Abstract
Nonactin, produced by Streptomyces griseus ETH A7796, is a macrotetrolide assembled from nonactic acid. It is an effective inhibitor of drug efflux in multidrug resistant erythroleukemia K562 cells at sub-toxic concentrations and has been shown to possess both antibacterial and antitumor activity. As total synthesis is impractical for the generation of nonactin analogs we have studied precursor-directed biosynthesis as an alternative as it is known that nonactic acid can serve as a nonactin precursor in vivo. To determine the scope of the approach we prepared and evaluated a furan-based nonactic acid derivative, 11. Although no new nonactin analogs were detected when 11 was administered to S. griseus fermentative cultures, a significant inhibition of nonactin biosynthesis was noted (IC50 ~ 100 μM). Cell mass, nonactic acid production and the generation of other secondary metabolites in the culture were unaffected by 11 demonstrating that 11 selectively inhibited the assembly of nonactin from nonactic acid. While we were unable to generate new nonactin analogs we have discovered, however, a useful inhibitor that we can use to probe the mechanism of nonactin assembly with the ultimate goal of developing more successful precursor-directed biosynthesis transformations.
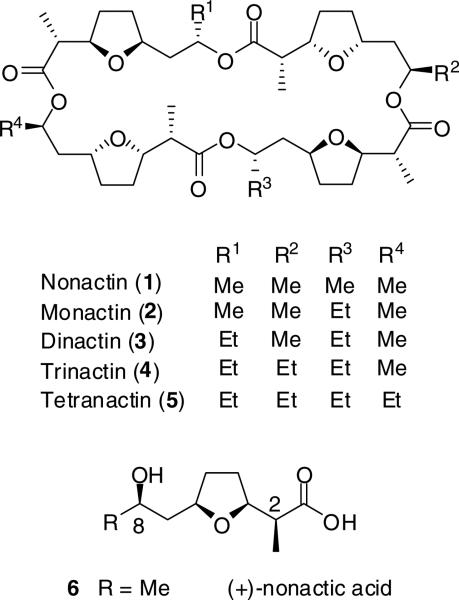
Streptomyces griseus subsp. griseus ETH A7796 (DSM40695) makes a series of ionophore antibiotics known as the macrotetrolides (Figure 1).1 Nonactin, the prototypical macrotetrolide (1) is assembled from two monomers of (–)-nonactic acid and two monomers of (+)-nonactic acid assembled (+)-(–)-(+)-(–) in a head-to-tail manner into a 32-membered macrocycle. Nonactin has both antibiotic and anticancer properties2 and has been shown to be an inhibitor of drug efflux in multiple drug resistant cancers.3 The natural macrotetrolide homologues produced by S. griseus show a wide range potency with the minimum inhibitory concentration of 1 being an order of magnitude greater than that of dinactin (3) against Staphylococcus aureus and Mycobacterium bovis, a difference that is related to the stability constants of their respective Na+ and K+ complexes.1,2,4
Figure 1.
The structures of the naturally occurring macrotetrolides and the monomer, nonactic acid.
Nonactin is far too hydrophobic and insufficiently soluble to be an effective therapeutic.5 The development of therapeutics based upon nonactin, therefore, will be dependent upon being able to make non-natural analogs in a direct and efficient manner. The total synthesis of nonactin has been achieved by a number of groups6-9 as has the synthesis of nonactic acid.10,11
The total synthesis of nonactin analogs is complicated as both enantiomers of nonactic acid, and its analogs, are required as is the sequential construction of a linear tetraester prior to a final macrolactonization reaction affording the nonactin analog. This complexity does not lend itself to straightforward drug development.
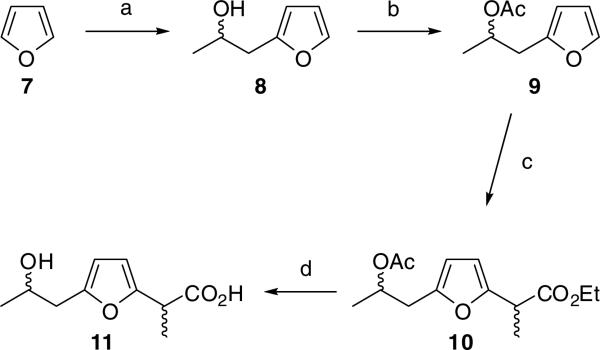
Fermentative cultures of S. griseus can generate 6 – 15 g/L of macrotetrolide mixtures (>90% 1 and 2). When nonactic acid is added to fermentative cultures of either S. griseus, or genetically altered strains of S. griseus that have been blocked in the early stages of nonactin biosynthesis, it can be readily and efficiently incorporated into nonactin.12 These observations strongly suggest that precursor-directed biosynthesis has the potential to generate new nonactin analogs.13,14 As we know that a complex structure such as nonactic acid will serve as a substrate for precursor-directed biosynthesis, our first task was to set bounds on the system by discovering the simplest, most straightforward nonactic acid analog that would work. To that end we completed the synthesis of the substituted furan derivative 11 (Scheme 1) and evaluated it in precursor-directed biosynthesis experiments.
Scheme 1.
Synthesis a furan-based nonactic acid analog 11. Reagents and conditions: a) nBuLi, -78 °C, THF then propylene oxide, 66 %; b) Ac2O, pyridine, THF, 79%; c) BEt3, Fe2(SO4)3, ethyl DL-2-iodopropionate, DMSO, 11%; d) 2.5 M LiOH, MeOH, THF, 81%.
Alkylation of a furan-derived anion with propylene oxide was achieved using White's method affording 8 in 66% yield after distillation.10 The secondary alcohol of 6 was protected as an acetate by reaction with acetic anhydride in pyridine to give 9 (79%).15 The furan derivative 10 was obtained by a free radical addition reaction with ethyl 2-iodopropionate, as described by Baciocchi and Muraglia, to give 10 in 11% yield.16,17 Saponification of 10 gave the free acid 11 (81%).18 Although the synthesis generated a mixture of diastereoisomers, the synthesis was quite short and effective and we hoped that new analogs would more likely be formed using such a mixture in a precursor-directed feeding experiment.
To assess the incorporation of compound 11 into new macrotetrolide analogs, two fermentative cultures of S. griseus ETH A7796 were prepared from a single vegetative culture and grown for 48 hours under standard conditions.19 At 48 hours after inoculation of the fermentative culture (50 mL), 56 mg of 11 (56 mg in 0.5 mL of ethanol) was added to one culture; a blank sample (0.5 mL ethanol) was added to the equivalent control culture. The cultures were allowed to grow for an additional 96 hours and the macrotetrolide mixture was isolated according to standard protocols.19 Analysis of the macrotetrolide mixtures by HPLC20 and LC-MS (TOF)21 showed an unexpected drastic reduction in macrotetrolide production in the fed culture compared to the control. Unfortunately, no likely nonactin analogs could be detected in the extract by both LC-MS and MS analysis of the complex mixture.
We decided to further evaluate the inhibitory effects of 11 on the production of nonactin to determine if adding less material might allow for the production of higher levels of nonactin in which a low incorporation of 11 might then be more evident. To this end a series of fermentative cultures were evaluated, with a range of concentrations of 11 ranging from 0.01 mM to 10 mM, together with a control culture receiving no exogenous compound. Mycelia were recovered from each culture and an approximate wet weight determined; addition of 11 had no effect upon biomass production. Macrotetrolides present in the extracts were quantified by reverse-phase HPLC.20 It was determined that nonactin production was inhibited by more than 90 % in fermentative cultures containing either 10 mM or 1 mM of 11. Nonactin production was at the same level as in the control in a culture containing 0.01 mM of 11; a concentration of 0.1 mM of 11 lead to an intermediate level of nonactin production. In all cases, no new macrotetrolide analogs were evident in either MS or LC-MS analyses of the extracts. In addition to showing that 11 had little effect on biomass production, each of the fermentative cultures reliably generated other secondary metabolites (phenazines) usually co-synthesized with macrotetrolides when secondary metabolism is initiated in S. griseus. Furthermore, analysis22 of the extracts demonstrated that both enantiomers of nonactic acid, the monomer precursor to nonactin, were generated in each culture irrespective of the concentration of 11 added. We have demonstrated, therefore, that while 11 does not serve as a precursor to new macrotetrolides, it is indeed a reasonably potent (IC50 ~ 100 μM) and selective inhibitor of nonactin biosynthesis. As nonactic acid production is not perturbed, the furan derivative 11 is interfering with the assembly of nonactin from its monomeric precursors, a process that requires only the products of the nonK and nonJ type II polyketide synthase-encoding genes and the nonL CoASH-dependent ligase-encoding gene.23,24 The inhibition shown by 11 is distinguished from that of our earlier acetylenic analogs which likely block the formation of the monomer, nonactic acid.25
While we were not successful in the developing new nonactin analogs through precursor-directed biosynthesis we have set limitations on the nonactic acid analogs that may be used; it is likely that the tetrahydrofuran ring of nonactic acid will be essential. We have discovered, however, an inhibitor that likely will be of use in our quest to determine how the macrocycle of nonactin is constructed in vivo from monomeric precursors which, in turn, will allow us to develop improved approaches for the discovery of nonactin analogs by precursor-directed biosynthesis.
Acknowledgments
Financial support of this work from the NIH (CA77347) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Keller-Schierlein W, Gerlach H. Fortschritte d. Chem.Org. Naturstoffe. 1968;26:161. [PubMed] [Google Scholar]
- 2.Meyers E, Pansy FE, Perlman D, Smith DA, Weisenborn FL. J. Antibiot. 1965;18:128. [PubMed] [Google Scholar]
- 3.Borrel MN, Pereira E, Fiallo M, Garnier-Suillerot A. Eur. J. Biochem. 1994;223:125. doi: 10.1111/j.1432-1033.1994.tb18973.x. [DOI] [PubMed] [Google Scholar]
- 4.Izatt RM, Bradshaw SA, Nielsen SA, Lamb JD, Christensen JJ, Sen D. Chem. Rev. 1985;85:271. [Google Scholar]
- 5.Lipinski CA. J. Pharm. Toxicol. Meth. 2000;44:239. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee JYK, Byeang H. Tetrahedron Lett. 1996;52:571. [Google Scholar]
- 7.Lee JYK, Byeang H. Tetrahedron Lett. 1995;36:3364. [Google Scholar]
- 8.Fleming IG, Sunil K. Journal of the Chemical Society, Perkin Transactions 1. 1998;17:2733–2748. [Google Scholar]
- 9.Fleming IG, Sunil K. J. Chem. Soc., Chem. Commun. 1994;19:2287. [Google Scholar]
- 10.Arco MJ, Trammell MH, White JD. Journal of Organic Chemistry. 1976;43:479. doi: 10.1021/jo00874a001. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett PAM, James D, Ottow Eckhard. J. Am. Chem. Soc. 1984;106:5304. [Google Scholar]
- 12.Woo AJ, Strohl WR, Priestley ND. Antimicr. Agents Chemother. 1997;43:1662. doi: 10.1128/aac.43.7.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritacco F, Graziani E, Summers M, Zabriskie T, Yu K, Bernan V, Carter G, Greenstein M. Appl Environ Microbiol. 2005;71:1971. doi: 10.1128/AEM.71.4.1971-1976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen J, Hutchinson CR, Cane DE, Khosla C. Science. 1997;277:367. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 15.1-(Furan-2-yl)propan-2-yl acetate (9). Acetic anhydride (12.8 mL, 135 mmol) and then pyridine (18.4 mL, 225 mmol) were added to a solution of 8 (5.7 g, 45 mmol) in THF (15 mL). The resulting solution was stirred at room temperature for 2 hours after which the reaction was quenched by the addition of water (100 mL). The resulting mixture was extracted with ethyl acetate (3 × 100 mL) and the organic phases were recovered, combined and washed with water (50 mL), aqueous saturated copper sulfate (2 × 100 mL) and finally ammonium chloride solution (2 × 100 mL). The extracts were dried (Na2SO4), filtered and concentrated to a residue which was purified by distillation (96 °C, 24 mmHg) to give (9) (6.0 g, 79 %). Rf = 0.50 (10% ethyl acetate/hexanes); 1H NMR (400 MHz, CDCl3) δ 7.24 (s, 1H), 6.21 (s, 1H), 5.99 (s, 1H), 5.08 (sextet, J = 12.7, 6.4 Hz, 1H), 2.85 (dd, J = 15.1, 6.4 Hz, 1H), 2.75 (dd, J = 15.1, 6.4 Hz, 1H) 1.93 (s, 3H) and 1.17 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 151.4, 141.2, 110.0, 106.7, 69.0, 34.2, 20.9 and 19.3 ; IR (ATR) 2982, 2937, 1736, 1372, and 1237 cm-1.
- 16.Baciocchi E, Muraglia E. Tet. Letts. 1993;34:5015. [Google Scholar]
- 17.Ethyl 2-(5-(2-acetoxypropyl)furan-2-yl)propanoate (10). Triethylborane (1.0 M, 182 mL, 182 mmol) was added to a suspension of 9 (30.6 g, 182 mmol), ethyl DL-2-iodopropionate (41.5 g, 182 mmol) and ferric sulfate hydrate (72.8 g, 182 mmol) in DMSO (250 mL). After 45 minutes of vigorous stirring at room temperature while open to the atmosphere, a second addition of triethylborane (1.0 M, 182 mL, 182 mmol) was added to the suspension and the suspension was allowed to stir vigorously for an additional 45 min. Brine (200 mL) was then added and the resultant solution was extracted with Et2O (3 × 300 mL). The combined organic phases were dried (Na2SO4), filtered and concentrated under vacuum to give an oil. The crude oil was purified by flash chromatography (1:4 EtOAc/hexanes) to give 10 (5.34 g, 11%) as a yellow-orange oil. 1H NMR (400 MHz, CDCl3) δ 6.01 (d, J = 3.3 Hz, 1H). 5.95 (d, J = 2.9 Hz, 1H), 5.06 (sext, J = 12.8, 6.6, 6.2 Hz, 1H), 4.10 (q, J = 14.3, 7.3, 7.0, 2H), 3.69 (q, J = 7.3 Hz, 1H), 2.73 (dd, J = 15, 6.2 Hz, 1H), 2.73 (dd, J = 15, 6.2 Hz, 1H), 1.96 (s, 3H), 1.43 (d, J = 7.3 Hz, 3H), 1.21-1.17 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 172.5, 170.3, 152.1, 150.7, 107.6, 106.4, 69.2, 60.8, 39.4, 34.3, 21.1, 19.4, 15.6 and 14.0; IR (ATR) 2983, 2940, and 1733 cm-1; ESITOF-HRMS m/z calcd for C14H20O5H+: 269.1398; found 269.1389.
- 18.2-(5-(2-Hydroxypropyl)furan-2-yl)propanoic acid (11). A solution of 10 (2.20 g, 8.2 mmol) in 40 mL of 2.5 M LiOH/MeOH/THF (1:2:3) was allowed to stir at room temperature for 1.5 hours. The reaction was acidified to pH 2 by the addition of HCl (1.0 M), extracted with EtOAc (3 × 100 mL), dried (Na2SO4), filtered and concentrated. The crude product was purified by chromatography on silica gel (97:2.5:0.5 EtOAc/MeOH/AcOH) to give 11 (1.32 g, 81.0%) as a clear yellow oil. Rf = 0.54 (99:1 EtOAc/TFA); 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 2H), 6.05 (d, 1H, J = 3.3), 5.98 (d, 1H, J = 3.3), 4.02 (sextet, J = 12.4, 6.2, 2H), 3.73 (q, 1H, J = 7.3), 2.68 (d, J = 6.2, 2H), 1.46 (d, J = 7.3. 3H) and 1.15 (d, J = 6.2, 3H); 13C NMR (100 MHz, CDCl3) δ 177.3, 151.9, 151.6, 107.6, 106.8, 66.8, 39.1, 37.4, 22.2 and 15.3; IR (ATR) 3625-2375, 2973, 2922 and 1712 cm-1; ESI-TOF-HRMS m/z calcd for C10H14O4H+: 199.0970; found 199.0971.
- 19.Ashworth DM, Clark CA, Robinson JA. J. Chem. Soc., Perkin Trans. I. 1989:1461. [Google Scholar]
- 20.HPLC resolution and quantification of macrotetrolide mixtures was accomplished using a Varian microsorbmv 100-5 250 × 4.6 mm C-18 column with an isocratic mobile phase of acetonitrile-water (86:14) containing 0.1 % trifluoroacetic acid at a flow rate of 1 mL/min. Macrotetrolide elution was monitored by measuring the absorbance of the eluate at 215 nm. Under these conditions the macrotetrolide retention times were: nonactin, 7.50 min; monactin, 8.75 min; dinactin, 10.50 min; trinactin, 13.00 min and tetranactin, 16.33 min.
- 21.Cox J,E, Priestley ND. J. Am. Chem. Soc. 2005;127:7976. doi: 10.1021/ja050068k. [DOI] [PubMed] [Google Scholar]
- 22.The culture broth was acidified to pH 1 – 2 with 6 M HCl and then extracted with EtOAc. The crude extracts were analyzed by TLC (95:4:1, EtOAc/MeOH/AcOH) and stained with anisaldehyde. The crude extracts containing nonactic and homononactic acids were also esterified by dissolving in 5% H2SO4 in methanol and then heating at 65 °C for 1 hr. The methyl nonactate and methyl homononactate that were produced were isolated by chromatography on silica gel (50:50 EtOAc/hexanes). Additional characterization of methyl nonactate and methyl homononactate was achieved by gas chromatography.
- 23.Walczak RJ, Woo AJ, Strohl WR, Priestley ND. FEMS Letts. 2000;183:171. doi: 10.1111/j.1574-6968.2000.tb08953.x. [DOI] [PubMed] [Google Scholar]
- 24.Kwon H-J, Smith WC, Scharon J, Hwang SH, Kurth MJ, Shen B. Science. 2002;297:1327. doi: 10.1126/science.1073175. [DOI] [PubMed] [Google Scholar]
- 25.Priestley ND, Earle M. Bioorg. Med. Chem. Letts. 1997;7:2187. [Google Scholar]