as ligand-receptor and antibody-antigen binding (40).
Phosphorylation of PDNF was determined by a phosphorylation site readout that depended on specific antibodies that recognized Akt-phosphorylated substrates, an extremely valuable tool used in the past few years to identify and characterize previously unidentified substrates of Akt (10). Phosphorylation of PDNF by Akt was also demonstrated by an in vitro kinase assay. Based on colocalization studies with antibodies specific for PDNF and for Akt-dependent phospho-peptides, most substrates phosphorylated by Akt in T. cruzi–infected Schwann cells colocalized with PDNF (Fig. 1D). However, PDNF may not be the only T. cruzi protein that is targeted for phosphorylation by Akt, as further analysis of T. cruzi proteomics with the Scansite program revealed at least 8 additional potential substrates of Akt, including Tc85-11, a member of the PDNF-trans-sialidase superfamily thought to mediate T. cruzi–host cell interactions (41) (table S2).
Akt phosphorylates PDNF of intracellular T. cruzi predominantly late in the infection cycle (Fig. 3B), when the parasite burden is large. The generation of phosphorylated PDNF correlated with the enhanced activation of Akt. Phosphorylation of PDNF and enhancement of the activation of Akt was reproduced by transfecting Schwann cells with a plasmid encoding PDNF (Fig. 4A). In addition, intracellular PDNF increased the expression of the gene encoding Akt and inhibited transcription of at least three genes that encode proapoptotic factors (caspase-9, the Bcl2-family member BAX, and the transcription factor FOXO) (Fig. 5). These biochemical, enzymatic, and genetic alterations in T. cruzi–infected and PDNF-transfected cells would likely endow the cells with several properties that promote host cell viability such as the resistance to apoptotic stimuli that we have demonstrated here (Figs. 6 and 7).
How could intracellular PDNF activate Akt? Normally, PI3K-Akt signaling is activated when PI3K, which resides in the cytoplasm, binds, through its regulatory p85 subunit, to either an RTK at the cell surface or to activated adaptor molecules. As a result, PI3K localizes to the plasma membrane, where it phosphorylates the membrane lipid phosphatidy linositol 4,5-bisphosphate (PIP2) to produce phosphatidylinositol 3,4,5-trisphosphate (PIP3). This leads to the activation of downstream signaling pathways that control cell growth and survival. Integrated cascades of phosphorylation of tyrosine and serine or threonine residues play an essential role in transducing signals through the PI3K-Akt pathway. The mechanism by which this occurs is through the pTyr-binding domains Src homology 2 (SH2) and phosphotyrosine binding (PTB) (42), and, although less well-studied, through pSer- and pThr-binding motifs (43). Phosphorylation on serine or threonine residues, initially discovered as a way to allosterically regulate catalytic activity, can also create sites for pSer- or pThr-binding signaling molecules, which result in their recruitment to signaling complexes. Such molecules currently include 14-3-3 proteins, WW domains, forkhead-associated regions, WD40 repeats, and Polo box domains (43, 44). In particular 14-3-3 proteins are implicated in the regulation of cell cycle, apoptosis, and activation of the Raf-MAPK pathway (43) and in PI3K-Akt signaling and cell survival (45). Proteins that bind to motifs that contain pSer or pThr sites generally recognize sequences that overlap with sites phosphorylated by Akt (46) and thus can potentially complex with PDNF through its pSer- and pThr-containing motifs. On the other hand, the C-terminal proline-rich region (PRR) of PDNF contains multiple PxxP repeats (P, proline; x, any aliphatic residue) (Fig. 1A), which suggest a capability to interact with signaling proteins that contain SH3 domains, such as Src and PI3K (47). For example, the polyproline motif PxxP of NS1, an influenza A virus protein, is essential for its binding to the p85β subunit of PI3K and the activation of the PI3K-Akt pathway in response to viral infection (48).
The biological functions of PDNF are mostly studied in the context of its sialic acid–binding properties (5, 49), largely because these studies are focused on the neuraminidase (4) and sialyltransferase (26, 50, 51) activities of the protein. However, PDNF has also intrinsic neurotrophic properties (11) that result from its binding to the neurotrophin receptors TrkA and TrkC (6, 7),which do not require its neuraminidase or trans-sialidase activities (11, 52). The interaction of T. cruzi with TrkA drives the invasion of neuronal and nonneuronal cells (12). Also independent of trans-sialidase activity is the promotion of the survival of endothelial cells through as yet unknown receptors (53).
The neuraminidase and trans-sialidase activities of PDNF require substrates (sialyl and galactosyl residues) that are available only in the extracellular environment. These extracellular actions of T. cruzi PDNF are short-lived because they occur when T. cruzi is establishing its long-term intracellular habitat. Therefore, it was most surprising to find that PDNF interacted with a signaling protein (Akt) located in the cell cytosol where sialo-glycoconjugates, the substrates of neuraminidase–trans-sialidase, are absent.
In summary, the results presented here are consistent with T. cruzi, through PDNF, interacting with and activating Akt signaling while residing in the cytosol of infected cells. Activation was sustained (lasted days) and was most prominent late in the infection cycle when parasite burden was maximal, a time when the intracellular niche of T. cruzi needs the most protection. In addition, T. cruzi activates Akt, albeit transiently, at the port of entry of the cell habitat, when it binds to the NGF receptor TrkA to invade cells (9, 11). Collectively, these findings characterize a parasite effector protein with a unique dual activity to modulate host signaling responses during infection, and present a previously undescribed paradigm for the interaction of a pathogen with its host.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin stock, fetal calf serum (FCS), and G418 were from GIBCO; DAPI (4′,6-diamidino-2-phenylindole), and LY294002 were from Sigma, and the Akt inhibitor Akti VIII was from EMD Chemicals. Antibodies against pAkt (Ser473 and Thr308), Akt, and phosphorylated substrates of Akt were from Cell Signaling Technology. The PDNF-specific mAB TCN-2 (T. cruzi neuraminidase monoclonal antibody-2) was isolated as described earlier (23). Alexa-conjugated secondary antibodies against mouse and against rabbit were from Molecular Probes and the horseradish peroxidase (HRP)–conjugated secondary antibody was from Chemicon. The ECL kit was purchased from PerkinElmer. The antiprotease cocktail was from Roche Molecular Biochemicals. Recombinant, full-length PDNF cDNA (clone 19Y) was expressed in E. coli and purified by affinity chromatography, and the trans-sialidase activity assay was performed as described before (26).
Cell culture and infections with T. cruzi
Immortalized human Schwann cells (54) were maintained in DMEM supplemented with 10% FCS and penicillin-streptomycin at 37°C in 5% CO2. Infections were performed with T. cruzi trypomastigotes (Silvio strain) at 2 × 105 parasites per milliliter or at a parasite-to-cell ratio of 50:1. After 2 or 3 hours, monolayers were washed to remove unattached parasites, and cells were then maintained in medium containing 2% FCS for 3 to 5 days to complete the intracellular infection cycle.
Cloning of TCNA and transfections
The coding region of the gene encoding PDNF (formerly T. cruzi neuraminidase, or TCNA) was amplified from the TS19y clone (20) with the primers TCNA-F, 5′-CCGCTCGAGATGGGTTTGGCACCCGGATCG-3′, and TCNA-R, 5′-TCCCCGCGGTCAGAAAACTGCCATAAA (restriction sites for Xho I and Sac II are underlined) and inserted into the pIRES2-DsRed-Express vector (Clontech). Selected recombinant plasmids were purified with the Qiagen kit, sequenced at Tufts University Core facility, and used for transfections. Cells were plated at 1 × 105 cells/ml in 100-mm plates 20 hours before their transfection with 10 µg of total DNA per plate with the FuGENE HD transfection reagent (Roche Diagnostics) as recommended by the manufacturer. Transfected cells were selected with G418 (1 mg/ml) and analyzed for the presence of PDNF by Western blotting and trans-sialidase activity assays (26). Schwann cells transfected with the plasmid encoding PDNF are named Sc-PDNF cells, whereas Schwann cells transfected with empty vector are named Sc-Red cells.
Immunoprecipitations and Western blotting
Cell monolayers were lysed with lysis buffer [20 mM tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM glycerophosphate, 1 mM Na3VO4, leupeptin (1 µg/ml), and 1 mM phenylmethylsulfonyl fluoride] on ice for 10 min, cleared by centrifugation (12,000g for 10min at 4°C), and immunoprecipitated with an antibody specific for Akt-phosphorylated substrate or with TCN-2 at 4°C overnight. Immunoprecipitates were collected with protein G–Sepharose for 2 hours at 4°C, washed with lysis buffer, and resuspended in SDS–sample buffer for SDS-PAGE and Western blotting analysis. In other experiments, total cell lysates (30 to 50 µg of protein) were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and incubated with the relevant primary antibodies, followed by incubation with HRP-conjugated secondary antibody, and visualization by ECL.
Immunocytochemistry
Monolayers of infected or transfected cells were fixed with 4% para-formaldehyde for 30 min at 4°C, permeabilized in 0.2% Triton X-100, and blocked in 10% goat serum overnight at 4°C. Cells were incubated with the appropriate primary antibody in 5% goat serum overnight at 4°C, washed in phosphate-buffered saline and incubated with Alexa Fluor 488–conjugated secondary antibody against rabbit and Alexa Fluor 568–conjugated secondary antibody against mouse (Molecular Probes). Cell nuclei were visualized with DAPI (250 ng/ml). Images were analyzed by fluorescence microscopy with Spot camera software (Diagnostic Instruments).
In vitro kinase assays
Akt was purified from lysates of serum-stimulated Schwann cell with an antibody specific for Akt and protein G–Sepharose. Isolated complexes were resuspended in kinase buffer [25 mM tris-HCl (pH 7.5), 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10mM MgCl2] supplemented with 1 mM adenosine triphosphate (ATP) and PDNF isolated from a bacterial expression system (bPDNF). After 30 min at 30°C, the kinase reaction was terminated with SDS-Laemmli sample buffer, and samples were analyzed by Western blotting by incubation with an antibody against Akt-phosphorylated substrates. PDNF was identified by comparison with a Coomassie blue-stained sample of bPDNF run in parallel with the samples for Western blotting.
Apoptosis assays
Infected Schwann cells, Sc-Red cells, and Sc-PDNF cells were treated for 24 hours with 100 to 500 µM H2O2 with or without pretreatment with the Akt inhibitor Akti VIII (10 µM), fixed, and visualized by fluorescence microscopy after staining with DAPI and TUNEL reagents with the In Situ Cell Death Detection kit (Roche). Cell survival was measured by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)-based CellTiter kit (Promega). Alternatively, Sc-Red and Sc-PDNF cells were grown for 4 days in DMEM, 1% bovine serum albumin (BSA) with or without TNF-α (20 ng/ml) and TGF-β1 (40 ng/ml) and with or without Akti VIII (10 µM).
cDNA array hybridizations
These analyses were performed as described previously (55, 56). The concentration and quality of total RNA isolated from Sc-Red cells and Sc-PDNF cells were estimated by spectrophotometry (A260 nm/A280 nm of 1.9 to 2.1) and by agarose gel electrophoresis. Hybridization and data analysis were performed by the Tufts Expression Array Core with cDNAs prepared from Sc-Red cells and Sc-PDNF cells labeled with aminoallyl (aa)-dUTP Cy3 or Cy5 dye, respectively. The two differently dye-labeled cDNAs were hybridized with the same microarray slide containing 48,500 human genes (Microarrays Inc) for 16 hours. After washing, the slide was scanned at 550 and 649 nm for Cy3 and Cy5 dyes, respectively, in a ScanArray 4000 scanner (PerkinElmer). Images were overlaid and analyzed with QuantArray spot quantitation software (Packard BioChip Technologies). The ratio of both fluorescence intensities for each spot reflected the ratio of each gene expressed in the control and treatment samples. Local background values were subtracted from the spot intensities and filtered on the basis of three standard deviations above background. For each gene, ratios of red (Cy5) over green (Cy3) intensities (I) were calculated and normalized through a Lowess fit of the log2 ratios [log2(ICy5/ICy3)] over the log2 of the total intensity [log2(ICy5 + ICy3)]. Mean ratios were calculated from the duplicate spots, and only values with a covariance (CV) b0.5 were further taken into account. Normalized ratios that were statistically significant with a two-tailed t test (5% level) between the dye-swap repeat and higher than 1 or lower than −1 (log2 scale) were considered differentially expressed.
Quantitative real-time PCR
These assays were performed as described previously (57, 58). In brief, RNA was isolated with the Trizol reagent and chloroform, cDNA was synthesized from500 ng of total RNA with Superscript III reverse transcriptase primed with random hexamers, and quantitative real-time PCR (qPCR) reactions were performed with QuantiTect SYBR Green PCR kit (Qiagen) on an Applied Biosystems 7300 Real-Time PCR system. Conditions for the PCR reactions were as follows: 95°C for 15 min, followed by 50 cycles at 94°C for 15 s, 54°C for 30 s, and 72°C for 45 s, concluding with a dissociation stage to measure amplification product specificity. Primers used to calculate Akt expression were synthesized as described previously (57). Experiments were performed in triplicate, and fold differences were calculated by the 2−ΔΔCt method [ΔΔCt = (Cttarget gene − Ctβ-actin)treated − (Cttarget gene − Ctβ-actin)untreated] as described previously (58).
Data analysis
The results from the experiments were expressed as the means ± SEM. Statistical difference was evaluated with the unpaired t test.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: Location, invasion, retention. Nat. Rev. Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 2.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas’ disease. Clin. Microbiol. Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE. The adipocyte as an important target cell for Trypanosoma cruzi infection. J. Biol. Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 4.Pereira ME. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983;219:1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- 5.Schenkman S, Eichinger D, Pereira ME, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 6.Chuenkova MV, PereiraPerrin M. Chagas’ disease parasite promotes neuron survival and differentiation through TrkA nerve growth factor receptor. J. Neurochem. 2004;91:385–394. doi: 10.1111/j.1471-4159.2004.02724.x. [DOI] [PubMed] [Google Scholar]
- 7.Weinkauf C, Pereiraperrin M. Trypanosoma cruzi promotes neuronal and glial cell survival through the neurotrophic receptor TrkC. Infect. Immun. 2009;77:1368–1375. doi: 10.1128/IAI.01450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 9.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuenkova MV, Pereira MA. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell. 2000;11:1487–1498. doi: 10.1091/mbc.11.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Melo-Jorge M, PereiraPerrin M. The Chagas’ disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe. 2007;1:251–261. doi: 10.1016/j.chom.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Golgher D, Gazzinelli RT. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity. 2004;37:399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg I, Prioli RP, Ortega-Barria E, Pereira ME. Stage-specific phospholipase C-mediated release of Trypanosoma cruzi neuraminidase. Mol. Biochem. Parasitol. 1991;46:303–305. doi: 10.1016/0166-6851(91)90054-a. [DOI] [PubMed] [Google Scholar]
- 15.Frevert U, Schenkman S, Nussenzweig V. Stage-specific expression and intracellular shedding of the cell surface trans-sialidase of Trypanosoma cruzi. Infect. Immun. 1992;60:2349–2360. doi: 10.1128/iai.60.6.2349-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg IA, Prioli RP, Mejia JS, Pereira ME. Differential expression of Trypanosoma cruzi neuraminidase in intra- and extracellular trypomastigotes. Infect. Immun. 1991;59:464–466. doi: 10.1128/iai.59.1.464-466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prioli RP, Mejia JS, Aji T, Aikawa M, Pereira ME. Trypanosoma cruzi: Localization of neuraminidase on the surface of trypomastigotes. Trop. Med. Parasitol. 1991;42:146–150. [PubMed] [Google Scholar]
- 18.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 19.Pereira ME, Mejia JS, Ortega-Barria E, Matzilevich D, Prioli RP. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J. Exp. Med. 1991;174:179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuenkova M, Pereira M, Taylor G. trans-Sialidase of Trypanosoma cruzi: Location of galactose-binding site(s) Biochem. Biophys. Res. Commun. 1999;262:549–556. doi: 10.1006/bbrc.1999.1154. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 2001;19:348–353. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]
- 22.Amaya MF, Watts AG, Damager I, Wehenkel A, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, Alzari PM. Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure. 2004;12:775–784. doi: 10.1016/j.str.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Prioli RP, Mejia JS, Pereira ME. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J. Immunol. 1990;144:4384–4391. [PubMed] [Google Scholar]
- 24.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 25.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: Structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scudder P, Doom JP, Chuenkova M, Manger ID, Pereira ME. Enzymatic characterization of β-d-galactoside α2,3-trans-sialidase from Trypanosoma cruzi. J. Biol. Chem. 1993;268:9886–9891. [PubMed] [Google Scholar]
- 27.Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3β pathway regulates apoptotic and cytoprotective signaling responses. J. Biol. Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenoy K, Wu Y, Pervaiz S. LY303511 enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via hydrogen peroxide–mediated mitogen-activated protein kinase activation and up-regulation of death receptors. Cancer Res. 2009;69:1941–1950. doi: 10.1158/0008-5472.CAN-08-1996. [DOI] [PubMed] [Google Scholar]
- 29.Docampo R, Moreno SN. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev. Infect. Dis. 1984;6:223–238. doi: 10.1093/clinids/6.2.223. [DOI] [PubMed] [Google Scholar]
- 30.Skoff AM, Lisak RP, Bealmear B, Benjamins JA. TNF-α and TGF-β act synergistically to kill Schwann cells. J. Neurosci. Res. 1998;53:747–756. doi: 10.1002/(SICI)1097-4547(19980915)53:6<747::AID-JNR12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Bonetti B, Valdo P, Ossi G, De Toni L, Masotto B, Marconi S, Rizzuto N, Nardelli E, Moretto G. T-cell cytotoxicity of human Schwann cells: TNFα promotes fasL-mediated apoptosis and IFNγ perforin-mediated lysis. Glia. 2003;43:141–148. doi: 10.1002/glia.10235. [DOI] [PubMed] [Google Scholar]
- 32.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 33.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 34.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci. STKE. 2003;2003 doi: 10.1126/stke.2003.172.re5. RE5. [DOI] [PubMed] [Google Scholar]
- 35.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 36.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 37.Romashkova JA, Makarov SS. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 38.Kim AH, Sasakic T, Chao MV. JNK-interacting protein 1 promotes Akt1 activation. J. Biol. Chem. 2003;278:29830–29836. doi: 10.1074/jbc.M305349200. [DOI] [PubMed] [Google Scholar]
- 39.Plas DR, Thompson CB. Akt-dependent transformation: There is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 40.Hughes RM, Waters ML. Model systems for β-hairpins and β-sheets. Curr. Opin. Struct. Biol. 2006;16:514–524. doi: 10.1016/j.sbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Marroquin-Quelopana M, Oyama S, Jr, Aguiar Pertinhez T, Spisni A, Aparecida Juliano M, Juliano L, Colli W, Alves MJ. Modeling the Trypanosoma cruzi Tc85-11 protein and mapping the laminin-binding site. Biochem. Biophys. Res. Commun. 2004;325:612–618. doi: 10.1016/j.bbrc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe MB. Phosphotyrosine-binding domains in signal transduction. Nat. Rev. Mol. Cell Biol. 2002;3:177–186. doi: 10.1038/nrm759. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe MB, Elia AE. Phosphoserine/threonine-binding domains. Curr. Opin. Cell Biol. 2001;13:131–138. doi: 10.1016/s0955-0674(00)00189-7. [DOI] [PubMed] [Google Scholar]
- 44.Seeburg DP, Pak D, Sheng M. Polo-like kinases in the nervous system. Oncogene. 2005;24:292–298. doi: 10.1038/sj.onc.1208277. [DOI] [PubMed] [Google Scholar]
- 45.Barry EF, Felquer FA, Powell JA, Biggs L, Stomski FC, Urbani A, Ramshaw H, Hoffmann P, Wilce MC, Grimbaldeston MA, Lopez AF, Guthridge MA. 14-3-3:SHC scaffolds integrate phoshoserine and phosphotyrosine signaling to regulate phosphatidylinositol 3-kinase activation and cell survival. J. Biol. Chem. 2009;284:12080–12090. doi: 10.1074/jbc.M807637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu MY, Cai S, Espejo A, Bedford MT, Walker CL. 14-3-3 interacts with the tumor suppressor tuberin at Akt phosphorylation site(s) Cancer Res. 2002;62:6475–6480. [PubMed] [Google Scholar]
- 47.Kaneko T, Li L, Li SS. The SH3 domain—A family of versatile peptide- and protein-recognition module. Front. Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- 48.Shin YK, Li Y, Liu Q, Anderson DH, Babiuk LA, Zhou Y. SH3 binding motif 1 in influenza A virus NS1 protein is essential for PI3K/Akt signaling pathway activation. J. Virol. 2007;81:12730–12739. doi: 10.1128/JVI.01427-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buscaglia CA, Campo VA, Frasch AC, Di Noia JM. Trypanosoma cruzi surface mucins: Host-dependent coat diversity. Nat. Rev. Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- 50.Buschiazzo A, Tavares GA, Campetella O, Spinelli S, Cremona ML, Paris G, Amaya MF, Frasch AC, Alzari PM. Structural basis of sialyltransferase activity in trypanosomal sialidases. EMBO J. 2000;19:16–24. doi: 10.1093/emboj/19.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schenkman S, Pontes de Carvalho L, Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J. Exp. Med. 1992;175:567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuenkova MV, PereiraPerrin M. A synthetic peptide modeled on PDNF, Chagas’ disease parasite neurotrophic factor, promotes survival and differentiation of neuronal cells through TrkA receptor. Biochemistry. 2005;44:15685–15694. doi: 10.1021/bi0512039. [DOI] [PubMed] [Google Scholar]
- 53.Dias WB, Fajardo FD, Graca-Souza AV, Freire-de-Lima L, Vieira F, Girard MF, Bouteille B, Previato JO, Mendonca-Previato L, Todeschini AR. Endothelial cell signalling induced by trans-sialidase from Trypanosoma cruzi. Cell. Microbiol. 2008;10:88–99. doi: 10.1111/j.1462-5822.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 54.Chuenkova MV, Furnari FB, Cavenee WK, Pereira MA. Trypanosoma cruzi trans-sialidase: A potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9936–9941. doi: 10.1073/pnas.161298398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 56.Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J. Biol. Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 57.Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecol. Oncol. 2004;94:785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Buschiazzo A, Amaya MF, Cremona ML, Frasch AC, Alzari PM. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol. Cell. 2002;10:757–768. doi: 10.1016/s1097-2765(02)00680-9. [DOI] [PubMed] [Google Scholar]
- 60.We thank J. Sharon, R. Lewis, and P. Tsichlis for reading the manuscript and for useful suggestions. NIH grants NS40574 and NS42960 supported this work.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.