Abstract
A key event in atherosclerosis is a maladaptive inflammatory response to subendothelial lipoproteins. A critical aspect of this response is failure of inflammation resolution, which normally consists of suppression of inflammatory cell influx, effective clearance of apoptotic cells (efferocytosis), and promotion of inflammatory cell egress. Defects in these processes promote the progression of atherosclerotic lesions into dangerous plaques, which can trigger atherothrombotic vascular disease, the leading cause of death in industrialized societies. This review will provide an overview of these concepts, with a focus on macrophage death, defective efferocytosis, and novel therapeutic strategies designed to boost inflammation resolution in atherosclerosis.
Successful resolution of inflammatory disease processes, often referred to as “catabasis,” requires a distinct series of processes, including inhibition of inflammatory cell recruitment, promotion of inflammatory cell egress, and clearance of apoptotic cells (efferocytosis)1, 2. These processes are mediated by a wide array of molecules, including anti-inflammatory cytokines, lipoxygenase-derived bioactive lipids, and transcription factors. Inflammation resolution also involves subsets of inflammatory cells, such alternatively activated, or M2, macrophages, that possess specific functional characteristics related to suppressing inflammation and cleaning up cellular debris3.
Understanding the principles of inflammation resolution is important in deciphering the complex process of atherosclerosis progression. Atherothrombotic vascular disease (ATVD) is the number one cause of death in the industrialized world, and this problem is growing annually due to the epidemic of obesity and insulin resistance world-wide4, 5. Atherogenesis is triggered by the retention of apolipoprotein B-containing lipoproteins in the subendothelium of the arterial wall (BOX 1) 6–8. These retained lipoproteins, perhaps after oxidative modification, trigger a chronic inflammatory response involving initially monocyte-derived macrophages and then other inflammatory cells, including T cells and mast cells. In the early stages, the lesions are relatively small and asymptomatic because they are not at risk to promote plaque disruption and lumenal thrombosis. Moreover, at least one key event in inflammation resolution—efferocytosis—seems to function well in these early lesions (see section of efferocytosis below). However, the minority of lesions that do progress to the type of dangerous plaque that can cause ATVD have all the hallmarks of defective resolution of inflammation, including defective efferocytosis, a persistent inflammatory state, and defective egress of inflammatory cells (FIG. 1) 9–12. Each of these defects promotes highly inflamed and necrotic plaques that are referred to as “vulnerable plaques,” because they vulnerable to structural disruption and thrombosis, which are the immediate precursors of acute cardiovascular clinical events13. For example, the failure of macrophage egress leads to prolonged production by these cells of collagen-degrading matrix proteases and coagulation-promoting tissue factor14. The failure of efferocytosis leads to post-apoptotic cellular necrosis, which amplifies the inflammatory response and eventually leads to the generation of the plaque-disrupting necrotic core of vulnerable plaques9, 15. In the following sections, the processes of immune cell entry and egress; anti-inflammatory signaling though inflammation resolution mediators; and the roles of macrophage death and defective efferocytosis in plaque progression will be explored.
Box 1. How atherosclerotic plaques develop.
There are certain areas of medium-sized arteries that are prone to the permeation and then subendothelial retention of apolipoprotein B-containing lipoproteins, such as low-density lipoprotein (LDL) and remnant lipoproteins. Permeation and retention depend on a number of variables, including the level, duration, and properties of circulating apoB lipoproteins in the bloodstream; focal alterations in the endothelial layer in susceptible areas of arteries, which occur most often at sites of disturbed blood flow; and the nature of subendothelial molecules that have the capacity to promote retention, notably chondroitin sulfate proteoglycans and molecules that “bridge” the lipoproteins to the proteoglycans, such as lipoprotein lipase6.
For reasons that are not completely understood, but are probably related to an initial innate immune response to the retained and often subendothelially modified lipoproteins, the overlying endothelium is activated to secrete chemokines and express adhesion molecules which attract and bind monocytes7, 8. These processes are followed by entry, or “diapedesis,” of the monocytes into the subendothelial space. Once in the subendothelium, or “intima,” the monocytes differentiate into macrophages and ingest the retained lipoproteins6–8. Lipoprotein uptake promotes the intracellular accumulation of a variety of lipids, including cholesterol, oxysterols, and fatty acids, which promotes the accumulation of lipid droplets in the cytoplasm (“foam cells”) and an inflammatory response in the cells6–8.
Over weeks, months, and even years, the process continues and even amplifies. For example, macrophage foam cells can secrete additional extracellular matrix molecules that further promote lipoprotein retention, and the inflammatory response leads to the attraction of more monocytes as well as T cells, mast cells, and possibly neutrophils6–8, 131. The lesion enlarges, but at this stage the arterial lumen remains patent enough to feed the distal organ due to outward remodeling of the arterial wall. Moreover, in the majority of cases, the lesion is contained by a subendothelial scar-like structure referred to as the “fibrous cap,” which is produced by collagen-secreting myofibroblasts that populate the intima and are derived from precursor cells in the media, adventitia, and/or blood stream131.
However, some of these lesions, fed by the aforementioned amplification process and the failure of inflammation resolution discussed in this review, undergo a process of arterial wall breakdown. The types of lesions that are susceptible, or “vulnerable,” to this process of arterial wall breakdown are often referred to as “vulnerable plaques” and are characterized by large areas of necrosis (see text), thin fibrous caps, and a heightened state of inflammation13. Disruption of the intima can expose pro-coagulant and pro-thrombotic contents in the intima, such as tissue factor, to coagulation factors and platelets in the bloodstream132. In the worse case scenario, an occlusive thrombus forms, leading to acute oxygen and nutrient deprivation of distal tissues fed by the artery. When these events occur in the coronary arteries, the region of heart muscle tissue fed by the involved artery becomes injured, and the result is unstable angina, myocardial infarction, or sudden cardiac death.
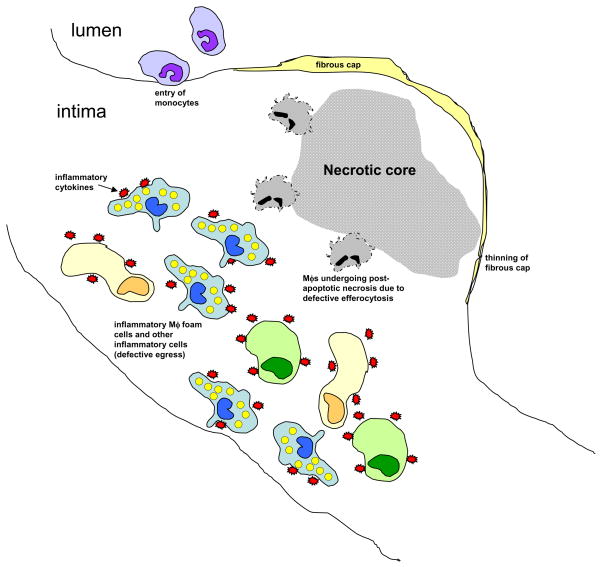
Figure 1. Schematic of a dangerous, or “vulnerable,” atherosclerotic plaque showing the hallmarks of defective resolution of inflammation.
Inflammatory cells, including lipid-laden macrophage (Mφ) foam cells, have accumulated in the intima, resulting from both persistent influx of new cells, particularly monocytes, and defective egress of the resident cells. Moreover, dead macrophages are not efficiently cleared by the process of efferocytosis, and so they undergo post- apoptotic necrosis. This process contributes to the formation of the necrotic core, which contributes to plaque disruption, particularly thinning of the fibrous cap. If the process continues, there will be a breach in the fibrous cap, leading to lumenal thrombosis and arterial occlusion.
Monocyte entry and macrophage egress
Two key processes in the resolution phase of inflammation are decreased entry of new inflammatory cells as well as exit, or egress, of living cells that still remain in the lesion1, 11. For example, entry of new monocytes into pre-existing inflammatory atherosclerotic lesions in mice is actually enhanced rather than being suppressed as would normally occur during inflammation resolution16. Moreover, Ly-6C(hi) monocytes, which are precursors of inflammatory macrophages, increase in number in the setting of hypercholesterolemia in mice, in part due to impaired conversion into less inflammatory Ly-6C(lo) monocytes17, 18. These studies suggest that there is persistent recruitment of inflammatory monocytes into established atherosclerotic lesions, particularly in the setting of hypercholesterolemia, consistent with defective inflammation resolution.
Monocytes can differentiate into two major types of macrophages, those that promote inflammation, referred to as classically activated, or M1, macrophages, and those that promote resolution, referred to as alternatively activated, or M2, macrophages3. Thus, an imbalance in the ratio of classically activated and alternatively activated macrophages in advanced atherosclerosis may cause or at least reflect impaired resolution19. Among the factors that can shift the balance in favor of M2 macrophages are an increase in the Th2 cell-secreted molecules, such as interleukin-43; the transcription factors peroxisome proliferators-activated receptors (PPARs) γ and δ 20, 21; and the bioactive lipid sphingosine-1-phosphate22. Studies in mice have shown, as predicted, that processes that promote Th2 cell polarization23, activate PPARγ24, or activate sphingosine-1-phosphate signaling22, 25 have beneficial effects on atherosclerosis, but the degree to which these results reflect M2 polarization, other aspects of inflammation resolution, or other processes in atherogenesis remains to be fully explored.
Egress of inflammatory cells from atheromata is also impaired in the setting of hypercholesterolemia26. An important step in inflammatory cell egress of myeloid cells is their conversion into migratory cells, such as dendritic cells (DCs)27. Many growth factors and cytokines, notably granulocyte-macrophage-colony stimulating factor (GM-CS), tumor necrosis factor-α (TNFα), and interleukin-4 and -6, have been shown to promote dendritic cells differentiation and/or maturation, perhaps representing a link between the initial inflammatory cytokine stage and subsequent resolution stage of inflammation28. In the atherosclerotic lesions of Apoe−/− mice, inflammatory cell egress is difficult to detect in the setting of hypercholesterolemia, but when plaques are exposed to a low cholesterol state, dendritic-like cells migrate through adventitial lymph vessels to local lymph nodes in a process that is dependent on the DC migratory molecule, CCR729. Thus, at least one factor that impairs key processes if inflammation resolution is the hypercholesterolemic state, perhaps through induction of inflammatory molecules that block the resolution phase.
Inflammation resolution mediators
Anti-inflammatory cytokines and transcription factors play important roles in the resolution of inflammation1, 2. Chief among these are interleukin-10 (IL-10), transforming growth factor-β (TGFβ), lipoxins, resolvins, protectins, maresins, prostaglandin E2 (PGE2), and, in the case of macrophages in atherosclerosis, a transcriptional factor called liver x receptor (LXR)30–34. The major target cells of IL-10 signaling are macrophages and dendritic cells, where IL-10 receptor signaling induces the anti-inflammatory molecule suppressor of cytokine signaling 3 (SOCS3) and inhibits the NF-κB pathway30. The net result is suppressed macrophage-mediated activation of inflammatory T cells and decreased production by macrophages of inflammatory cytokines (interleukins-1, 6, and 12; TNFα), and matrix metalloproteinases. IL-10 also activates signal transducer and activator of transcription-3 (STAT3), which inhibits endoplasmic reticulum (ER) stress-induced apoptosis in macrophages by inducing cell-survival molecules35, and it down-regulates CD3636, which triggers apoptosis in ER-stressed macrophages exposed to toll-like receptor 2 (TLR2) ligands (T. Seimon and I. Tabas, manuscript in preparation). IL-10 is also a potent enhancer of efferocytosis both in vitro and in vivo (D. Schrijvers and I. Tabas, unpublished data). In mouse models of atherosclerosis, genetic targeting of IL-10 worsens atherosclerosis, and genetic or pharmacologic over-expression is beneficial37–40. For example, Western diet-fed Ldlr−/− mice transplanted with IL-10 transgenic bone marrow showed a 47% decrease in lesion size and a marked decrease in lesion complexity with a 80% reduction in necrotic core area compared with mice receiving wild-type bone marrow39. Moreover, serum IL-10 levels in humans are reduced in subjects with acute coronary syndromes and are inversely correlated with future atherothrombotic events in survivors of myocardial infarction41–43. Thus, deficiency in the amounts and/or actions of IL-10 may contribute to the defect in inflammation resolution in atherosclerosis.
TGFβ plays two key roles in inflammation resolution: anti-inflammation, particularly as mediated by CD4(+)CD25(+) regulatory T cells and efferocytes44, 45, and stimulation of a protective “scar” response in resolving lesions by inducing collagen production in fibroblasts46. A defect in this pathway in intimal smooth muscle cells may contribute to a key feature of vulnerable atherosclerotic plaques, namely, thinning of the fibrous cap47. When TGFβ signaling was interrupted in Apoe−/− mice by administration of a decoy soluble TGFβ receptor or anti-TGFβ neutralizing antibodies, plaque progression toward a vulnerable phenotype was accelerated, with a heightened state of inflammation, large necrotic cores, and thin fibrous caps48, 49. In contrast, transgenic overexpression of TGFβ in Apoe−/− mice stabilized atheromata by decreasing these three detrimental endpoints 46.
Macrophage 12/15-lipoxygenase (12/15-LO) plays a critical role in lesional inflammation resolution in chow-fed Apoe−/− mice through synthesis of lipoxin A4 (LXA4), resolvin D1 (RvD1), and protectin D1 (PD1)10. In particular, 12/15-LO deficiency promoted lesion formation, and there was an inverse correlation between 12/15-LO expression and plasma level of certain inflammatory cytokines. In vitro experiments in this study showed that LXA4, PD1, and RvD1 suppressed atherosclerosis-relevant inflammatory cytokine production by lipopolysaccharide (LPS)-activated macrophages, and they enhanced the ability of macrophages to ingest apoptotic cells10. Moreover, TNFα-mediated activation of endothelial cells, which plays a critical role in monocyte chemotaxis and adhesion during atherogenesis, was also suppressed by RvD1 and LXA4. In particular, RvD1 suppressed the production of monocyte chemotactic protein-1 (MCP-1) and interleukin-8 and induced anti-inflammatory platelet-derived growth factor-β (PDGFβ); PD1 suppressed MCP-1 and vascular cell adhesion molecule-1 (VCAM-1); and LXA4 down-regulated P-selectin10. Other models have substantiated these findings. For example, in a rabbit model of inflammatory periodontal disease, a significant risk factor for ATVD in humans50, overexpression of 15-LO and subsequent production of LXA4 was associated with a decrease in high fat diet-induced atherosclerosis51. In a study relevant to joint inflammation in systemic lupus erythrematosis, LXA4 was found to stimulate the efferocytosis of apoptotic neutrophils, which is a topic relevant to 52. In addition to the 12/15-LO pathway, there is evidence that a 14-LO pathway can generate a molecule called maresin, which has potent anti-inflammatory effects33. The cyclooxygenase pathway, through the generation of prostaglandin E2 (PGE2), can also participate in the suppression of the inflammatory response after an acute infection31. PGE2, through interaction with guanine nucleotide protein-coupled receptors of the Gαs subtype, triggers a cyclic AMP-mediated pathway that induces IL-10 production and suppresses the NF-κB pathway31.
Transcription factors known as liver X receptors (LXRs) have anti-inflammatory effects in macrophages34. The anti-inflammatory mechanisms of LXR include inhibition of NF-κB-mediated gene induction, including pro-atherogenic interleukin-6, and suppression of antigen-induced T cell proliferation53, 54. In addition, LXR induces arginase II, an enzyme that can prevent inflammatory nitric oxide production and one that is characteristic of the M2 macrophage phenotype55. LXR can also block the induction of cycloxygenase-2 in LPS-activated macrophages in vitro34 and the expression of matrix metalloproteinase-9, which is thought to mediated plaque disruption, in the aorta of aged Apoe−/− mice56. In terms of atherosclerosis progression, treatment of atherosclerosis mouse models with LXR agonists markedly suppresses lesion progression, even after lesions have become established, and gene targeting of LXR has the opposite effect57–59, although some of this effect may be related to the role of LXR in cholesterol efflux from macrophages. LXR can also induce another key feature of inflammation resolution, macrophage egress from atheromata (J. Feig et al., submitted for publication).
Importantly, the various cellular processes of inflammation resolution can be linked by these inflammation resolution factors. For example, IL-10 not only directly blocks inflammatory responses in M1 macrophages but also stimulates the conversion of M1 macrophages into the M2 subtype and enhances efferocytosis3, 60. LXR activation in macrophages links three key features of inflammation resolution: suppression of inflammatory cytokine production34; egress of inflammatory macrophages from atheromata (J. Feig et al., submitted for publication); and enhancement of efferocytosis through the induction of at least two efferocytosis receptors, transglutaminase-261 and Mertk62. As a third example of integration, successful efferocytosis leads to the production of TGFβ{2590}, which stimulates formation of the protective fibrous cap in atheromata46. 12/15-LO not only leads to the synthesis of lipid mediators of inflammation resolution32, but also mediates the induction of pro-resolution PPARγ and its ligands, such as 13-hydroxyoctadecadienoic acid (13-HODE) and 15-hydroxyeicosatetraenoic acid (15-HETE), by interelukin-4, a marker of M2 macrophages63. These integrative responses suggest that therapeutic manipulations that affect one of these factors may have multiple beneficial effects in enhancing inflammation resolution in atheromata.
Macrophage death and efferocytosis
Early lesional macrophage death
Although the mechanisms of macrophage death in early lesions are not well known, apoptosis is present in macrophage-rich regions of early lesions64. Several studies have used genetic engineering in mice to either increase or decrease early lesional macrophage apoptosis to determine its effect on lesion size and progression. In Ldlr−/− mice in which bone marrow-derived cells, including lesional macrophages, had a deficiency of the pro-apoptotic protein Bax, aortic root lesions had decreased macrophage apoptosis and were larger and more macrophage-rich 65. Similar results were obtained in Apoe−/− mice lacking the pro-apoptotic protein p53 in macrophages66. Conversely, Ldlr−/− mice lacking the pro-survival protein AIM (Spα) showed an increase in early lesional macrophage apoptosis and developed smaller atherosclerotic lesions67. Similar findings were observed in studies in which early lesional macrophage apoptosis was increased through stimulation of pro-apoptotic nitric oxide production by L-arginine68 and through gene targeting of the prostaglandin E(2) receptor, EP4, which has pro-survival signaling in macrophages69. Thus, early lesional macrophage apoptosis is associated with smaller lesion size and less plaque progression. A likely explanation for this phenomenon is that apoptotic cells in this setting are efficiently cleared by neighboring macrophages, resulting in lesions with less macrophages, which is known to be associated with less lesion progression,70 in a manner that avoids post-apoptotic necrosis. Support for this concept comes from a study in which the efferocytosis mediator complement C1q was genetically targeted in Ldlr−/− mice71. In these mice, the appearance of apoptotic macrophages in early lesions increased dramatically, suggesting that normal paucity of detectable apoptotic cells in early lesions is due to efficient efferocytosis. The corollary of this concept is that the elements of defective inflammation resolution known to exist in advanced lesions, notably defective efferocytosis, have not yet taken hold in early atherosclerosis 9, 15 (FIG. 2A).
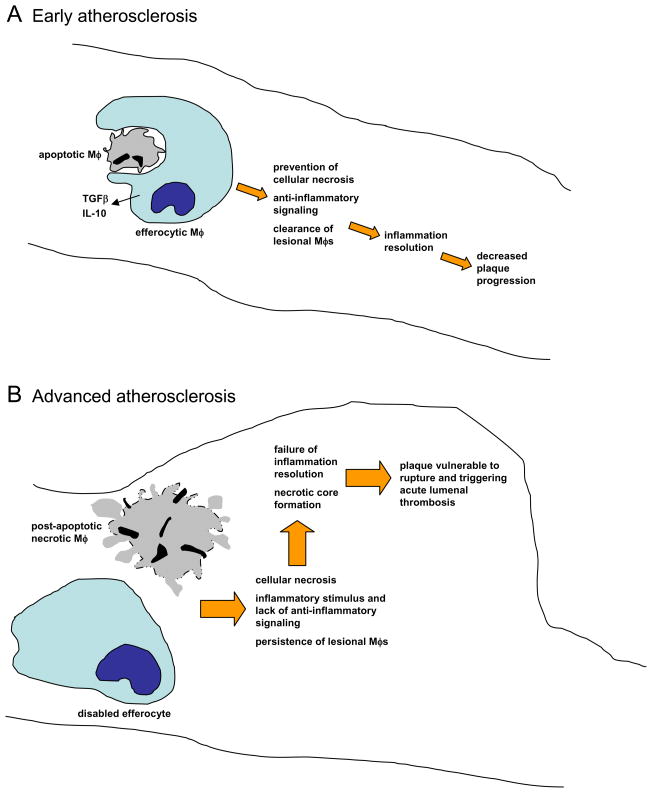
Figure 2. Efferocytosis and inflammation resolution in early and advanced atherosclerosis.
A In early atherosclerotic lesions, efferocytosis is efficient, leading to rapid clearing of apoptotic macrophages (Mφs). This process prevents post-apoptotic cellular necrosis, elicits the production of anti-inflammatory cytokines, and clears macrophages from the lesions. The result of this inflammation resolution process is decreased plaque progression. B In advanced lesions, efferocytes do not function properly and this apoptotic macrophages become secondarily necrotic. The necrotic material is a stimulus for inflammation, and the normal anti-inflammatory signaling associated with efferocytosis does not occur. Moreover, an important mean for ridding the lesion of inflammatory macrophages is lost. Thus, inflammation resolution fails to occur normally, and necrotic macrophages coalesce into necrotic cores. These features define plaques that are vulnerable to rupture. which in turn can trigger acute lumenal thrombosis and arterial occlusion.
Advanced lesional macrophage death
A number of hypotheses have been advanced to explain macrophage apoptosis in advanced lesions, and undoubtedly more than one mechanism is involved. Examples include growth factor deprivation, toxic cytokines, and oxidized lipids or lipoproteins,72 but there is as yet little proof for these ideas in vivo. However, there is increasing in vivo evidence supports the concept that ER stress may contribute significantly to advanced lesional macrophage death (FIG. 2B). ER stress activates a branch of the so-called Unfolded Protein Response (UPR) pathway that increases the expression of a pro-apoptotic protein called CEBP-homologous protein (CHOP), also know as growth arrest and DNA damage protein-153 (GADD153)73. CHOP can lead to apoptosis by a number of mechanisms, but recent work in our laboratory points to a specific apoptotic mechanism involving release of calcium from the ER lumen74, 75. Released ER calcium can trigger apoptosis through excess uptake by mitochondria, which triggers the release of pro-apoptotic effectors from mitochondria, and by activation of a calcium-responsive enzyme called calcium/calmodulin-dependent protein kinase II (CaMKII), which promotes cell death by activating both extrinsic (death receptor) and intrinsic (mitochondrial) pathways of apoptosis75. There are many ER stressors in advanced atherosclerotic lesions, and expression of ER stress proteins, including CHOP, are closely correlated with apoptosis and plaque vulnerability in human coronary arteries76. In vitro studies have suggested that some of these atherosclerosis-relevant ER stressors, such as 7-ketocholesterol, are sufficient by themselves to trigger this apoptotic pathway if they are present in high enough amounts74. However, another setting may be more subtle ER stress, and in this case a “second hit” is needed to trigger apoptosis77, 78. An example includes excess accumulation of lipoprotein-derived cholesterol by macrophages, where the ER stress “hit” arises from excess cholesterol accumulation in the ER membrane, and the “second hit” is engagement of pattern recognition receptors (PRRs), such as the type A scavenger receptor, CD36, and toll-like receptors, by the lipoproteins themselves77, 78 (T. Seimon and I. Tabas, unpublished data). PRR activation acts as a second hit by enhancing pro-apoptotic processes and suppressing compensatory cell-survival signaling in the setting of ER stress77, 78 (T. Seimon and I. Tabas, unpublished data).
Most importantly, there are in vivo molecular-genetic causation data supporting these concepts. While murine models of atherosclerosis, such as Western diet-fed Apoe−/− and Ldlr−/− mice, are not useful for studying plaque disruption or acute atherothrombosis, they are very good for studying atherosclerosis up to and including the stage of plaque necrosis79, 80. Using these models, it has been possible to test the pathophysiologic relevance of specific mechanisms of macrophage apoptosis as well as the overall concept that advanced lesional macrophage death promotes plaque necrosis (TABLE 1A). With regard to the lipoprotein-cholesterol model mentioned above, a mutation in an intracellular cholesterol trafficking protein that protects the ER from cholesterol accumulation suppresses advanced lesional macrophage apoptosis and plaque necrosis in Apoe−/− mice81. Similar results were found in mice lacking a critical pro-apoptotic signaling molecule, signal transducer and activator of transcription-1 (STAT-1), which is downstream of the CHOP pathway82. Most importantly, deficiency of CHOP in two models of advanced murine atherosclerosis, the Ldlr−/− mouse and the Apoe−/− mouse, protect advanced lesions from apoptosis and plaque necrosis, as does combined CD36 and SRA deficiency in Apoe−/− mice83. Moreover, in vitro studies have shown that the macrophage ER stress-apoptosis pathway outlined above is enhanced in the setting of insulin resistance84, 85, which in humans is a major force behind plaque necrosis and atherothrombotic vascular disease86, 87. A direct comparison of Ldlr−/− mice with macrophages that have intact vs. defective insulin signaling revealed a substantial impact of macrophage insulin resistance on macrophage apoptosis and plaque necrosis85. The role of SRA and CD36 as “second hits” for ER stress-induced apoptosis was illustrated by a mouse model in which both PRRs were genetically targeted, with the result that advanced lesional macrophage apoptosis and plaque necrosis were markedly deceased88. Finally, as alluded to above, apoptotic processes in general, and ER stress-induced apoptosis in particular, is usually represent and imbalance between apoptotic and compensatory cell-survival pathways. For example, ER stress activates the mitogen-activated protein kinase p38, which promotes cell-survival in certain settings of ER stress by activating Akt, a known mediator of cell survival89. Macrophage-targeted knockout of p38 in Apoe−/− mice enhanced both advanced lesional macrophage apoptosis and plaque necrosis89.
Table 1. Evidence that ER stress-induced macrophage (Mφ) apoptosis and defective efferocytic clearance of apoptotic Mφs are important in advanced plaque progression in mouse models of atherosclerosis.
| A. Models affecting ER stress-induced Mφ apoptosis | |||
|---|---|---|---|
| Mouse model | Description of mutation | Weeks on high-fat diet | Effect of mutation on Mφ apoptosis and plaque necrosis in advanced aortic root lesions |
| Npc1+/−;Apoe−/−81 | NPC1 is a protein involved in intracellular cholesterol trafficking. The heterozygous mutation results in a partial defect of cholesterol trafficking to the ER, and thus protects the ER from cholesterol-induced ER stress | 18, 25 | ↓ |
| Stat1−/− → Ldlr−/−*82 | STAT1 is activated during ER stress and promotes Mφ apoptosis | 10, 12 | ↓ |
| Chop−/−;Apoe−/−83 Chop−/−;Ldlr−/−83 | CHOP is induced during ER stress and triggers apoptosis in ER-stressed Mφs | 10, 12 | ↓ |
| Insr−/− → Ldlr−/−85 | Mφs lacking insulin receptors, as a model of Mφ insulin resistance, undergo ER stress and are more susceptible to ER stress-induced apoptosis | 8, 12 | ↑ |
| Sra−/−;Cd36−/−; Apoe−/−88 | Activation of the type A scavenger receptor (SRA) and CD36 synergize with ER stress to cause apoptosis in Mφs | 12 | ↓ |
| p38afl/fl;LysMCre+/−; Apoe−/−89 | ER stress activates p38 in Mφs, which can trigger a compensatory cell survival pathway through activation of Akt | 9 | ↑ |
| B. Models affecting efferocytosis | |||
| Mouse model | Description of mutation | Weeks on high-fat diet | Effect of mutation on Mφ apoptosis and plaque necrosis in advanced aortic root lesions |
| Tg2−/− → Ldlr−/−102 | Transglutaminase 2 mediates recognition of apoptotic cells by efferocytic Mφs | 16 | ↑ |
| Mfge8−/− → Ldlr−/−103 | MFG-E8 is a molecule that can bridge apoptotic cells and efferocytes, thus facilitating efferocytosis | 8, 15, 20 | ↑ |
| MertkKD;Apoe−/−104, 105 | Mertk is a receptor on efferocytes for apoptotic cells. The KD mutation renders the receptor non-functional | 8, 10, 15, 16 | ↑ |
| Gld;Apoe−/−106 | The Gld mutation inactivates the ligand for the Fas receptor. Mice with this mutation acquire an autoimmune syndrome characterized by defective efferocytosis. | 12 | ↑ |
This designation indicates transplantation of bone marrow from mutant mice into lethally irradiated Ldlr−/− mice to create chimeric mice in which only bone marrow-derived cells carry the mutation. Bone marrow from wild-type mice are used for the control arm in these types of studies. For atherosclerosis studies using this method, the major type of lesional cell carrying the mutation is the macrophage.
Human advanced plaques, like those in mice, show markers of ER stress and evidence of macrophage apoptosis90–92. In one study, autopsy specimens and fresh atherectomy specimens of human coronary arteries from patients with heart disease. The specimens were divided into five stages of atherosclerosis, from very early lesions to vulnerable plaques, and analyzed them for UPR markers and apoptosis. The results showed a very close correlation among UPR marker expression, including CHOP, apoptosis, and advanced plaque stage91. These correlative findings set the stage for genetic studies, such as genome wide association studies, to see evidence for a more causative relationship between genes in the ER stress-apoptosis pathway and human coronary artery disease.
Efferocytosis
A key step in the resolution phase of inflammation is efferocytosis of apoptotic inflammatory cells45, 93, 94. In acute inflammation, this process is concerned mostly with the clearance of short-lived apoptotic neutrophils. However, in the chronic inflammatory process of atherosclerosis, most of the apoptotic cells that need to be cleared are apoptotic macrophages93–95. Efficient clearance of apoptotic cells prevents secondary, or post-apoptotic, cellular necrosis, and it triggers an anti-inflammatory response through the induction of TGFβ, IL-10, and other anti-inflammatory cytokines94. Inflammation can also be suppressed in efferocytes by molecules released by the apoptotic cells themselves, as is the case with protein S1009 (migration inhibitory factor-related protein-8 [MRP-8]) released by apoptotic neutrophils96. In the context of an area of critical relevance to ATVD, uncleared apoptotic cells also shed plasma membrane microparticles, which can stimulate thrombosis97. In early atherosclerotic lesions, efferocytosis appears to be efficient, because (a) genetic manipulations that increase apoptosis lead to a decrease in the number of macrophages, slower lesions progression, and no increase in necrosis; (b) vice versa for manipulations that decrease apoptosis; and (c) when early lesional efferocytosis is compromised by genetic engineering, apoptotic macrophages become much more abundant9, 71. In advanced lesions, however, increased apoptosis is associated with plaque necrosis, which suggests inefficient efferocytosis9, 15 (FIG. 2B). Indeed, a clever study in which in situ efferocytosis was assessed by quantification of apoptotic cells either associated with or free from neighboring phagocytes showed a cleared defect in advanced human atherosclerotic lesions compared to a control tissue, human tonsils98.
What are the molecular mechanisms of efferocytosis in advanced atheromata, and what goes wrong in this setting? Efferocytosis involves a complex interplay among several sets of proteins that enable the recognition and engulfment of apoptotic cells by phagocytes93–95. In the early stages of apoptosis, cells secrete factors, referred to as “find-me” signals, that attract phagocytes (e.g., lysophosphatidylcholine99; suppress the secretion of other molecules, referred to as “don’t-eat-me” signals, that normally prevent the phagocytosis of non-apoptotic cells (e.g., CD47100; and display a unique set of molecules on their surface that recognize and bind to cognate “receptors” on the apoptotic cell (e.g., phosphatidylserine101. In several cases, the interaction involves an intermediary bridging molecule. For example, αvβ3 integrin and Mertk on phagocytes interact with apoptotic cells through the bridging molecules milk fat globulin E8 (MFG-E8; lactadherin) and Gas6, respectively93–95. Contact between apoptotic cells and phagocytes enables engulfment of the apoptotic cells, leading eventually to digestion in phagolysosomes. In addition, as mentioned above, receptor engagement on the phagocyte often triggers an anti-inflammatory response93–95. Mouse studies have revealed specific roles for a number of receptors or bridging molecules in advanced lesional efferocytosis, including transglutaminase 2102, milk fat globule-EGF factor 8 (MFG-E8)103, Mertk104, 105, and Fas ligand106 (TABLE 1B). For example, Apoe−/− mice lacking the Mertk receptor have a defect in efferocytosis in advanced atheromata that correlates with an increase in plaque inflammation and plaque necrosis104, 105.
In advanced atheromata, “defective efferocytosis” could, in theory, represent overwhelming apoptosis that saturates the efferocytic capacity in advanced lesions. However, the very high capacity of efferocytosis when it is functioning properly suggests that this explanation is, at most, only part of the explanation94. For example, as mentioned in the previous sections, when apoptosis is increased through genetic manipulation in early atherosclerotic lesions, where efferocytosis is appropriately efficient, the apoptotic cells are efficiently cleared67. Another possibility is that advanced lesional macrophage death per se limits efferocytosis by limiting the pool of competent efferocytes. However, advanced lesions have a large population of living macrophages that could presumably carry out this role107. Furthermore, we have shown that the quintessential macrophage alteration in atheromata, namely, foam cell formation, does not compromise the ability of the cells to recognize and ingest apoptotic cells108. A third possibility is that the apoptotic macrophages themselves in advanced lesions are poor substrates for efferocytic recognition and engulfment. For example, the nature of the apoptotic stimulus or some other feature in these lesions might lead to poor display of efferocytosis ligands or “find-me” signals and/or inappropriately increased display of “don’t-eat-me” signals on the apoptotic cells. A fourth possibility is that bridging molecules or efferocytosis receptors are deficient in atheromata. In this context, the Mertk receptor, which plays a critical role in advanced lesional macrophage apoptosis104, 105, is susceptible to sheddase-mediated cleavage, probably by ADAM17, which inhibits efferocytosis by two mechanisms—destruction of Mertk and competitive inhibition of efferocytosis by soluble Mer109. As another example, the bridging molecules GAS6 and MFG-E8 may be down-regulated in advanced lesions by the processes of lesional smooth muscle death and inflammation, respectively110–113. Finally, to the extent that some degree of efferocytosis does occur in advanced lesions, the critical component of anti-inflammatory signaling may be defective, e.g., due to a defect in secretion of or response to anti-inflammatory cytokines. Whatever the mechanisms, increasing evidence suggests that defective efferocytosis in advanced atheromata is a major cause of necrotic core formation and thus contributes substantially to the formation of vulnerable plaques.
Future directions and therapeutic implications
The ability to translate the complex process of plaque progression into an integrated molecular-cellular concept of defective inflammation resolution provides a useful way to understand how atherosclerosis leads to clinical disease and how plaque progression may be prevented by novel therapeutic approaches. In this light, it is important to outline areas within this paradigm that need further investigation. Most importantly, we need a more complete understanding of the molecular and cellular mechanisms for defective inflammation resolution in advanced atherosclerosis. While this review has focused on upstream mediators of inflammation resolution, complex post-inflammation negative feedback mechanisms at the transcriptional and translational levels represent critical sequelae of the inflammatory response114–116. At a fundamental level, it will be important to elucidate what portion of the defect in inflammation resolution in advanced atherosclerosis is due to defective production of inflammation resolution effectors and what portion is due to defective action of these effectors at the cellular level, e.g., due to decreased activity of receptor signal transduction pathways. Now that we possess a reasonably complete list of these effectors, and with a good knowledge of how they act on cells, we can probe plaques during the most critical period of progression to the necrotic/thin-cap stage for transcription factors and enzymes involved in effector formation and for the receptors and signaling transducers that are activated by these effectors. A good knowledge of the cell types involved is also critical, and more work is needed in this area. T cells are involved in production of certain inflammation resolution effectors, e.g., IL-10, and in factors that determine their action. For example, Th2 and Th1 cell cytokines influence the balance between M2 and M1 macrophages, respectively (above)3. The major inflammation resolution effector cells in lesions are macrophages and dendritic cells, and understanding how each of the various subsets of these cell types influences the overall resolution response—and the defects in advanced lesions—is an important goal for the future. Moreover, it will be interesting to assess the roles of mast cells and neutrophils in normal and defective inflammation resolution, because studies are increasingly implicating these two cell types in atherogenesis and plaque progression117, 118.
The answers to these questions will increasingly inform the field as to the best therapeutic approaches to prevent defective inflammation resolution in progressing atherosclerotic plaques and to reverse the defect in those plaques that are already advanced119. In our current state of knowledge, we can already begin propose strategies. With regard to lipid mediators of inflammation resolution, omega-3 long-chain fatty acids, which occur naturally in fish oils, are precursors of protectins, resolvins, and maresins32, 33, and the fish oil EPA has been shown to have beneficial effects on ATVD in humans120, 121. More in-depth understanding of the mechanisms of these effects may suggest more specific and potent drugs to enhance inflammation resolution in advanced atherosclerotic lesions. In this context, exposure of macrophages to saturated fatty acids in vitro and exposure of obese mice to a saturated fatty acid-enriched diet lead to defects in macrophage efferocytosis, including in advanced atherosclerotic lesions, and one mechanism may be displacement of omega-3 fatty acids in macrophages by the saturated fatty acids (S. Li et al., submitted for publication). LXR and PPARγ activators are already available as oral-based formulations, although off-target systemic effects, notably hepatosteatosis and heart failure, respectively, limit their current usefulness53. As another example, the orally available drug FTY720, a mimetic of the pro-resolution lipid sphingosine-1-phosphate, has been shown to increase the proportion of M2 macrophages in atherosclerotic lesions and retard lesion progression in mice22. In a mouse model of peritonitis, an analogue of the pro-resolution lipid LXA4 enhanced efferocytic clearance of apoptotic neutrophils122. Endogenous and exogenous cannabinoids, which are fatty acid derivatives, can trigger inflammation resolution, perhaps through stimulation of biosynthesis of lipid mediators123. Indeed, oral administrative of the cannabinoid receptor-1 agonist rimonabant suppressed atherosclerosis and plaque inflammation in fat-fed Ldlr−/− mice124. Here, as with LXR and PPARγ agonists, the safest application of these potent agents would be in a setting that optimizes the specific accumulation of the drug in atherosclerotic plaques. Thus, future developments in plaque-targeted drug technology will be important with these agents.
Therapeutic administration of bioactive proteins represents an even greater technical challenge but one with great promise. For example, there are abundant data that the inflammation resolution effectors IL-10 and TGFβ can prevent plaque progression37–40, 46, 48, 49. The protein annexin I, which is an anti-inflammatory effector of glucocorticoid action, has been shown to increase the efferocytosis of apoptotic neutrophils in the inflamed joints of mouse model of lupus125, 126. The challenge in these cases is how to administer a bioactive protein and how to do it in a plaque-targeted manner. New approaches in plaque-targeted protein delivery systems, such as nanoparticle-based therapy, may offer great promise in this area127. A different type of innovative strategy to enhance efferocytosis was suggested by Silverman and colleagues128, who showed that immunization of mice with apoptotic cells boosted the production of natural IgM antibodies to apoptotic cell surface proteins, leading to C1q recruitment and increased efferocytosis. Others have suggested that if efferocytosis could be enhanced, purposefully inducing inflammatory cell apoptosis could speed the process of inflammation resolution129.
To the extent that successful inflammation resolution-based therapies for atherosclerosis may necessitate non-oral therapeutic regimens, the primary niche may be that of “bridging” therapy for subjects with severe atherothrombotic risk factors, including post-acute coronary event. The foundation of chronic ATVD preventative therapy is risk reduction through cholesterol-lowering, mainly via statins; blood pressure control; treatment of insulin resistance, including lifestyle changes; and elimination of environmental risk factors, notably smoking. However, it takes up to two years for such therapy to show maximum beneficial effects, and even with these therapies, many of patients still are at high risk for recurrent disease130. In this context, one can envision that safe and effective therapy directed specifically at preventing further plaque progression and promoting plaque regression could substantially lower events during this period. Moreover, therapy directed at improving inflammation resolution would be expected to alter the overall inflammatory milieu of vulnerable plaques in a manner that should improve the effectiveness of conventional, chronic plaque-stabilizing therapies, such as statins. Given the very high risk of morbidity and mortality during this period, the benefits of periodic systemic therapy should outweigh the inconvenience.
Summary and conclusions
In industrialized societies, almost all individuals have subclinical atherosclerotic lesions by the time they enter their teenage years. Although only a minority of the lesions in these individuals will progress to the state of causing ATVD, the number of lesions is so high that this progression accounts for the leading cause of morbidity and mortality in this population. Therefore, defining the molecular and cellular processes that cause the transition of subclinical atherosclerotic lesions to dangerous plaques is essential. This review has provided evidence that defects in specific molecular and cellular events that normally function to limit, or resolve, inflammatory processes can account for a substantial portion of this transition. In contemplating how this knowledge can lead to useful therapy, it is important to understand that atherosclerosis progression in general, and defective inflammation resolution in particular, are complex processes, and it is unlikely that only one approach will be effective. Rather, therapeutic approaches that attack complementary processes in defective inflammation resolution are likely to have the most success. Fortunately, as is evident from the information in this review, there are a number of complementary processes in inflammation resolution, and some of these are likely to be feasible targets for both conventional types of drugs and for those that will require novel means of formulation, delivery, and tissue targeting. Given the increasingly prevalent forces of aging and obesity on ATVD in our society, even modest progress in this area over the next decade could have a huge beneficial effect.
Acknowledgments
The author acknowledges stimulating conversations with Drs. Edward A. Fisher, Edward Thorp, Dorien Schrijvers, Gwen Randolph, Larry Chan, and Ajay Chawla during the conception and writing of this review. The work from the author’s laboratory cited herein was supported by National Institutes of Health grants HL54591 and HL75662 from the National Heart, Lung, and Blood Institute.
Glossary
- Atherosclerosis
A process whereby lipids, inflammatory cells, and extracellular matrix accumulate in the subendothelial space, or intima, of focal areas of medium-sized arteries
- Atherothrombotic vascular disease (ATVD)
Disease caused by acute occlusive arterial thrombosis overlying areas of chronic atherosclerosis. The occlusive thrombosis starves the tissue that the involved artery feeds of oxygen and nutrients. For example, if the involved artery feeds the heart muscle, myocardial infraction, or death of heart muscle tissue, can ensue
- Apoptosis
Programmed cell death that is triggered by receptor-mediated signaling from so-called “death receptors” (e.g., Fas) and/or by release of cell death-inducing molecules from the mitochondria. Both of the pathways eventually activate proteases called caspases, which execute the cell death process
- Efferocytosis
A term used to described the phagocytic clearance of apoptotic cells, from the Latin to take to the grave or bury. Efferocytosis ensures clearance of dead cells before they become necrotic, and the process usually triggers an anti-inflammatory response
- ER stress
A term used to describe a condition in which the equilibrium of the endoplasmic reticulum (ER) is disturbed by any one of a number of processes. ER stress triggers an integrated signal transduction pathway called the Unfolded Protein Response (UPR), which through alterations of protein translation and induction of protein chaperones and other effectors helps restore equilibrium in the ER
- Apoe−/− mice
A widely used mouse model of atherosclerosis that is prone to develop atherosclerosis because they have high levels of a type of atherogenic lipoprotein called remnant lipoproteins. The lipoprotein abnormality is cause by genetic absence of apolipoprotein E, which normally functions to clear remnant lipoproteins from the blood stream by interacting with hepatocytes
- Ldlr−/− mice
Another widely used mouse model of atherosclerosis. These mice develop high levels of LDL when placed on a high-fat diet, because their hepatocytes lack LDL receptors and this cannot efficiently rid the bloodstream of atherogenic LDL particles
- Insulin resistance
A state in which insulin receptor signaling in cells is impaired. The cause can be exposure to high levels of insulin, which down-regulates insulin receptors via a negative homeostatic mechanism, or disruption of signaling molecules downstream of the insulin receptor, such as insulin receptor substrate (IRS) 1 and 2. Insulin resistance, caused by high levels of insulin in the bloodstream, is responsible for a substantial portion of the pathology associated with type 2 diabetes, including ATVD
References
- 1.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 5.NHLBI Morbidity and Mortality Chart Book. (2004).
- 6.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 7.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 10.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 13.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 14.Libby P, Clinton SK. The role of macrophages in atherogenesis. Curr Opin Lipidol. 1993;4:355–363. [Google Scholar]
- 15.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Swirski FK, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajnoczky G, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vila-Petroff M, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Garlanda C, Locati M. Macrophage Diversity and Polarization in Atherosclerosis. A Question of Balance. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 20.Chawla A, et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 21.Odegaard JI, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nofer JR, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 23.Laurat E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 24.Li AC, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keul P, et al. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 26.Angeli V, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Llodra J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou GM, Tam YK. Cytokines in the generation and maturation of dendritic cells: recent advances. Eur Cytokine Netw. 2002;13:186–199. [PubMed] [Google Scholar]
- 29.Adams CWM, Bayliss OB, Ibrahim MZM. The distribution of lipids and enzymes in the aortic wall in dietary rabbit atheroma and human atherosclerosis. J Path Bact. 1963;86:421–430. doi: 10.1002/path.1700860216. [DOI] [PubMed] [Google Scholar]
- 30.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters-Golden M. Putting on the brakes: cyclic AMP as a multipronged controller of macrophage function. Sci Signal. 2009;2:e37. doi: 10.1126/scisignal.275pe37. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubic T, Lorenz RL. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc Res. 2006;69:527–535. doi: 10.1016/j.cardiores.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Pinderski Oslund LJ, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 38.Caligiuri G, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 39.Pinderski LJ, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 40.Potteaux S, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 41.Tziakas DN, et al. Anti-inflammatory cytokine profile in acute coronary syndromes: behavior of interleukin-10 in association with serum metalloproteinases and proinflammatory cytokines. Int J Cardiol. 2003;92:169–175. doi: 10.1016/s0167-5273(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 42.Wojakowski W, et al. The pro- and anti-inflammatory markers in patients with acute myocardial infarction and chronic stable angina. Int J Mol Med. 2004;14:317–322. [PubMed] [Google Scholar]
- 43.Seljeflot I, Hurlen M, Solheim S, Arnesen H. Serum levels of interleukin-10 are inversely related to future events in patients with acute myocardial infarction. J Thromb Haemost. 2004;2:350–352. doi: 10.1111/j.1538-7933.2004.0584c.x. [DOI] [PubMed] [Google Scholar]
- 44.Wahl SM, Swisher J, Cartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 45.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frutkin AD, et al. TGF-{beta}1 Limits Plaque Growth, Stabilizes Plaque Structure, and Prevents Aortic Dilation in Apolipoprotein E-Null Mice. Arterioscler Thromb Vasc Biol. 2009;29:1251–1257. doi: 10.1161/ATVBAHA.109.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873–880. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Lutgens E, et al. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- 49.Mallat Z, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 50.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 51.Van Dyke TE. Resolution of inflammation-unraveling mechanistic links between periodontitis and cardiovascular disease. J Dent. 2009;37:S582–S583. doi: 10.1016/j.jdent.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godson C, et al. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 53.Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marathe C, et al. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J Biol Chem. 2006;281:32197–32206. doi: 10.1074/jbc.M605237200. [DOI] [PubMed] [Google Scholar]
- 56.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 57.Joseph SB, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin N, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 59.Bradley MN, et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 61.Rebe C, et al. Induction of Transglutaminase 2 by a Liver X Receptor/Retinoic Acid Receptor {alpha} Pathway Increases the Clearance of Apoptotic Cells by Human Macrophages. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.201855. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang JT, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 64.Kockx MM, et al. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, et al. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boesten LS, et al. Macrophage p53 controls macrophage death in atherosclerotic lesions of apolipoprotein E deficient mice. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Arai S, et al. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metabolism. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Wang BY, et al. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation. 1999;99:1236–1241. doi: 10.1161/01.cir.99.9.1236. [DOI] [PubMed] [Google Scholar]
- 69.Babaev VR, et al. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith JD, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatia VK, et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabas I. Apoptosis and plaque destabilization: the role of macrophage apoptosis induced by cholesterol. Cell Death & Differentiation. 2004;11:S12–S16. doi: 10.1038/sj.cdd.4401444. [DOI] [PubMed] [Google Scholar]
- 73.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G, et al. Role of ERO1α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009 doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links endoplasmic reticulum stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009 doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009:S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVries-Seimon T, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenfeld ME, et al. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. doi: 10.1007/s11883-002-0025-3. [DOI] [PubMed] [Google Scholar]
- 80.Tabas I. Mouse models of apoptosis and efferocytosis. Curr Drug Targets. 2008;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 81.Feng B, et al. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim WS, et al. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thorp E, et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang CP, et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han S, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metabolism. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 86.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 87.Burke AP, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 88.Manning-Tobin JJ, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seimon TA, et al. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gargalovic PS, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 91.Myoishi M, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 92.Sanson M, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 93.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Brit Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 94.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 95.Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–656. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 96.De Lorenzo BH, et al. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2009 doi: 10.1016/j.imbio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Mallat Z, et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 98.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 99.Peter C, et al. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 100.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Z. New phosphatidylserine receptors: clearance of apoptotic cells and more. Dev Cell. 2007;13:759–760. doi: 10.1016/j.devcel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 102.Boisvert WA, et al. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 103.Ait-Oufella H, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 104.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.it-Oufella H, et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 106.Aprahamian T, et al. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Libby P, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, et al. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 109.Sather S, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakano T, et al. Vascular smooth muscle cell-derived, Gla-containing growth-potentiating factor for Ca(2+)-mobilizing growth factors. J Biol Chem. 1995;270:5702–5705. doi: 10.1074/jbc.270.11.5702. [DOI] [PubMed] [Google Scholar]
- 111.Bennett MR, Boyle JJ. Apoptosis of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 1998;138:3–9. doi: 10.1016/s0021-9150(98)00013-6. [DOI] [PubMed] [Google Scholar]
- 112.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Curtiss LK, Tobias PS. Emerging role of toll-like receptors in atherosclerosis. J Lipid Res. 2008 doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 115.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 116.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zernecke A, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 119.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 120.Verboom CN. Highly purified omega-3 polyunsaturated fatty acids are effective as adjunct therapy for secondary prevention of myocardial infarction. Herz. 2006;31 (Suppl 3):49–59. [PubMed] [Google Scholar]
- 121.Saito Y, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS) Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 122.O’Sullivan TP, et al. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. J Med Chem. 2007;50:5894–5902. doi: 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- 123.Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009;11:109–119. doi: 10.1208/s12248-009-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dol-Gleizes F, et al. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. doi: 10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- 125.Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26) J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- 126.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 127.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 128.Chen Y, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hallett JM, et al. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29:250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Rouleau J. Improved outcome after acute coronary syndromes with an intensive versus standard lipid-lowering regimen: results from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial. Am J Med. 2005;118(Suppl 12A):28–35. doi: 10.1016/j.amjmed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 131.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 132.Kruth HS, Fry DL. Histochemical detection and differentiation of free and esterified cholesterol in swine atherosclerosis using filipin. Exp Mol Pathol. 1984;40:288–294. doi: 10.1016/0014-4800(84)90046-7. [DOI] [PubMed] [Google Scholar]