Conductin/axin2 and Wnt signalling regulates centrosome cohesion
Wnt signalling regulates centrosome cohesion. Work by the Behrens group shows that conductin/axin2, a negative regulator of β-catenin, localizes to centrosomes by binding to the centriole-associated component C-Nap1. Conductin/axin2 promotes centrosome cohesion by phosphorylating β-catenin at centrosomes and the authors propose a model for the regulation of centrosome separation by conductin and Wnt signalling.
Keywords: conductin/axin2, β-catenin, centrosomes, Wnt signalling, APC
Abstract
Activated Wnt/β-catenin signalling is a characteristic of many cancers and drives cell-cycle progression. Here, we report a mechanism linking Wnt/β-catenin signalling to centrosome separation. We show that conductin/axin2, a negative regulator of β-catenin, localizes at the centrosomes by binding to the centriole-associated component C-Nap1. Knockout or knockdown of conductin leads to premature centrosome separation—that is, splitting—which is abolished by knockdown of β-catenin. Conductin promotes phosphorylation of the amino-terminal serine (Ser 33/37) and threonine (Thr 41) residues of centrosome-associated β-catenin. β-Catenin mutated at these residues causes centrosomal splitting, whereas a phospho-mimicking mutant of β-catenin does not. Importantly, β-catenin-induced splitting is not inhibited by blocking β-catenin-dependent transcription. Treatment with Wnts and inhibition of glycogen synthase kinase 3 block β-catenin phosphorylation and induce centrosomal splitting. These data indicate that Wnt/β-catenin signalling and conductin regulate centrosomal cohesion by altering the phosphorylation status of β-catenin at the centrosomes.
Introduction
The Wnt/β-catenin signalling pathway controls the stability of β-catenin. In the absence of Wnts, β-catenin is sequentially phosphorylated by kinases casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β) at the amino-terminal residues Ser 45 and Ser 33/37/Thr 41 respectively, in a multiprotein complex composed of the adenomatous polyposis coli protein and scaffold proteins axin1 and conductin/axin2 (Behrens et al, 1998; Kikuchi, 1999). Phosphorylated β-catenin is ubiquitylated and degraded in the proteosomes (Behrens, 2005). In the presence of Wnts or mutations in the degradation complex, β-catenin is stabilized, enters the nucleus and promotes the expression of target genes driving cell proliferation by binding to T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors (Behrens et al, 1996; Clevers, 2006). It has been shown that phospho-β-catenin has a role in microtubule regrowth at centrosomes (Huang et al, 2007). Recently, β-catenin was also shown to function in centrosome cohesion (Bahmanyar et al, 2008). The mechanistic and regulatory frameworks of these new functions, however, remain unclear.
A centrosome consists of two centrioles surrounded by a dense pericentriolar material. As cells progress from G1 into S phase, centriole duplication begins, resulting in cells with two centrosomes that each contains two closely juxtaposed centrioles by the end of S/G2 (Nigg, 2004). The two centrosomes are held together by a linker and function as one microtubule nucleation centre. Before onset of mitosis the linker is severed, followed by centrosome separation and formation of spindle poles (Tsou & Stearns, 2006). After mitosis, daughter cells inherit a single centrosome with two centrioles ready to start the duplication cycle again. The two interphase centrosomes are held together by a physical linker composed of centrosomal NIMA-related kinase 2 (Nek2)-associated protein 1 (C-Nap1) and rootletin, which forms filaments and anchors itself on binding sites provided by C-Nap1 on the proximal end of daughter centrioles (Fry et al, 1998a,1998b; Bahe et al, 2005). Phosphorylation of rootletin and C-Nap1 by Nek2 leads to separation of centrosome pairs (Mayor et al, 1999). The activity of Nek2 is modulated during the cell cycle and this is thought to ensure that events pertinent to the centrosomal cycle take place in a timely manner (Lim et al, 2009). The premature separation of a centrosomal pair is referred to as splitting (Bahe et al, 2005).
Conductin acts as a scaffold protein in the β-catenin degradation complex by binding to adenomatous polyposis coli, CK1, GSK3β and β-catenin with distinct binding domains (Behrens et al, 1998). We have shown previously that conductin localizes at the centrosomes and the mitotic spindle, and regulates the spindle checkpoint (Hadjihannas & Behrens, 2006; Hadjihannas et al, 2006). Here, we report that conductin localizes at centrosomes through C-Nap1, where it controls the GSK3β-mediated phosphorylation of β-catenin to regulate centrosome cohesion. The data indicate that pathways, such as the one controlled by Wnts, have evolved hubs at centrosomes, which might act to couple mitogenic signals to centrosome function.
Results And Discussion
C-Nap1 mediates centrosomal localization of conductin
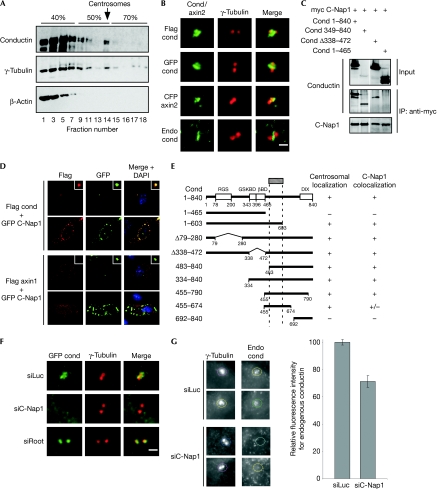
To verify the presence of endogenous conductin at the centrosomes, we used discontinuous sucrose gradients from lysates of SW480 colon cancer cells, which contain a relatively high amount of conductin as compared with other cell lines owing to misregulated Wnt signalling. Conductin co-fractionated with γ-tubulin in the centrosomal fractions, whereas β-actin did not (Fig 1A). High-magnification ( × 100) microscopy and two-dimensional deconvolution showed that in all cell lines tested, conductin localized at the centrosomes in more than 90% of the cells, independent of the tag used (Fig 1B; supplementary Fig S1A online). Conductin localized mainly between the centrosomes, at instances engulfing them irrespective of microtubules and the actin cytoskeleton (Fig 1B; supplementary Fig S1B and supplementary Movies S1,S2 online). Importantly, staining with two conductin antibodies showed endogenous conductin at centrosomes, which was diminished after knockdown of conductin (Fig 1B; supplementary Fig S1C online). Endogenous conductin localized similarly to exogenous conductin, suggesting that centrosomal localization of conductin constructs reflects the endogenous situation (Fig 1B; supplementary Fig S1C online).
Figure 1.
Conductin localizes at the centrosomes by binding to C-Nap1. (A) Western blotting for conductin, γ-tubulin and β-actin in sucrose density-gradient fractions from SW480 cells. The sucrose percentage is indicated. (B) Immunofluorescence co-staining of Flag conductin, GFP conductin and CFP axin2 (human conductin sequence) in green, together with γ-tubulin (red), in U2OS cells. The bottom panels show co-staining of endogenous conductin (green) with γ-tubulin (red) in SW480 cells, Scale bar, 2 μm (C) Western blotting for conductin and C-Nap1 in anti-myc immunoprecipitations using U2OS cells co-transfected with the indicated plasmids. The top panel shows the lysates (input). (D) Immunofluorescence staining of U2OS cells co-transfected with GFP C-Nap1 (green) and Flag conductin or Flag axin1 (top and bottom half, respectively; red). The insets show C-Nap1-positive centrosomes. The lower panels in the top and bottom halves show GFP C-Nap1 localization in the cytoplasmic patches. (E) Centrosomal localization and C-Nap1 colocalization of conductin mutants. (F) GFP and γ-tubulin (red) staining in U2OS cells co-transfected with GFP conductin (green) and siRNAs against luciferase (siLuc), C-Nap1 (siC-Nap1) or rootletin (siRoot). Scale bar, 2 μm (G) Co-staining of γ-tubulin and endogenous conductin in SW480 cells transfected with siLuc or siC-Nap1 (two examples shown). The coloured circles indicate the centrosomes used for quantification of the fluorescence intensities of conductin normalized to γ-tubulin intensities (shown in the bar chart). Error bars indicate the s.e.m. (n=100) of two independent experiments. C-Nap1, centrosomal NIMA-related kinase 2 (Nek2)-associated protein 1; CFP, cyan fluorescent protein; GFP, green fluorescent protein; siRNAs, small interfering RNAs.
By using conductin deletions mutants, we identified the domain required for centrosomal localization in the carboxy-terminal region of conductin (amino acids 455–674; Fig 1E). In co-immunoprecipitation experiments, we observed clear co-immunoprecipitation of conductin with C-Nap1, whereas rootletin and Nek2 showed a weak, albeit above background, interaction (Fig 1C; supplementary Fig S2A online). The C-terminal half of conductin was sufficient for binding to C-Nap1, whereas N-terminal sequences could not mediate binding (Fig 1C). Overexpressed C-Nap1 appeared in three types of cytoplasmic localization—a fine centrosomal localization, big patches or many speckles—and conductin showed striking colocalization with all three types (Mayor et al, 2002; Fig 1D). We narrowed down the conductin domain sufficient for C-Nap1 colocalization to amino acids 455–790 (Fig 1E). Axin1, a protein related to conductin, did not colocalize with C-Nap1 (Fig 1D). Importantly, centrosomal localization of conductin was diminished or abolished in most cells where C-Nap1 expression was knocked down (Fig 1F; supplementary Fig S3A online). Conversely, in rootletin-knockdown cells, conductin localized on rather than in between centrosomes (Fig 1F; supplementary Figs S2B,S3A online). In addition, knockdown of C-Nap1 in SW480 cells reduced the amount of endogenous conductin at centrosomes (Fig 1G). Collectively, these results show that conductin localizes at centrosomes by binding to C-Nap1, most probably through a domain in the C terminus of conductin.
Loss of conductin leads to centrosomal splitting
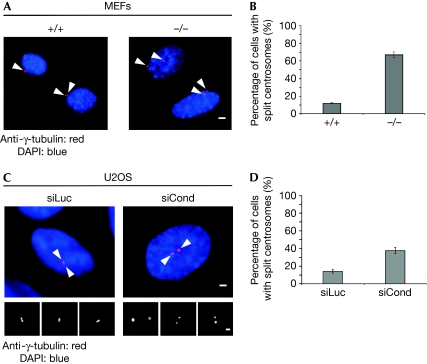
Depletion of β-catenin produces monopolar spindles with unseparated centrosomes (Kaplan et al, 2004). Reciprocally, β-catenin localizes at the centrosomes in a complex with rootletin and Nek2, and leads to centrosome splitting (Bahmanyar et al, 2008). To investigate whether conductin has an effect on centrosome cohesion, we assayed wild-type (+/+) and conductin-knockout (−/−) mouse embryonic fibroblasts (MEFs). We noticed a significant difference in the distance separating the two centrosomes between the two cell populations (Fig 2A,B). Whereas in most (+/+) MEFs centrosomes were separated by distances ranging from 2 to 3 μm, in most conductin (−/−) MEFs centrosomes were separated by more than 3 μm (Fig 2A,B). In addition, knockdown of conductin in human osteosarcoma U2OS cells reproducibly increased the percentage of cells with centrosomes separated by distances greater than 2 μm, as compared with that in control transfected cells (Fig 2C,D; supplementary Fig S3B online). We conclude that absence of conductin impairs centrosome cohesion, leading to the splitting of centrosomes.
Figure 2.
Loss or knockdown of conductin causes centrosomal splitting. (A) Anti-γ-tubulin staining using wild-type (+/+) and conductin-knockout (−/−) MEFs. The arrowheads indicate the centrosomes. Scale bar, 4 μm. (B) Quantification of split centrosomes from (A). (C) Anti-γ-tubulin staining in U2OS cells transfected with siRNAs against luciferase (siLuc) and conductin (siCond). The arrowheads indicate the centrosomes. Scale bars, 2 μm. (D) Quantification of split centrosomes from (C). GFP centrin2 was co-transfected to identify transfected cells from which percentages were calculated. Error bars indicate the s.e.m. (n>300) of at least three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole; MEFs, mouse embryonic fibroblasts; GFP, green fluorescent protein; siRNAs, small interfering RNAs.
Conductin alters centrosomal β-catenin phosphorylation
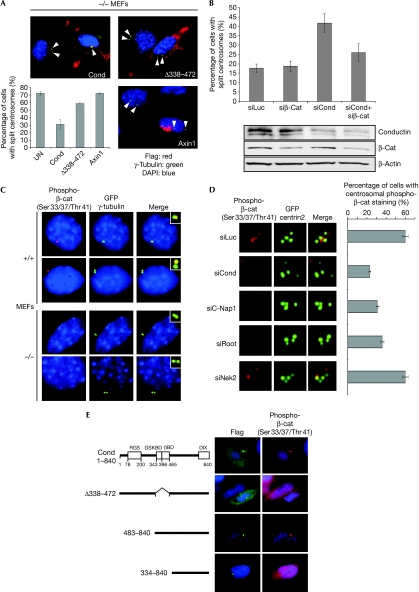
Reconstitution of conductin in conductin (−/−) MEFs rescued centrosomal cohesion, whereas axin1 did not, which is in line with the fact that axin1 does not associate with C-Nap1 (Fig 3A). Expression of a conductin mutant lacking the β-catenin- and GSK3β-binding domains was not able to promote centrosomal cohesion (Fig 3A). Furthermore, knockdown of β-catenin reduced the splitting induced by knockdown of conductin in U2OS cells (Fig 3B). β-Catenin is therefore required for centrosomal splitting to occur when conductin is absent. Knockdown of conductin did not increase the β-catenin levels under these conditions, suggesting that β-catenin stabilization is not the trigger for centrosomal splitting (Fig 3B). We therefore determined whether conductin regulates the phosphorylation of β-catenin at centrosomes. Staining with an antibody against β-catenin triple-phosphorylated on Ser 33 and Ser 37, and Thr 41, clearly indicated centrosomal localization in (+/+) MEFs (Fig 3C). Additionally, phospho-β-catenin appeared at the spindle poles in other cell lines (supplementary Fig S4A online). Importantly, in conductin (−/−) MEFs, phospho-β-catenin was absent or greatly reduced from centrosomes as compared with that in (+/+) MEFs, whereas total β-catenin at the centrosomes was not altered (Fig 3C; supplementary Fig S4B online). Similarly, knockdown of conductin in U2OS cells reduced the phosphorylation of β-catenin at centrosomes (Fig 3D). Knockdown of C-Nap1 or rootletin also abolished the phosphorylation of β-catenin at centrosomes, in line with the fact that C-Nap1 and rootletin are required for proper centrosomal localization of conductin, whereas knockdown of Nek2 did not affect β-catenin phosphorylation at the centrosomes (Fig 3D; supplementary Fig S3C online). Overexpression of conductin in SW480 cells led to accumulation of phospho-β-catenin at centrosomes, whereas conductin constructs lacking β-catenin- and GSK3β-binding domains did not (Fig 3E). These data show that conductin induces β-catenin phosphorylation at centrosomes and promotes centrosomal cohesion.
Figure 3.
Conductin regulates phosphorylation of β-catenin at the centrosomes. (A) Immunofluorescence co-staining of transfected Flag-tagged conductin, Δ338–472 mutant and axin1 (red) together with γ-tubulin (green, indicated by arrowheads) in conductin-knockout (−/−) MEFs. Bottom left panel: quantification of split centrosomes. (B) Quantification of split centrosomes in U2OS cells transfected with the indicated siRNAs. The bottom panels show western blotting from these cells using the indicated antibodies. (C) Immunofluorescence co-staining for phospho-β-catenin (red) and γ-tubulin (green) in wild-type (+/+) and conductin-knockout (−/−) MEFs. The insets show a magnified view of the centrosomes. (D) Immunofluorescence staining of phospho-β-catenin (red) in U2OS cells co-transfected with the indicated siRNAs and GFP centrin2 (green). The percentage of cells showing staining for phospho-β-catenin at the centrosomes is indicated on the right. (E) Immunofluorescence co-staining of phospho-β-catenin (red) and transfected full-length conductin and deletion mutants (green) in SW480 cells. (A, B, D) Error bars indicate the s.e.m. of at least three experiments; n>200, n>400, n>300, respectively. C-Nap1, centrosomal NIMA-related kinase 2 (Nek2)-associated protein 1; DAPI, 4′,6-diamidino-2-phenylindole; MEFs, mouse embryonic fibroblasts; GFP, green fluorescent protein; siRNAs, small interfering RNAs.
β-Catenin phosphorylation and centrosomal cohesion
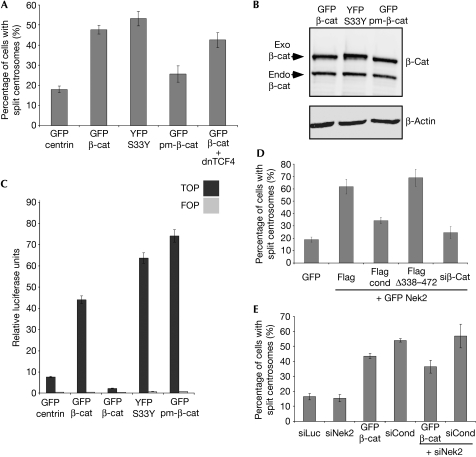
We quantified centrosome splitting in U2OS cells transfected with wild-type β-catenin, an S33Y mutant of β-catenin, in which Ser 33 was mutated to tyrosine, rendering this site resistant to GSK3β-mediated phosphorylation, and with a phospho-mimicking version of β-catenin (pm-β-catenin), in which all three GSK3β phosphorylation sites were substituted with aspartic acid. Wild-type as well as S33Y β-catenin or a similar S33A mutant, were able to induce splitting in these cells, in agreement with reports in a recent study (Fig 4A and data not shown; Bahmanyar et al, 2008). By contrast, pm-β-catenin was not able to induce splitting, despite being expressed at comparable levels as the wild-type and S33Y/S33A versions of β-catenin (Fig 4A,B). All β-catenin constructs localized at the centrosomes (supplementary Fig S4C online). TOP/FOP assays showed that transcriptional activation by Wnt signalling can be induced by all three β-catenin plasmids, indicating that any difference in their ability to cause centrosomal splitting hinges neither on their expression levels nor on their transcriptional activity (Fig 4C). In line with this, transfection of a dominant-negative mutant of TCF4 (dnTCF4) did not interfere with the ability of β-catenin to cause centrosome splitting, although it blocked the transcriptional activity of β-catenin (Fig 4A,C; Dehner et al, 2008). We conclude that β-catenin regulates centrosomal cohesion through a mechanism that depends on its phosphorylation by GSK3β but is distinct from its stabilization and transcriptional activity. Overexpression of Nek2 leads to centrosomal splitting (Fig 4D; Fry et al, 1998b). Strikingly, either co-transfection of conductin or knockdown of β-catenin could counteract this effect, whereas a conductin mutant lacking the β-catenin- and GSK3β-binding domains could not (Fig 4D). Reciprocally, splitting induced by conductin knockdown or β-catenin overexpression was not inhibited by concurrent knockdown of Nek2 (Fig 4E). Additionally, conductin was not required for Nek2-induced phosphorylation of the β-catenin arm repeats (Bahmanyar et al, 2008; supplementary Fig S4D online). Collectively, these results suggest that Nek2 acts upstream from or in parallel to the conductin and β-catenin mechanism. As expected, conductin did not suppress splitting induced by knockdown of rootletin or C-Nap1 (supplementary Fig S4E online).
Figure 4.
Phosphorylation of β-catenin regulates centrosome cohesion independently from stability and transcriptional activity. (A) Quantification of U2OS cells with split centrosomes, transfected with the indicated plasmids. (B) Western blotting for transfected β-catenin constructs (exo) in U2OS cells as indicated using antibodies against total β-catenin and β-actin. Endogenous β-catenin is also indicated. (C) TOP/FOP assays of 293T cells transfected with the indicated plasmids. (D,E) Quantification of split centrosomes in U2OS cells transfected with the indicated plasmids and siRNAs. Error bars indicate the s.e.m. (n=300–600) of at least three independent experiments. GFP, green fluorescent protein; Nek2, NIMA-realted kinase 2; pm, phospho-mimicking; siRNAs, small interfering RNAs; YFP, yellow fluorescent protein.
Wnt signalling regulates centrosome cohesion
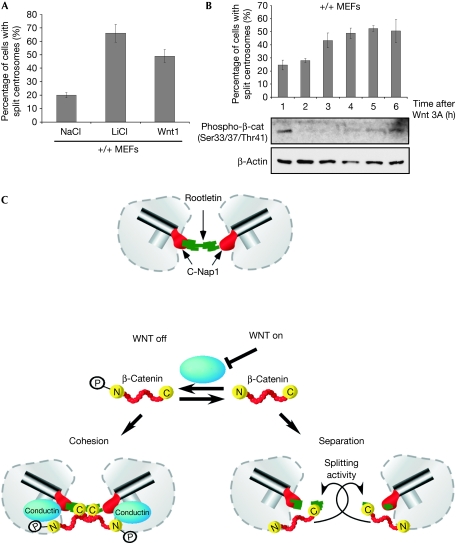
Wnt signalling activation leads to inhibition of GSK3β-mediated β-catenin phosphorylation. Treatment of MEFs with Wnt1-conditioned media or LiCl to block GSK3β directly caused centrosomal splitting (Fig 5A). Additionally, phosphorylation of β-catenin was abolished within 2 h of Wnt3A treatment, and within 3 h there was a twofold increase in the number of cells showing centrosomal splitting (Fig 5B). Together, these data indicate that inhibition of β-catenin phosphorylation through activation of the Wnt signalling cascade induces centrosomal splitting. We conclude that phosphorylation is the molecular switch that regulates the ability of β-catenin to promote centrosomal splitting.
Figure 5.
Centrosome cohesion is regulated by Wnt signalling. (A) Quantification of split centrosomes in MEFs after treatment with sodium chloride (NaCl), lithium chloride (LiCl) or Wnt1-conditioned media for 18 h. (B) Quantification of split centrosomes in MEFs after treatment with Wnt3A-conditioned media for the indicated time points. Lower panels: western blotting for phospho-β-catenin and β-actin of lysates from the MEFs above. (C) A model for the regulation of centrosome cohesion by Wnt signalling. Error bars indicate the s.e.m. (n>300) of at least three independent experiments. MEFs, mouse embryonic fibroblasts.
We propose a model for Wnt-controlled centrosome separation (Fig 5C), in which dephosphorylated β-catenin acts to disrupt cohesion, whereas phosphorylated β-catenin is devoid of such activity. Conductin, which localizes at the centrosomes, induces β-catenin phosphorylation, thereby promoting cohesion. Conversely Wnt signalling abolishes the phosphorylation of β-catenin and leads to centrosome splitting. In this model, the splitting activity of β-catenin is independent from its transcriptional activity and stabilization, as under conditions that induced centrosome splitting we did not notice increased β-catenin levels in conductin (−/−) MEFs or in cells treated with a small interfering RNA (siRNA) against conductin. It remains to be determined how dephosphorylated β-catenin promotes centrosomal splitting. It is unlikely that β-catenin possesses enzymatic activity that would dissolve cohesive linkers such as rootletin. We speculate that phosphorylated and dephosphorylated β-catenin molecules differ in their interactions with centrosomal proteins to alter centrosomal cohesion, a scenario supported by the localization of all β-catenin constructs at the centrosomes. In line with this, a phosphorylation-resistant mutant of β-catenin has a longer half-life at centrosomes than wild-type β-catenin (Bahmanyar et al, 2008). Interestingly, β-catenin is also required for Nek2-induced splitting, indicating that it is an integral component of the centrosome separation process.
Notably, axin1 was recently found at the centrosomes where it binds to the γ-tubulin ring complex proteins and functions in microtubule nucleation, whereas conductin does not (Fumoto et al, 2009). We show that conductin localizes to the centrosomes by binding to C-Nap1 and acts on centrosomal cohesion, whereas axin1 does not, indicating that the two proteins are targeted to the centrosome through different routes and serve distinct functions.
This study shows that a mitogenic pathway such as Wnt/β-catenin signalling has evolved arms to regulate cell-cycle-related processes such as centrosomal cohesion. These findings could be relevant in cancers where both Wnt/β-catenin signalling and centrosomes are altered.
Methods
Cell culture and transfections. SW480, U2OS cells and MEFs were cultured as described by Hadjihannas et al (2006). For transfection of siRNAs, previously published sequences (supplementary information online) were transfected with oligofectamin (Invitrogen, Carlsbad, CA, USA). Plasmids were transfected with ESCORT IV (Sigma, St Louis, MO, USA) or polyethylenimine (Dehner et al, 2008). siRNAs and plasmids were co-transfected using Lipofectamine 2000 (Invitrogen).
Immunofluorescence microscopy, antibodies and fluorescence intensity measurements. Stainings were performed as described by Hadjihannas et al (2006) using antibodies against γ-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-β-catenin (Cell Signaling, Danvers, MA, USA), β-catenin (Nathke et al, 1994), Flag (Sigma) and conductin (Lustig et al, 2002). For endogenous stainings, cells were pre-extracted for 40 s in a buffer (containing 80 mM PIPES (pH 6.8), 1 mM MgCl2, and 1 mM EGTA, 0.5% Triton X-100) for 30 s before fixation. Endogenous stainings of conductin were performed by using mouse (Lustig et al, 2002) or rabbit (USBiological, Swampscott, MA, USA) monoclonal antibodies on methanol-fixed cells, which were then treated for 10 min at 95°C in an antigen retrieval buffer (100 mM Tris, 5% urea). Coverslips were viewed by using an Axioplan2 and images were acquired using Metamorph (Zeiss, Jena, Thuringen, Germany). For fluorescence intensity measurements, background-subtracted fluorescence intensities for conductin staining were divided by those for γ-tubulin in the same cell. Exposure times were kept constant.
Centrosomal splitting assays. Asynchronous cell cultures on coverslips were transfected for 24–48 h and processed for immunofluorescence staining. The percentage of cells showing splitting was determined in cells containing two well-defined γ-tubulin-marked centrosomes and the distance between them was measured using a × 100 objective with oil immersion. For U2OS cells, a 2 μm threshold was used as described previously (Bahe et al, 2005). For MEFs a 3 μm threshold was used owing to the bigger cell size and the fact that centrosomes in more than 85% of the cells were separated by 2–3 μm. Experiments were performed at least three times and error bars indicate the standard error of the mean (s.e.m.). The various treatments used in our experiments did not alter the cell-cycle stage of the cells, as judged by fluorescence-activated cell sorter analysis (data not shown).
Centrosomal fractionation and western blotting. Fractionations were performed as described by Mitchison & Kirschner (1986). Briefly, 10 × 15 cm2 dishes of 70–90% confluent SW480 cells were incubated for 2 h with 10 μg/ml nocodazole and 5 μg/ml cytochalasin-D at 37°C. Subsequent steps were performed as detailed in the Mitchison protocol. Gradients were centrifuged (120 000g at 8°C) by using a Beckman Optima LE-80K Ultracentrifuge (Beckman Coulter, Brea, CA, USA). Fractions of 200 μl were removed from the top of the column starting at the 40% sucrose phase and 15 μl of each fraction were used for western blotting. Western blotting and immunoprecipitation were performed as described by Hadjihannas et al (2006).
TOP/FOP assays. Cells were transfected with TOP/FOPFlash reporters and the indicated plasmids for 24 h, followed by measurement of luciferase activity in the cell lysates (supplementary information online).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank E. Nigg for the plasmids, A. Barth for the β-catenin antibody, M. Apel for help with cloning pm-β-catenin, B. Jerchow for MEFs and D. Bernkopf for reading the paper. This work was supported by Deutsche Forschungsgemeinschaft (DFG) and IZKF-Erlangen grants to J.B. and M.V.H., respectively.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA (2005) Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J Cell Biol 171: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH Jr, O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AI (2008) Beta-catenin is a Nek2 substrate involved in centrosome separation. Genes Dev 22: 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J (2005) The role of the Wnt signalling pathway in colorectal tumorigenesis. Biochem Soc Trans 33: 672–675 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280: 596–599 [DOI] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J (2008) Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through upregulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem 283: 19201–19210 [DOI] [PubMed] [Google Scholar]
- Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA (1998a) C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell-cycle-regulated protein kinase Nek2. J Cell Biol 141: 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Meraldi P, Nigg EA (1998b) A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J 17: 470–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumoto K, Kadono M, Izumi N, Kikuchi A (2009) Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep 10: 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjihannas MV, Behrens J (2006) CIN by WNT: growth pathways, mitotic control and chromosomal instability in cancer. Cell Cycle 5: 2077–2081 [DOI] [PubMed] [Google Scholar]
- Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J (2006) Aberrant Wnt/β-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci USA 103: 10747–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Senga T, Hamaguchi M (2007) A novel role of phospho-β-catenin in microtubule regrowth at centrosome. Oncogene 26: 4357–4371 [DOI] [PubMed] [Google Scholar]
- Kaplan DD, Meigs TE, Kelly P, Casey PJ (2004) Identification of a role for β-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem 279: 10829–10832 [DOI] [PubMed] [Google Scholar]
- Kikuchi A (1999) Roles of axin in the Wnt signalling pathway. Cell Signal 11: 777–788 [DOI] [PubMed] [Google Scholar]
- Lim HH, Zhang T, Surana U (2009) Regulation of centrosome separation in yeast and vertebrates: common threads. Trends Cell Biol 19: 325–333 [DOI] [PubMed] [Google Scholar]
- Lustig B et al. (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor T, Meraldi P, Stierhof YD, Nigg EA, Fry AM (1999) Protein kinases in control of the centrosome cycle. FEBS Lett 452: 92–95 [DOI] [PubMed] [Google Scholar]
- Mayor T, Hacker U, Stierhof YD, Nigg EA (2002) The mechanism regulating the dissociation of the centrosomal protein C-Nap1 from mitotic spindle poles. J Cell Sci 115: 3275–3284 [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW (1986) Isolation of mammalian centrosomes. Methods Enzymol 134: 261–268 [DOI] [PubMed] [Google Scholar]
- Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ (1994) Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol 125: 1341–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA (2004) Centrosomes in Development and Disease. Weinheim, Germany: Wiley [Google Scholar]
- Tsou MF, Stearns T (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442: 947–951 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.