Abstract
Changes in cerebral blood flow (CBF), volume (CBV), and oxygenation (blood-oxygenation level dependent (BOLD)) during functional activation are important for calculating changes in cerebral metabolic rate of oxygen consumption (CMRo2) from calibrated functional MRI (fMRI). An important part of this process is the CBF/CBV relationship, which is signified by a power-law parameter: γ=ln (1+ΔCBV/CBV)/ln (1+ΔCBF/CBF). Because of difficulty in measuring CBF and CBV with MRI, the value of γ is therefore assumed to be ∼0.4 from a prior primate study under hypercapnia. For dynamic fMRI calibration, it is important to know if the value of γ varies after stimulation onset. We measured transient relationships between ΔCBF, ΔCBV, and ΔBOLD by multimodal MRI with temporal resolution of 500 ms (at 7.0 T) from the rat somatosensory cortex during forepaw stimulation, where the stimulus duration ranged from 4 to 32 secs. Changes in CBF and BOLD were measured before the administration of the contrast agent for CBV measurements in the same subjects. We observed that the relationship between ΔCBF and ΔCBV varied dynamically from stimulation onset for all stimulus durations. Typically after stimulation onset and at the peak or plateau of the ΔCBF, the value of γ ranged between 0.1 and 0.2. However, after stimulation offset, the value of γ increased to 0.4 primarily because of rapid and slow decays in ΔCBF and ΔCBV, respectively. These results suggest caution in using dynamic measurements of ΔCBF and ΔBOLD required for calculating ΔCMRo2 for functional stimulation, when either ΔCBV has not been accurately measured or a fixed value of γ during hypercapnia perturbation is used.
Keywords: neuronal activity, oxygen consumption, perfusion
Introduction
For quantitative functional MRI (fMRI), the relationship between stimulus-induced changes in cerebral blood flow (CBF) and volume (CBV) are important because changes in cerebral metabolic rate of oxygen consumption (CMRo2) can be calculated from calibrating the blood-oxygenation level-dependent (BOLD) contrast (Kida et al, 2000; Hyder et al, 2001). The relationship between ΔCBF and ΔCBV signified by γ =ln(1+ΔCBV/CBV)/ln(1+ΔCBF/CBF) is an important part of the calibration because in many studies it is either not measured or cannot be easily measured (e.g., in humans). Although recent human fMRI studies (Davis et al, 1998; Kim et al, 1999; Hoge et al, 1999) have calculated ΔCMRo2 using ΔBOLD and ΔCBF measured during stimulation, these predictions were generated using the fixed value of γ of 0.38 obtained from a prior PET study in primates under hypercapnia (Grubb et al, 1974). Several groups have observed different values of γ when measured by MRI and other non-MRI methods during functional challenges as opposed to hypercapnia (Jones et al, 2001; 2002; Mandeville et al, 1999a; Ito et al, 2001).
For dynamic fMRI calibration, it is essential to know how the value of γ varies after stimulation onset. Very few studies have examined the transient relationships between ΔCBF and ΔCBV because of difficulties in measuring CBF and CBV from the same subject with high temporal resolution. For example, studies in both humans (Feng et al, 2003) and rats (Liu et al, 2004) have reported the prediction of ΔCMRo2 by measuring high temporal resolution ΔCBF, but had to use a fixed value of γ derived from the PET hypercapnia study. A more direct prediction of ΔCMRo2 had been made by Mandeville et al (1999a) using MRI measurement for ΔCBV with a superparamagnetic contrast agent and laser-Doppler flowmetry (LDF) measurement for ΔCBF. While optical imaging techniques can measure changes in CBV (from oxy-and deoxyhemoglobin) and CBF (from LDF) concurrently in the same subject to estimate ΔCMRo2 (Jones et al, 2001, 2002), the approach suffers from restricted field of view of the cortical surface (i.e., surface pial vessels) and potential partial volume effects between optical imaging and LDF techniques. Here we measured transient relationships between stimulation-induced ΔCBF, ΔCBV, and ΔBOLD with temporal resolution of 500 ms from the rat somatosensory cortex in the same subjects, using multimodal MRI. A preliminary account of this work has appeared earlier (Kida and Hyder, 2004).
Materials and Methods
Animal Preparation
Experiments were conducted on 8 male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing between 220 and 260 g. Rats were initially anesthetized with 1.5% halothane in 70%/30% N2O/O2 and then tracheotomized for artificial ventilation and maintained on ∼1% halothane in 70%/30% N2O/O2 for all surgical procedures. A femoral artery was cannulated with a polyethylene catheter (PE-50) to withdraw blood samples for blood gas analysis and to monitor blood pressure. A femoral vein was cannulated with a polyethylene catheter (PE-10) to inject contrast agent (AMI-227, Advanced Magnetics Inc., Cambridge, MA, USA). Intraperitoneal catheters (PE-50) were placed for the administration of drugs. Ventilation parameters were adjusted to maintain arterial blood gas tension within normal range for all animals in this study (pH 7.38±0.05, pCO2 38.7±5.2 mm Hg, pO2 138±28 mm Hg). The scalp was retracted and removed to reveal the skull around the bregma. A pair of needle copper electrodes was inserted beneath the skin of the forepaw. All whiskers of rats were cut to avoid contaminating a signal from whisker region of somatosensory. A temperature-controlled water blanket was placed under the rat's torso to maintain body temperature (∼37°C) throughout the experiments.
After all surgical procedures, halothane anesthesia was discontinued and anesthesia was maintained with an 80 mg/kg intraperitoneal bolus of α-chloralose, followed by every half hour intraperitoneal injection at 20 mg/kg, and as shown previously (Mandeville et al, 1999a, 1999b; Kida et al, 2001; 2006), maintains stable physiology for repeated scans on the same subject. Rats were immobilized with d-tubocurarine chloride (initial 0.5 mg/kg; supplemental 0.25 mg/kg/h; intraperitoneally). Before fMRI measurement, approximately 2 h was allowed for clearance of halothane (Venkatasubramanian et al, 1996) after switching anesthesia to α-chloralose. A block design of forepaw stimulation (intensity 2 mA, pulsed duration 0.3 ms, frequency 3 Hz) was applied with different durations (4, 8, 16, and 32 secs).
MRI Experiments
All fMRI data were obtained on a modified 7.0 T horizontal-bore spectrometer (Bruker Instruments, Billerica, MA, USA) using an 80 mm volume-coil for homogeneous transmission and 10 mm surface-coil for local reception. Multislice gradient echo images were acquired to adjust the fMRI slice positioning over the somatosensory cortex. After positioning, shimming was performed manually with waterline band width of <20 Hz across a 10 mm slice.
Single-shot gradient echo echo planar imaging (EPI) for BOLD fMRI experiment were used: data matrix=32 ×32, in-plane resolution=430 ×430 μm, slice thickness=2 mm, echo time (TE)=20 ms, and repetition time (TR) =500 ms. Multislice fMRI data were obtained with two flip angles, lower 20° and higher 60°, providing BOLD- and CBF-weighted data, respectively (Smith et al, 2002; Kida et al, 2004).
The absolute CBF under resting condition was acquired with arterial spin labeling technique (Schwarzbauer et al, 1996) using gradient echo EPI data and multiple inversion recovery times ranging from 100 to 2000 ms to calculate slice-selective and nonslice-selective maps for longitudinal relaxation time: slice excitation=2 mm, slice inversion= 10 mm, data matrix=32 ×32, in-plane resolution= 430 ×430 μm, TE=20 ms, and TR=8 secs. A single-exponential recovery fit to the inversion recovery time data was used to create slice-selective and nonslice-selective tissue water longitudinal relaxation time images (Hyder et al, 2000). The BOLD data with the two flip angles in conjunction with the arterial spin labeling data provided the magnitude of CBF change (Kida et al, 2004).
Changes in CBV were measured by a superparamagnetic contrast agent after the CBF and BOLD measurements. Blood volume susceptibility was raised through serial injections (2 mg/kg ×4≈8 mg/kg) of an iron oxide contrast agent AMI-227, which remains in the intravascular space for several hours (Kennan et al, 1998), and errors associated with this CBV technique have been discussed in the past (Kida et al, 2000). The magnitude of CBV change was calculated by combining the absolute and relative values of apparent transverse relaxation time (T2*) during forepaw stimulation without and with administration of contrast agent (Kida et al, 2000; Hyder et al, 2001). Absolute values in T2* were obtained by gradient echo EPI data and multiple TE values ranging from 10 to 100 ms: slice excitation=2 mm, a data matrix=32 ×32, in-plane resolution=430 ×430 μm, and TR=8 secs. A single-exponential fit to the TE data provided observed values of T2*. The relative change in T2* during forepaw stimulation was obtained from the equation, ΔS/S∼ –TE ×Δ(1/T2*), where ΔS/S is the change in the BOLD signal and Δ(1/T2*) is the change in relaxation rate. The low flip angle BOLD data were used for the magnitude of CBV change because inflow effects from large blood vessels are minimal with this contrast at high field (Menon, 2002; Kida et al, 2004).
Data Analysis
All fMRI data were processed with MATLAB software and programs written in-house (Math Works, Natick, MA, USA). For each fMRI experiment, t-statistic and ΔS/S maps were created on a pixel-by-pixel basis from images in the prestimulation and the stimulation periods. Head movement artifacts were identified by a center-of-mass analysis and data with movement artifacts were discarded from analysis (Yang et al, 1998).
High temporal resolution data for ΔBOLD (low flip angle) and ΔCBV were analyzed without any additional postprocessing of the respective data, as shown previously (Mandeville et al, 1999a). However, the high temporal resolution CBF-weighted data were subject to a deconvolution process with a previously measured transfer function for each given stimulus duration, as shown previously (Kida et al, 2004), to obtain high temporal resolution data for ΔCBF. For each rat, three or four voxels located in the middle layers of the somatosensory cortex were selected, averaged, and compared. The chosen voxels, based on high statistical threshold, had to be contiguous and be present for all stimulus durations in all modalities. The peak values of ΔCBF, ΔCBV, and ΔBOLD responses were used to calculate ΔCMRo2 as described previously (Kida et al, 2000; Hyder et al, 2001). Since the current experiments were conducted at high field, the intravascular contributions to BOLD calibration are negligible, as discussed previously (Kida et al, 2000; Hyder et al, 2001). All data are presented as mean=s.d.
Results
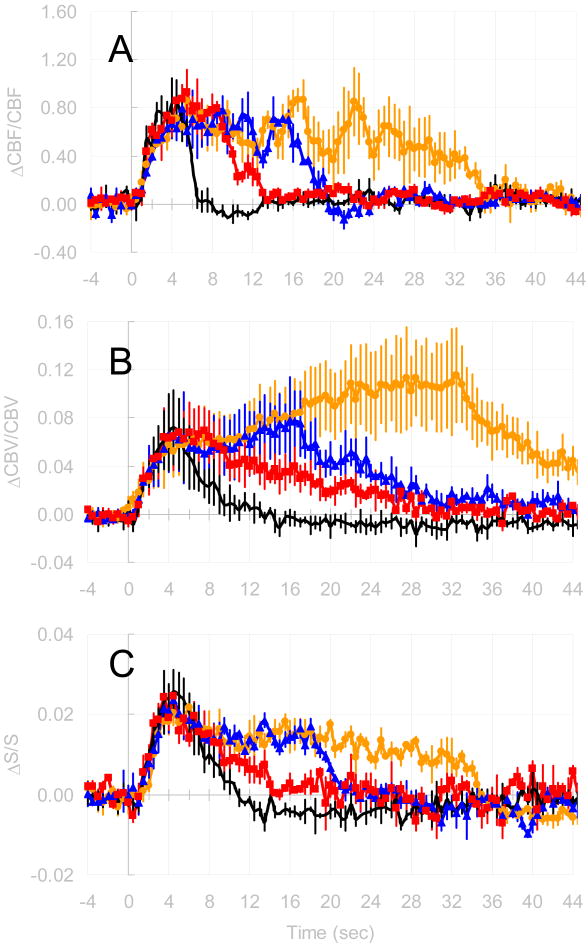
We measured ΔCBF, ΔCBV, and ΔBOLD responses to forepaw stimulation in the rat somatosensory cortex with temporal resolution of 500 ms. Figure 1 shows the time courses of ΔCBF, ΔCBV, and ΔBOLD for the same voxels, in a single rat, for all stimulus durations. These time courses were averaged over three voxels, located in the central layers of the somatosensory cortex, which showed significant changes to forepaw stimulation. The increases in the ΔCBF and ΔBOLD reached peak levels faster than the ΔCBV. The averaged peak values of changes in CBF, BOLD, and CBV from all stimulus duration were 98±31%, 2±1%, and 11±4%, respectively. The greatest mismatch of time course between ΔCBF, ΔCBV, and ΔBOLD were detected after stimulation offset: the CBF signal reached baseline levels the fastest (<5 secs); the CBV signal reached baseline levels the slowest (>30 secs); and the BOLD signal showed a prolonged poststimulation undershoot (>20 secs). It should be noted, however, that the contrast-to-noise ratio of the undershoot portion of the BOLD signal was lower than the overshoot and was detected with significance by averaging across subjects (data not shown). The slow poststimulus dynamics of CBV with MRI contrast agent, as described in the past (Kennan et al, 1998; Mandeville et al, 1999a), is attributed to a delayed venous compliance (Mandeville et al, 1999b). Comparison of values of ΔCMRo2 calculated with γ determined experimentally in this study (see below) or γ set to 0.38 obtained from the prior PET study shows that ΔCMRo2/CMRo2 has the potential to be underestimated by as much as 30% if γ is not measured directly (data not shown).
Figure 1.
Time courses of (a) ΔCBF, (b) ΔCBV and (c) ΔBOLD during forepaw stimulation from a single rat. Time courses were averaged from three significant voxels in the middle layers of the somatosensory cortex. The different colors indicate the different stimulus durations (—— 4 s, —■— 8 s, —▲— 16 s, and —●— 32 s).
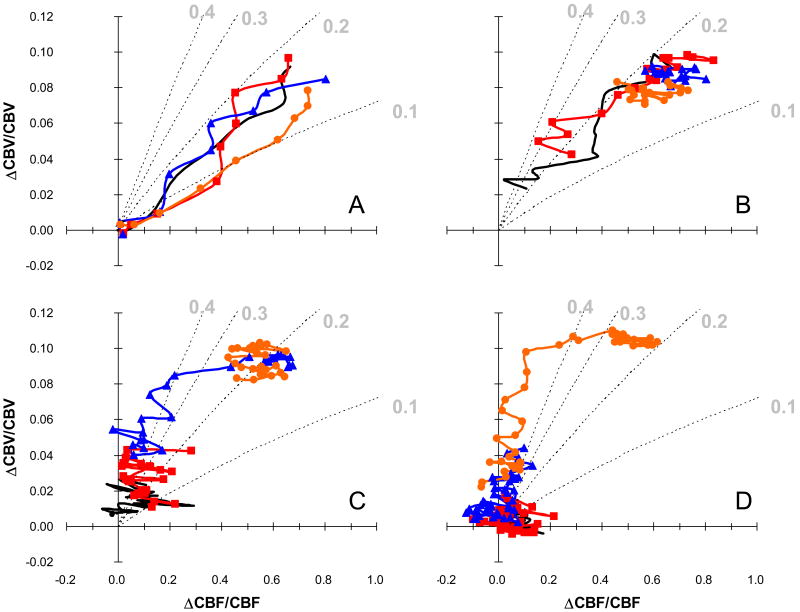
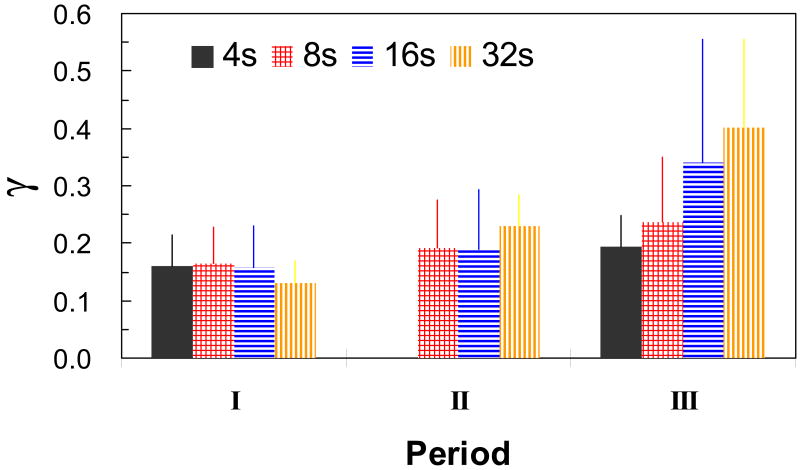
The concurrent measurements of ΔCBF and ΔCBV in the same subjects allowed us to compare directly the flow–volume relationship with the value of γ. Figure 2 represents the detailed comparison between changes in CBF and CBV during the different stimulus durations from all animals (i.e., 12 data sets from 8 rats where 4 rats had both forepaws stimulated but each individually). Since after stimulation onset and offset ΔCBF always preceded ΔCBV, the γ value was initially small and gradually increased. For a clearer illustration of these trends, the time course of CBF changes was divided into three regimes, as shown in Figure 3: periods I, II, and III represented the phases of CBF rise, CBF peak or plateau (≥4 secs), and CBF decline, respectively. In period I, the values of γ, ranging from 0.13 to 0.17, were assessed to be independent of the stimulus duration as the differences were not statistically significant. Similarly in period II, the values of γ, ranging from 0.19 to 0.23, were also determined to be independent of the stimulus duration because the differences were not statistically significant, but absolute values increased in magnitude slightly from period I. Note that for period II, the stimulus duration of 4 secs was not included because the peak value had not been reached. In period III, the values of γ, ranging from 0.17 to 0.39, were found to be dependent on the stimulus duration and the values increased as the stimulus duration increased from 4 to 32 secs. Between the stimulus durations of 4 and 8 secs, no significant differences were found in the γ values for the different periods I, II, and III. However, for stimulus durations of 16 and 32 secs, the values of γ increased between periods I, II, and III quite significantly (P<0.03; P<0.01, respectively). The most marked changes were found in the 32 secs stimulation protocol between periods I to II and II to III (P<0.01).
Figure 2.
Detailed comparison between ΔCBF and ΔCBV for all stimulus durations in terms of the γ value. The relationships are shown for (a) 0 to 4 s, (b) 4 to 12 s, (c) 12 to 24 s, and (d) 24 to 44 s after stimulation onset. Data were averaged over all animals (n = 12 from 8 animals). The dotted contour lines indicate the theoretical relationship between ΔCBF and ΔCBV for different γ values (i.e., 0.1, 0.2, 0.3, and 0.4). The different colors indicate the different stimulus durations (—— 4 s, —■— 8 s, —▲— 16 s, and —●— 32 s).
Figure 3.
The averaged values of γ in three periods following stimulation onset (n = 12 from 8 animals). Periods I, II, and III represent the phases of CBF rise, CBF plateau, and CBF decline, respectively. Note there were no γ values reported period II for the 4 s duration stimulation because of rapid rise and fall of the signal. The different colors indicate the different stimulus durations (filled 4 s, checkered 8 s, horizontal lines 16 s, and vertical lines 32 s).
Discussion
In the present study, we have shown the temporal relationships between changes in CBF, CBV, and BOLD responses to forepaw stimulation in the rat (Figure 1). Of particular importance is the relationship between time courses of CBF and CBV (Figures 2 and 3). These dynamic measurements are critical for the steps needed to estimate CMRo2 changes from calibrated fMRI. The observed changes in CBF (Silva et al, 2000; Kida et al, 2004), CBV (Kennan et al, 1998; Mandeville et al, 1999a), and BOLD (Silva et al, 1999; Kida et al, 2004) are similar to prior observations from the rat brain made with similar MRI methods as used in this study. However, in this study, we report the different measurements, all by MRI, in the same subject for various stimulus durations in the α-chloralose anesthetized model.
While MRI contrast agent-based CBV method measures changes in total blood volume, it is the venous fraction that is needed for ΔCMRo2 calculations with BOLD calibration. A first-order assumption is that the venous fraction is the dominant factor contributing to the total change. If the arterial fraction does contribute in the same extent as the venous fraction, then the venous CBV contribution for the ΔCMRo2 calculations (from calibrated fMRI) would be reduced by approximately half and which in turn would increase the value of ΔCMRo2 even more than present estimations. In partial support of this proposal, there are recent PET (Ito et al, 2005) and MRI (Lee et al, 2001) studies under hypercapnia, which show that the dominant changes in the vascular bed are in the arterial side, and in addition, there is a recent suggestion that during neural stimulation the arterial side may be quite dominant (Kim et al, 2006).
Using the steady-state BOLD model suggested by Ogawa et al (1993), several groups (Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999) have calculated ΔCMRo2 for functional activation using ΔCBF and ΔBOLD measurements. The approach is, first, to obtain a calibrating factor (or constant) under hypercapnia, where it is assumed that ΔCMRo2 is negligible, by measuring ΔCBF and ΔBOLD. Similarly, ACBF and ΔBOLD are measured again, but during functional activation. However, in both experiments, the ΔCBV is assumed from a fixed γ value, usually 0.38. When these different ΔCBF and ΔBOLD measurements are used together, ΔCMRo2 can be calculated during functional activation. However, the approach with hypercapnia perturbation for calibration of fMRI must be treated with caution because two critical assumptions are used. To obtain the calibrating factor (or constant), the approach applies hypercapnia perturbation with the assumption that CMRo2 is unchanged (Yang and Krasney, 1995). However, other reports have shown significant increases in CMRo2 during hypercapnia (Hovarth et al, 1994; Berntman et al, 1978; Hemmingsen et al, 1979), although these studies were performed under anesthesia. In addition, it is well known that there are astrocytic enzymes that can use CO2 as an alternative energy source when it is present in abundance (Hyder et al, 2006) and hypercapnic levels that exceed the normal CO2 arteriovenous difference, even by a few units of mm Hg, can be considered in excess of what the tissue needs because of the high solubility of CO2 (Ursino et al, 1989a, 1989b).
If the CMRo2 increases by even 10% during mild hypercapnia, the calibrating factor may be miscalculated, resulting in the underestimation of change in CMRo2 during functional activation. The other assumption in this approach is that the value of γ of 0.38 obtained during hypercapnia perturbation is fixed to calculate change in CMRo2 for functional stimulation. In the current study, the value of γ during forepaw stimulation was found to be significantly smaller than the value during hypercapnia. Under steady-state condition, if the value of γ of 0.38 is used for estimation of CMRo2, the ratio of changes in CMRo2 to CBF is ∼0.4 (Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999), as opposed to when the measured values of γ are used and the ratio is ∼0.7. It should be pointed out that the latter approach (i.e., the ratio of changes in CMRo2 to CBF is ∼0.7) has been validated by 13C-MRS measurements previously (Kida et al, 2000; Hyder et al, 2001). These results clearly indicate that the use of higher γ value, that is, overestimation of changes in CBV, during functional stimulation, significantly underestimates changes in CMRo2 by calibrating fMRI.
The value of γ during functional challenge (i.e., sensory stimulation) has been measured by MRI and other methods in the rat. Mandeville et al (1999a) reported the values of γ ranged from 0.18 and 0.36 using CBV changes measured by MRI contrast agents and CBF changes measured by LDF. Recently, Jones et al (2001, 2002) have shown the values of γ ranged from 0.25 to 0.30 (i.e., for 20 secs whisker stimulation and 60 secs CO2 perturbation) using optical imaging for CBV and LDF for CBF under similar anesthetized conditions, but slightly higher stimulation frequency than in the present and prior MRI studies investigating flow–volume relationship of the same model (i.e., Mandeville et al, 1999a; Silva et al, 1999). It is well known that the dynamic CBF changes measured by LDF are quantitative (Kida et al, 2004), but it may underestimate the actual fractional change in CBF because the technique does not allow absolute measure of CBF (Silva et al, 1999). Thus, the value of γ may be potentially overestimated using LDF for ΔCBF in conjunction with other methods for ΔCBV during functional challenges. Despite these technical differences, the γ values for short stimulus durations are comparable between our current MRI results and the prior optical studies in this animal model. While our results are more directly applicable for rodent studies, it is valuable to compare with some prior human studies.
A PET study has also reported smaller values of γ, ∼0.3, during visual stimulation in human (Ito et al, 2001). More recently, a new MRI method that measures a complex mixture of oxygen extraction and blood volume has shown CBV-related dynamics (Lu et al, 2003) mimic the time courses of CBF. Although these variations from different studies may be, in part, because of varied spatial resolutions for the different methods (i.e., MRI, PET, LDF, and optical imaging) and the actual source for the measurement of CBV (i.e., hemoglobin content versus plasma content), these studies and the current study suggest that the value of γ during functional challenge is significantly smaller than the prior hypercapnia PET results (Grubb et al, 1974). In part, because of these concerns and others, there has been a revived interest in developing new MRI methods for CBV measurements, which do not completely rely on contrast agents (Lu et al, 2003; Stefanovic and Pike, 2005; Kim and Kim, 2005). However, these methods do not provide the temporal resolution for dynamic values of γ, but future studies will have to address the implications of possible hematocrit changes associated with CBV measurements. In addition, the issue of varying flow–volume relationship with the stimulus frequency (Ito et al, 2001) has to be studied more closely in animal models.
Because the event-related fMRI studies using repeatedly short stimulus duration are widely employed in cognitive studies, dynamic calibration of fMRI for changes in CMRo2 representing fast responses in neuronal activity requires a detailed understanding of transient changes in CBF and CBV in conjunction with BOLD. Temporal mismatch between CBF and CBV after stimulus offset was found for long stimulus durations, which is in agreement with prior observations in the anesthetized rat (Mandeville et al, 1999a, 1999b) and the awake primate (Leite et al, 2002). Since short stimulus durations of 4 secs, which is usually used in event-related studies, indicates that the similar trend was observed in the period after onset and offset of stimulation, the usage of the single value of γ, but smaller than traditionally used, may be reasonable for estimation of transient changes in CMRo2. Because the steady-state BOLD model assumes that CMRo2 is proportional to CBF and the arteriovenous oxygen difference, the steady-state model cannot be directly applied for transient changes in CMRo2 unless more information about direct tissue oxygen measurements are used. Since dynamic changes in CBF and CMRo2 are mediated by oxygen transport processes (Herman et al, 2006) within the capillary, between the capillary and the tissue, and within the tissue, new approach will be needed for transient calibration of fMRI (Maciejewski et al, 2004).
Acknowledgments
We thank engineers Drs Fuqiang Xu and Kevin Behar for helpful discussions and extend appreciation for continuous support from QNMR (qnmr.yale.edu) and MRRC (mrrc.yale.edu) staff. FH thanks Arman and Leila for motivation.
This research is supported by grants from the National Institutes of Health (DC-03710 to FH, MH-67528 to FH) and National Science Foundation (DBI-0095173 to FH).
References
- 1.Berntman L, Dahlgren N, Siesjo BK. Cerebral blood flow and oxygen consumption in the rat brain during extreme hypercarbia. Anesthesiology. 1978;50:299–305. doi: 10.1097/00000542-197904000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI—mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng CM, Liu HL, Fox PT, Gao JH. Dynamic changes in the cerebral metabolic rate of O2 and oxygen extraction ratio in event-related functional MRI. Neuroimage. 2003;18:257–262. doi: 10.1016/s1053-8119(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 4.Grubb RL, Raichle ME, Eichling JO, Ter Pogosian MM. The effects of changes in pCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- 5.Hemmingsen R, Barry DI, Hertz MM. Cerebrovascular effects of central depressants—a study of nitrous oxide, halothane, pentobarbital and ethanol during normocapnia and hypercapnia in the rat. Acta Pharmacol Toxicol. 1979;45:287–295. doi: 10.1111/j.1600-0773.1979.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 6.Herman P, Trubel HK, Hyder F. A multiparametric assessment of oxygen efflux from the brain. J Cereb Blood Flow Metab. 2006;26:79–91. doi: 10.1038/sj.jcbfm.9600165. [DOI] [PubMed] [Google Scholar]
- 7.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovarth I, Sandor NT, Ruttner Z, McLaughlin AC. Role of nitric oxide in regulating cerebrocortical oxygen consumption and blood flow during hypercapnia. J Cereb Blood Flow Metab. 1994;14:503–509. doi: 10.1038/jcbfm.1994.62. [DOI] [PubMed] [Google Scholar]
- 9.Hyder F, Renken R, Kennan RP, Rothman DL. Quantitative multi-modal functional MRI with blood oxygenation level dependent exponential decays adjusted for flow attenuated inversion recoveries (BOLDED AFFAIR) Magn Reson Imag. 2000;18:227–235. doi: 10.1016/s0730-725x(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 10.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain—towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 11.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal–glial glucose oxidation and glutamatergic–GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S. Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:852–857. doi: 10.1038/sj.jcbfm.9600076. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Takahashi K, Hatazawa J, Kim SG, Kanno I. Changes in human regional cerebral blood flow and cerebral blood volume during visual stimulation measured by positron emission tomography. J Cereb Blood Flow Metab. 2001;21:608–612. doi: 10.1097/00004647-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry—the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- 15.Jones M, Berwick J, Mayhew J. Changes in blood flow, oxygenation, and volume following extended stimulation of rodent barrel cortex. Neuroimage. 2002;15:474–487. doi: 10.1006/nimg.2001.1000. [DOI] [PubMed] [Google Scholar]
- 16.Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation—separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–846. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- 17.Kida I, Hyder F. The relationship between changes in CBF and CBV is dynamically varying throughout the stimulus duration but complex following stimulation offset. Proc Int Soc Magn Reson Med. 2004;12:1008. [Google Scholar]
- 18.Kida I, Hyder F, Behar KL. Inhibition of voltage-dependent sodium channels suppresses the functional magnetic resonance imaging response to forepaw somatosensory activation in the rodent. J Cereb Blood Flow Metab. 2001;21:585–591. doi: 10.1097/00004647-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F. High-resolution CMRo2 mapping in rat cortex—a multi-parametric approach to calibration of BOLD image contrast at 7 Tesla. J Cereb Blood Flow Metab. 2000;20:847–860. doi: 10.1097/00004647-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kida I, Maciejewski PK, Hyder F. Dynamic imaging of perfusion and oxygenation by functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2004;24:1369–1381. doi: 10.1097/01.WCB.0000141501.12558.9B. [DOI] [PubMed] [Google Scholar]
- 21.Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29:216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRo2 from CBF and BOLD changes—significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Hendrich K, Kim SG. Quantification of arterial and total cerebral blood volume—arterial changes dominate during neural activation. Proc Int Soc Magn Reson Med. 2006;12:376. [Google Scholar]
- 24.Kim T, Kim SG. Quantification of cerebral arterial blood volume and cerebral blood flow using MRI with modulation of tissue and vessel (MOTIVE) signals. Magn Reson Med. 2005;54:333–342. doi: 10.1002/mrm.20550. [DOI] [PubMed] [Google Scholar]
- 25.Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow—implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- 26.Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263–274. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski PK, Kida I, Hyder F. Estimating dynamic CMRo2 from dynamic CBF and BOLD fMRI measurements. Proc Int Soc Magn Reson Med. 2004;12:272. [Google Scholar]
- 30.Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRo2 during rat forepaw stimulation. Magn Reson Med. 1999a;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999b;19:679–689. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Menon RS. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn Reson Med. 2002;47:1–9. doi: 10.1002/mrm.10041. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa S, Menon RS, Tank RE, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarzbauer C, Morrissey SP, Haase A. Quantitative magnetic resonance imaging of perfusion using magnetic labeling of water proton spins within the detection slice. Magn Reson Med. 1996;35:540–546. doi: 10.1002/mrm.1910350413. [DOI] [PubMed] [Google Scholar]
- 35.Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency—The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanovic B, Pike GB. Venous refocusing for volume estimation—VERVE functional magnetic resonance imaging. Magn Reson Med. 2005;53:339–347. doi: 10.1002/mrm.20352. [DOI] [PubMed] [Google Scholar]
- 39.Ursino M, Di Giammarco P, Belardinelli E. A mathematical model of cerebral blood flow chemical regulation—Part I—Diffusion processes. IEEE Trans Biomed Eng. 1989a;36:183–191. doi: 10.1109/10.16465. [DOI] [PubMed] [Google Scholar]
- 40.Ursino M, Di Giammarco P, Belardinelli E. A mathematical model of cerebral blood flow chemical regulation—Part II—Reactivity of cerebral vascular bed. IEEE Trans Biomed Eng. 1989b;36:192–201. doi: 10.1109/10.16466. [DOI] [PubMed] [Google Scholar]
- 41.Venkatasubramanian PN, Shen YJ, Wyrwicz AM. Characterization of the cerebral distribution of general anesthetics in vivo by two-dimensional 19F chemical shift imaging. Magn Reson Med. 1996;35:626–630. doi: 10.1002/mrm.1910350426. [DOI] [PubMed] [Google Scholar]; Yang SP, Krasney JA. Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J Cereb Blood Flow Metab. 1995;15:115–123. doi: 10.1038/jcbfm.1995.13. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor-elicited responses in rat olfactory bulb by functional MRI. Proc Natl Acad Sci USA. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]