Abstract
Objective
Amyotrophic lateral sclerosis (ALS) is a devastating, and currently incurable, neuromuscular disease in which oxidative stress and mitochondrial impairment are contributing to neuronal loss. Coenzyme Q10 (CoQ10), an antioxidant and mitochondrial cofactor, has shown promise in ALS transgenic mice, and in clinical trials for neurodegenerative diseases other than ALS. Our aims were to choose between two high doses of CoQ10 for ALS, and to determine if it merits testing in a Phase III clinical trial.
Methods
We designed and implemented a multi-center trial with an adaptive, two-stage, bias-adjusted, randomized, placebo-controlled, double-blind, Phase II design (n=185). The primary outcome in both stages was decline in the ALS Functional Rating Scale-revised (ALSFRSr) score over 9 months. Stage 1 (dose selection, 35 participants per group) compared CoQ10 doses of 1,800 and 2,700 mg/day. Stage 2 (futility test, 75 patients per group) compared the dose selected in Stage 1 against placebo.
Results
Stage 1 selected the 2,700 mg dose. In Stage 2, the pre-specified primary null hypothesis that this dose is superior to placebo was not rejected. It was rejected, however, in an accompanying pre-specified sensitivity test, and further supplementary analyses. Pre-specified secondary analyses showed no significant differences between CoQ10 at 2,700 mg/day and placebo. There were no safety concerns.
Interpretation
CoQ10 at 2,700 mg daily for 9 months shows insufficient promise to warrant Phase III testing. Given this outcome, the adaptive Phase II design incorporating a dose selection and a futility test avoided the need for a much larger conventional Phase III trial.
ALS is an orphan disease with an average annual incidence rate of 1 to 2 per 100,000 person-years,1-3 and, because the disease typically leads to death within 2 to 4 years of onset,4 a relatively low prevalence of 4-6 per 100,000 people.5 About 10% of ALS cases are familial, and about 15 to 20% of autosomal dominant familial ALS patients have mutations the superoxide dismutase 1 (SOD1) gene.6 The pathogenesis remains incompletely understood, but several lines of evidence suggest that oxidative stress plays an important role. SOD 1 is an enzyme that plays a role in detoxifying free radicals7. In a transgenic mouse model of familial ALS, there is increased oxidative stress. 8 In patients with sporadic ALS, oxidative stress indicators were found in plasma, urine, and CSF.9-13 Mitochondrial impairment in ALS is supported by several findings in vitro, animal studies, and patients. In an ALS cell culture model, motor neuron cell lines harboring SOD1 mutations have morphologically abnormal mitochondria and impaired respiratory chain function.14 Respiratory chain dysfunction has also been described in an ALS mouse model.15 In spinal cord tissue of ALS patients, mutant mtDNA molecules were increased, and respiratory chain enzyme activities were decreased compared to control tissue.16
Coenzyme Q10 (CoQ10), a naturally occurring compound and electron acceptor in the mitochondrial respiratory chain, is a candidate drug in the treatment of ALS for two reasons. First, it is a mitochondrial cofactor with the potential to boost mitochondrial function.17, 18 Second, it is a powerful free radical scavenger that can mitigate membrane damage, DNA damage and lipid peroxidation caused by oxidative stress.19, 20 This suggests a rationale for its benefit in the treatment of ALS, which is further supported by animal studies showing prolonged survival in A93G ALS transgenic mice treated with CoQ10.21 In human trials, CoQ10 conferred benefit in Phase II studies of Parkinson’s Disease22-24, like ALS a neurodegenerative diseases so that some downstream disease mechanisms may be common.19 In pilot studies, CoQ10 was well tolerated and safe in ALS patients.25 Although relatively costly, it has become one of the supplements frequently taken by ALS patients.26 Its efficacy for ALS has not been studied in clinical trials, however, and the appropriate dose is unknown.
Trials in neurodegenerative diseases other than ALS suggest that very high doses of CoQ10 would be needed to slow disease progression.22, 27 Given no clear dose-limiting toxicities, and uncertainty as to the highest dose ALS patients can consume with reasonable compliance, we decided that a conventional sequential dose-escalation design ramping up to the maximum tolerated dose would be impractical. Instead, we identified two doses, moderately high (1,800 mg daily) and high (2,700 mg daily), that appeared both feasible and justified in terms of their safety profile, given the available data from preclinical toxicity studies and human trials. A study in Parkinson’s disease patients had found that doses up to 1,600mg daily were well tolerated.22 In a pilot trial of 17 patients receiving escalating doses of CoQ10 (1,200mg, 1,800mg, 2,400mg and 3,000mg daily) the plasma levels reached a plateau at the 2,400mg dose and did not increase further at the 3,000mg dose.27 Based on these studies, we selected 1,800mg as a moderately high and 2,700mg as a high dose level. Our first goal was to select one dose for continued study. Our second goal was to establish if the selected dose of CoQ10 showed some promise of efficacy. Given the small pool of potential ALS trial participants at any given time, and the anticipation that several candidate drugs for ALS will be available simultaneously for testing in clinical trials,28 we considered it important to evaluate CoQ10 for ALS in a Phase II trial with as few patients as possible, rather than committing immediately to a large Phase III effort.
Methods
SETTING AND PARTICIPANTS
Setting
QALS (the clinical trial of high dose Coenzyme Q10 in ALS) was conducted in 19 US centers. Clinical coordination was by the Eleanor and Lou Gehrig MDA/ALS Research Center of Columbia University, and statistical design, analysis, and data management by the Statistical Analysis Center (SAC) in the Department of Biostatistics at Columbia University. The trial was approved by the Institutional Review Boards (IRBs) of Columbia University and the participating clinical sites, and conducted under an IND (71,297). An independent Data and Safety Monitoring Board (DSMB) was appointed by the sponsor, the National Institute of Neurological Disorders and Stroke (NINDS). Participants, all of whom provided voluntary informed consent, were randomized between April, 2005 and May, 2007. The last participant completed the trial protocol in March 2008. The protocol was filed at www.clinicaltrials.gov (identifier NCT00243932).
Participants
Participants were aged 21 to 85 years with a clinical diagnosis of definite, probable, or laboratory-supported probable ALS, either sporadic or familial, by El Escorial guidelines29. Exclusion criteria were forced vital capacity (FVC) of less than 60%, severe medical illness, or disease onset more than 5 years before study entry.
Study Drug
Drug was dispensed as wafers containing 200mg or 300mg of CoQ10 without Vitamin E. Vitamin E may increase CoQ10 absorption30, but has itself been investigated for possible benefts in ALS.31, 32 Wafers identical in yellow color and maple flavor but without CoQ10 were provided for the placebo group. Subjects were given 3 chewable wafers TID (Vitaline Formulas, Green Bay, Wisconsin).
STUDY DESIGN AND STATISTICAL ANALYSIS
Primary Outcome Measure
The primary outcome in both stages was decline in the ALS Functional Rating Scale-revised (ALSFRSr) score33, 34 from baseline to 9 months. The ALSFRSr, a questionnaire-based scale assessing daily living function ranging from 48 (best score) to 0 (worst), was administered to the patient, or to a proxy if the patient could not communicate effectively. Decline was defined as ALSFRSr at baseline minus ALSFRSr at month 9. Thus a positive value indicates worsening.
Randomization
Participants were randomized in permuted blocks, stratified by clinical site and riluzole use.
Two-Stage Design
QALS utilized a multi-center, randomized, stratified, placebo-controlled, double-blind, two-stage, adaptive, bias-corrected, intent-to-treat (ITT) design. Stage 1 selected one of two doses CoQ10 (1,800 or 2,700 mg daily) based on an initial sample comparison. Stage 2 assessed whether the preferred dose shows sufficient promise to warrant Phase III testing. The trial. The trial design has been reported by us previously in detail.35
Stage 1: Dose Selection
The preferred dose (1,800 or 2,700 mg daily) was identified using a selection procedure wherein one prefers the dose group with the smaller mean 9-month ALSFRSr decline.36, 37 In a selection procedure, the goal is to select the dose with truly better efficacy, termed a “correct selection,” assuming one exists. The trial was designed so that the probability of a correct selection is pre-specified to be at least 80% if the true absolute difference in mean ALSFRSr decline over 9 months were 1.7 or more, assuming a standard deviation of 8.4. The difference of 1.7 points represents a 20% slowing in decline, which was deemed the minimum clinically worthwhile effect. This criterion requires 35 patients at each of the two doses. If the true difference were smaller than 1.7 units, the selection procedure would still more likely than not select the dose with truly better efficacy. Unlike a conventional hypothesis test procedure, no statements of statistical significance are contemplated in a selection procedure, so control of type I error is irrelevant. This allowed use of the relatively small sample size of n=35 per group.
Stage 2: Efficacy
Efficacy was assessed by comparing the dose preferred in Stage 1 against placebo in a one-sided non-superiority (“futility”) test.38-40 The primary null hypothesis was that CoQ10 reduces the mean ALSFRSr decline over 9 months by at least 20% compared to placebo—in short, that CoQ10 is “promising.” It was tested against the alternative that CoQ10 reduces the mean ALSFRSr decline by less than 20% over 9 months compared to placebo, at one-sided alpha = 0.10. The 20% criterion was deemed the minimum worthwhile improvement over placebo. Therefore, rejection of the null hypothesis would indicate that proceeding to Phase III is not sufficiently promising to warrant further investigation, and, in that sense, is futile. The design specified power of more than 80% to reject the null hypothesis if the true mean ALSFRSr decline in the preferred dose of CoQ10 were at least 10% higher than in the placebo group. For this, 75 patients in each group (preferred dose and placebo) were required.
In Stage 2 the preferred CoQ10 dose and placebo were compared on the change from baseline to month 9 for three secondary outcomes: Forced Vital Capacity (FVC); quality of life; and fatigue severity, by t-tests at two-sided alpha = 0.05. These outcomes were not used in the Stage 1 selection; they were analyzed in Stage 2 only.
Secondary Outcome Measures
As with the ALSFRSr, secondary outcomes were scored so that a positive value indicates worsening. FVC is analyzed as achieved percent of vital capacity predicted on the basis of age and height.41 Fatigue severity was measured by the 9-item Fatigue Severity Scale (FSS).42 Quality of life was recorded on the SF36™ Health Survey.43 Its physical (PCS) and mental (MCS) components were analyzed separately. Oxidative stress in plasma was assessed by 8-hydroxy-2 deoxyguanosine (8OH2dG).44 (The results for this measure are not currently available for analysis and will be reported separately.) The secondary outcomes were analyzed in a conventional hypothesis construct, rather than in the futility construct used for the primary outcome.
Visit Schedule
Participants had seven in-person visits scheduled at screening, baseline, month 1, 3, 5, 7, and 9, and a month 10 post-treatment phone call. The ALSFRS-R, FVC, and FSS data were collected at all in-person visits. The SF36™ Health Survey and plasma 80H2dG data were obtained at visits month 1,5, and 9. CoQ10 plasma levels were measured at visits month 1,5, and 9.
Safety and Compliance
Safety outcomes were (1) clinical Adverse Events (AEs) classified according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 3.045, augmented with specific events especially relevant to ALS; and (2) laboratory test data for complete blood count, basic chemistry panel, and liver function testing. Follow-up for safety outcomes continued through 10 months. Compliance was measured by pill counts and plasma levels of CoQ10.
STATISTICAL ANALYSIS
Treatment allocation and comparisons
Given the adaptive, placebo-controlled design, randomization of patients and analysis of their data did not always occur during the same stage.35 In Stage 1, 105 participants were randomized 1:1:1 to 3 groups (CoQ10 1,800 mg/day, CoQ10 2,700 mg/day, and placebo, 35 per group), although only the two active groups were compared in the dose selection procedure. The adaptive elements of the QALS design are that, (i) additional patients need not be randomized to the dose not selected as preferable and (ii) to minimize total recruitment, once the preferred dose is selected, data from the 35 patients randomized to that dose, and the 35 placebo controls, are carried forward from Stage 1 for inclusion in the stage 2 analysis.
In Stage 2, 80 additional patients were randomized 1:1 to 2 groups (the preferred dose, and placebo, 40 per group). The additional patients were combined with the patients carried forward from Stage 1 to provide the 75 cases per group required for the Stage 2 analysis.
Statistical Tests and Bias Correction
Stage 1 data were analyzed using a statistical selection procedure with high probability to correctly select the truly superior dose. 35 The Stage 2 futility test was a bias-adjusted t-test. adjusted to allow for a selection bias resulting from the inclusion of cases from Stage 1 based on their apparently better outcomes.35
Scoring for deceased patients
Although the ALSFRSr directly scores only living patients, valid assessment of interventions for ALS requires a score for patients who die during follow-up. Omitting deceased patients would grossly violate intention-to-treat, and cause bias if death differs between groups.46 For the primary analysis, we assigned the lowest score of zero at 9 months, given that death is obviously a poor outcome, and there is no consensus on an alternative score. To assess the impact of assigning a less extreme score than zero for deaths on the primary outcome, we used an alternative scoring procedure in a pre-specified analysis, we assigned deceased patients the 10th percentile score from the distribution of 9-month ALSFRSr scores among patients who survived through 9 months. We used a similar procedure to code deceased participants on the secondary outcomes. Using the distribution of the 9-month value for all 3 treatment groups combined, we assigned the 10th percentile as the 9-month value for deceased patients. (For FSS, we used the 90th percentile, given that the higher the FSS score, the worse the outcome.) The change over 9 months for each deceased patient was calculated using the patient’s baseline value and this 9-month value.
Imputation for missing data
Given the ITT design, an imputation or some other procedure for handling missing primary endpoints was required. Imputation procedures and key sensitivity analyses were pre-specified by the investigators and approved by the DSMB.
We used a “nearest neighbor” procedure to impute ALSFRSr decline over 9- months for participants who were lost to follow-up (LTF). For each such participant, we identified the five other participants within the same treatment group whose baseline ALSFRSr scores were nearest to the baseline score of the LTF patient. The largest 9-month ALSFRSr decline observed among these five neighbors was imputed for the missing participant (worst-case imputation). The smallest observed 9-month ALSFRSr decline among the same five neighbors was used in a sensitivity analysis (best-case imputation).
Secondary Analysis
We performed, post hoc, a slope analysis using a mixed effects model with random intercept and random slope following the method employed in previous clinical trials for ALS.47 The dependent variable was the ALSFRS-R score at baseline and visits 1, 3, 5, 7, and 9. Time was treated as a continuous variable and took the values 0, 1, 3, 5, 7, and 9. Deaths were omitted from the slope analysis, as usual in the ALS literature. There were only three cases which were lost to follow-up (2%), and their data were included in the analysis up to the time of their loss to follow-up. For data sporadically missing during the course of follow-up, we assumed they were missing at random. This assumption is tenable given that subsequent data were available for such patients.
Quality Control
The quality of trial operations was enhanced by a comprehensive web-based data management system developed specifically for QALS.dss In-person training for ALSFRSr evaluators before trial start, and video-based training throughout its course, contributed to excellent ALSFRSr reliability, both in person (0.93 for inter- and 0.95 for intra-rater reliability), and in telephone administration for patients unable to attend in-person visits (0.97 reliability).48 A staff member from the Clinical Coordinating Center (CCC) monitored randomly selected charts in person at 15of the 19 sites.
RESULTS
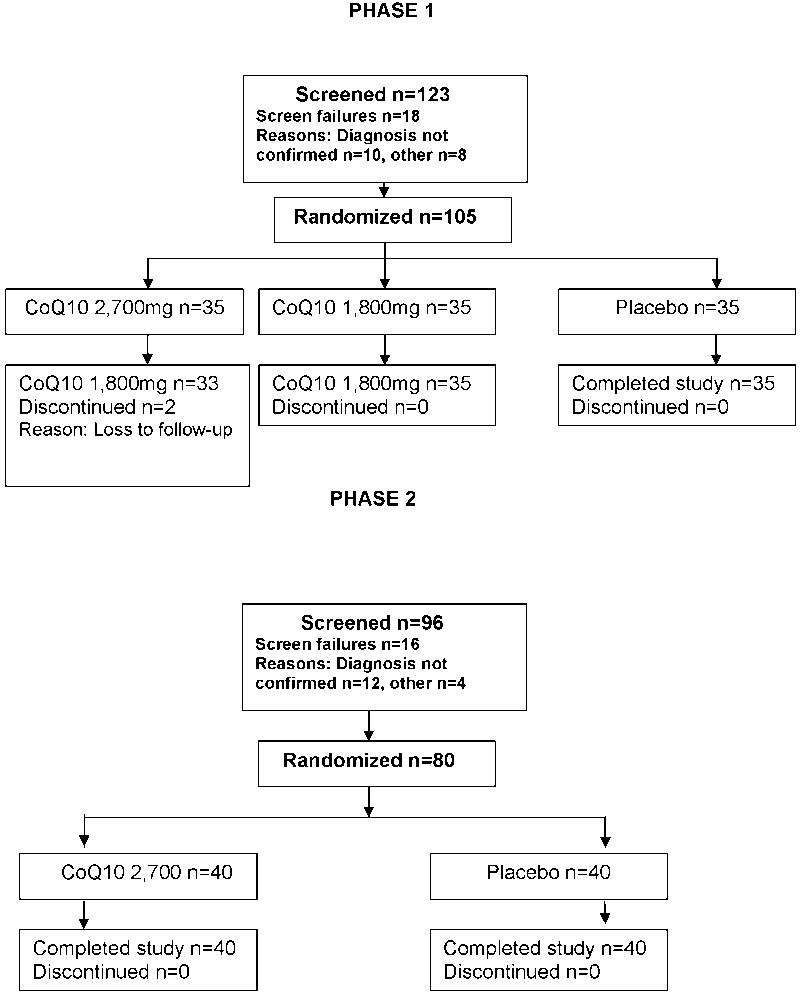
Figure 1 shows the progress of participants through the trial. No participant discontinued follow-up because of adverse events, withdrawal of consent, major protocol violations, or noncompliance. Two participants were lost to follow-up, both in the CoQ10 2,700 mg/day group.
Figure 1.
Participant Flow Diagram
Stage 1: Dose Selection
The 105 Stage 1 patients were randomized between April, 2005 and February, 2006. Follow-up through 9 months ended in November, 2006. The baseline characteristics by treatment group are shown in Supplementary Table 1. Patients in both groups were similar, but in the 1,800mg dose group were more likely to be male (71% vs. 43%). In the selection procedure, the mean ± SD ALSFRSr decline over 9 months was lower for the 2,700 mg/day dose (9.0 ± 8.2) than for 1,800 mg/day (10.9 ± 9.3). This result did not change with best-case rather than worst-case nearest-neighbor imputation for the 2 missing month 9 outcomes. Under the best-case scenario, the decline was unchanged for the 1,800 mg/day group, and 8.4 ± 8.4 for the 2,700 mg/day group. There were no safety concerns (Supplementary Table 2). CoQ10 plasma levels in the placebo group differed significantly from those in both active dose groups at all follow-up visits, but the levels in the two active groups themselves did not differ consistently. The mean CoQ10 level in the placebo group did not change significantly over time, suggesting that there was little un-assigned CoQ10 use (“drop-in”) (Supplementary Table 3 and Figure 2).
Figure 2.
Graphs with box plots of change in plasma CoQ10 concentrations at months 1,5, and 9 by treatment group for Stages 1 and 2 combined
In December 2006 the Clinical Principal Investigator was given results by treatment group without identifying which group was which. The results were prepared by the study statistician and shared with the DSMB before they were provided to the clinical principal investigator. The investigators proposed, and the DSMB and NINDS accepted after data review, that in the absence of safety concerns, the group with the lower ALSFRSr decline should be selected, per the Stage 1 design.
Stage 2: Efficacy
The 35 participants who had received the 2,700 mg/day dose in Stage 1, and the 35 concurrent placebo controls, were carried forward for inclusion in the bias-corrected Stage 2 futility analysis. The 80 additional patients needed for stage 2 (40 at CoQ10 2,700 mg/day, and 40 placebo controls) were randomized between February and May, 2007. Follow-up for these patients through 9 months ended in February, 2008. The two groups were similar with regards to demographic and clinical characteristics at baseline (Table 2).
Table 2.
Stage 2 Baseline Characteristics by treatment group
| CoQ10 2,700mg n=75 | Placebo n=75 | |
|---|---|---|
| Mean age in years (SD) | 56.5 (10.8) | 57.4 (11.0) |
| Gender, n (%) | ||
| Male | 40 (53.3%) | 46 (61.3%) |
| Female | 35 (46.7%) | 29 (38.7%) |
| Race, n (%) | ||
| White | 71 (94.7%) | 71 (94.7%) |
| Black | 3 (4.0%) | 1 (1.3%) |
| Asian | 1 (1.3%) | 1 (1.3%) |
| More than one | 0 (0.0%) | 1 (1.3%) |
| Other | 0 (0.0%) | 1 (1.3%) |
| Ethnicity, n (%) | ||
| Not Hispanic/Latino | 73 (97.3%) | 72 (96.0%) |
| Hispanic/Latino | 2 (2.7%) | 3 (4.0%) |
| Mean Symptom duration in years (SD) | 2.3 (1.1) | 2.0 (1.3) |
| Site of onset, n (%) | ||
| Lower extremity | 30 (40.0%) | 34 (45.3%) |
| Upper extremity | 34 (45.3%) | 28 (37.3%) |
| Head | 11 (14.7%) | 13 (17.3%) |
| Mean ALSFRS-R score (SD) | 35.3 (5.5) | 35.6 (5.0) |
| Mean FVC, % of predicted (SD) | 87.7 (23.6) | 89.2 (18.2) |
| Mean BMI‡ (SD) | 26.8 (7.0) | 26.2 (4.6) |
| Riluzole use, n (%) | 57 (76.0%) | 50 (66.7%) |
| Mean CoQ10 level (SD) | 1.0 (0.44) | 0.90 (0.35) |
Total n=149
SD=Standard Deviation
Futility analysis
The mean 9-month ALSFRSr decline in the 2,700 mg/day CoQ10 group (8.4 ± 7.3) was lower than for placebo (9.4 ± 8.8). The null hypothesis that CoQ10 is at least 20% superior to placebo was not rejected (Table 3), so that proceeding to Phase III was not immediately ruled out. This result was unaffected by best-case rather than worst-case imputation for the two LTF participants. However, when the 6 patients who died before the end of 9-month follow-up (1 active, 5 placebo) were assigned an ALSFRSr score of 16, the 10th percentile score pre-specified for the sensitivity analysis (see Methods), the mean ALSFRSr declines in the 2,700 mg/day CoQ10 and placebo groups were similar (8.2 ± 6.5 vs. 8.4 ± 6.6). Using these data, the null hypothesis that CoQ10 is at least 20% superior to placebo was rejected, suggesting that proceeding to Phase III would be futile (Table 3).
Table 3.
Stage 2 Efficacy Results
| Primary Outcome: Mean 9-month ALSFRSr decline (SD) by treatment group | |||||
|---|---|---|---|---|---|
| CoQ10 2,700mg* n=75 | Placebo n=75 | Delta (CI)** | t statistic | p-value | |
| Primary analysis1 | 8.80 (7.34) | 9.44 (8.82) | -1.25 (< 0.22) | -1.09 | 0.14 |
| Sensitivity 1 – LTF2 | 8.52 (7.41) | 9.44 (8.82) | -0.97(< 0.51) | -0.84 | 0.20 |
| Sensitivity 2 – Death3 | 8.52 (6.54) | 8.37 (6.56) | -1.82 (< -0.63) | -1.97 | 0.025 |
| Secondary Outcomes: Mean 9-month Decline (SD) by treatment group | |||||
| Forced Vital Capacity (% of predicted) | 0.20 (0.15) | 0.17 (0.18) | -0.03 (-0.08, 0.02) | -1.12 | 0.27 |
| SF-36 – PCS (physical) | 4.22 (8.02) | 6.04 (6.85)*** | 1.83 (-0.60, 4.26) | 1.50 | 0.14 |
| SF-36 – MCS (mental) | 2.87 (11.78) | 5.01 (11.69) | 2.14 (-1.67, 5.95) | 1.11 | 0.27 |
| Fatigue Severity | 0.71 (1.21) | 0.88 (1.51) | 0.17 (-0.27, 0.61) | 0.76 | 0.45 |
Means (SD) include abias correction.
Delta (CI) is consistent with the respective hypothesis tests for the primary and secondary outcomes. For the primary outcome, delta = (0.8 × placebo group mean decline in 9-month ALSFRSr) -CoQ10 group mean decline in 9-month ALSFRSr. For the secondary outcomes delta is the simple difference between placebo and CoQ10 group mean declines. Confidence intervals (CI) are one-sided 90% CIs for the primary outcome and two-sided 95% CIs for the secondary outcomes. For the primary outcome, a 90% upper confidence bound for delta that is negative (less than zero) indicates that the data are not consistent with the hypothesis that CoQ10 is associated with a ≥20% slowing in mean decline on the ALSFRSr. For the primary outcome, the true percent reduction in mean ALSFRSr is related to the true delta via the formula true % reduction = 100% × (0.2 + true delta / true placebo group mean). Given a fixed value of the true placebo group mean such as 9.0 (one point per month) or 9.4 (as observed), the conditional 90% upper confidence limit for the true percent reduction is 100% × (0.2 + upper confidence limit for true delta / assumed true placebo group mean).
n=73
Worst-scenario imputation for the two CoQ10 cases lost to follow-up.
Best-scenarioimputation for the two CoQ10 cases lost to follow-up.
The 6 deceased patients (1 CoQ10, 5 placebo) are scored 16 instead of 0 on the 9-month ALSFRSr. Worst-scenario imputation for the two CoQ10 cases lost to follow-up.
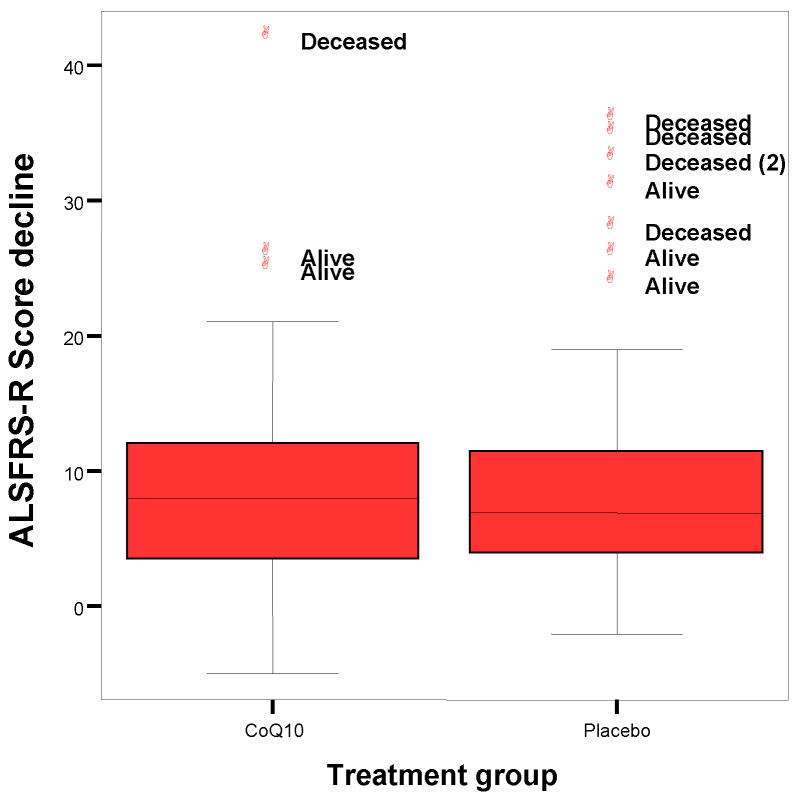
Further analysis (not pre-specified) shows that the lower mean ALSFRSr decline for CoQ10 in the primary analysis is driven almost entirely by outlying values (large declines) for the 5 deceased placebo patients. The median decline, which is insensitive to outliers, is slightly greater for CoQ10 than for placebo (Figure 3).
Figure 3.
Stage 2: Distribution of ALSFRSr score declines over 9 months, by treatment group
We also computed p-values for the primary analysis using 17 different 9-month ALSFRSr scores for deceased patients, starting with zero, as used in the primary analysis, and extending in one-unit increments to 16, as used in the sensitivity analysis (see Figure 4). In four of the tests (for the lowest scores, 0 through 3), p exceeds 0.10, and the futility boundary is not crossed; but for most of them (the other 13 scores, 4 through 16), p is less than 0.10, implying futility, and no progression to Phase III.
Figure 4.
Stage 2: p-values for futility analysis when deceased patients are scored from 0 to 16 in the 9-month ALSFRSr
Although the result of the primary analysis is sensitive to the scoring of deceased patients on the ALSFRSr, the difference in deaths between the two groups in the primary analysis (1 on CoQ10 2,700 mg/day, and 5 on placebo) is not statistically significant. When the two participants who died during month 10 post-treatment follow-up for safety are included in the analysis (both in the active group), the mortality difference between the groups is further removed from significance.
The post hoc slope analysis comparing the placebo and 2700mg groups was significant at the one-sided 0.10 significance level (p=0.097), consistent with futility.
Secondary Outcomes
There were no significant differences between the CoQ10 and placebo groups with regard to the available secondary outcomes (Table 3). The mean decline in physical and mental quality of life scores was less in the CoQ10 2,700 mg/day, but the comparison to the placebo group did not reach statistical significance. Fatigue severity worsened to a lesser degree in the CoQ10 2,700 mg/day group, but the difference was not significant.
Safety
AEs are summarized in Table 4. Forty-two serious adverse events (SAEs) occurred, none of which was judged to be related to the study drug. Almost al of the 158 non-serious AEs occurred were in CTCAE grades 1-2. The most common AE categories were gastrointestinal and respiratory events, followed by falls. There were no significant differences in AEs between the placebo and CoQ10 groups.
Table 4.
Stage 2 Adverse Events by treatment group*
| CoQ10 2,700mg n=75 | Placebo n=75 | |
|---|---|---|
| Patients with any AE | 63 (84.0%) | 64 (85.3%) |
| Fall | 28 (37.3%) | 17 (22.7%) |
| Pain | 22 (29.3%) | 19 (25.3%) |
| Nausea | 13 (17.3%) | 16 (21.3%) |
| Constipation | 18 (24.0%) | 10 (13.3%) |
| Diarrhea | 8 (10.7%) | 18 (24.0%) |
| Mood alteration | 16 (21.3%) | 7 (9.3%) |
| PEG placement | 8 (10.7%) | 10 (13.3%) |
| BIPAP/NIPPV | 9 (12.0%) | 6 (8.0%) |
| Edema: limb | 8 (10.7%) | 5 (6.7%) |
| Patients with dental AEs | 4 (5.3%) | 2 (2.7%) |
| Patients with AEs possibly related to treatment | 32 (42.7%) | 31 (41.3%) |
| Patients with any serious AE | 18 (24.0%) | 19 (25.3%) |
| Death | 3 (4.0%) | 5 (6.7%) |
Events reported in more than 10% of patients in a single arm. AE follow-up is through 10 months.
Compliance
By wafer count, the proportion of participants who met the trial definition of compliance (consumed >80% of prescribed medication) was 73% in the 2,700mg/day CoQ10 group, 71% in the placebo group. The proportion of participants who were severely noncompliant (defined as having consumed less than 10% of prescribed medication) was 5% in the 2,700 mg/day CoQ10 group and 3% in the placebo group.
CoQ10 plasma levels increased significantly after the baseline visit for the two active CoQ10 groups, but not for the placebo group (suggesting little drop-in). There were statistically significant differences between each CoQ10 group and placebo at months 1, 5, and 9, but not between the 2 doses of CoQ10 (Table 1 and Supplementary Table 3). Figure 2 shows that, compared to baseline, the changes in plasma levels for the 2,700 mg/day group were somewhat greater at months 5 and 9 than for the 1,800 mg/day group, as expected, but smaller at month 1, contrary to expectation.
Table 1.
CoQ10 Plasma Levels: Mean mmol/l (SD) by treatment group
| Visit | CoQ10 1,800mg n=35 | CoQ10 2,700mg n=73 | Placebo n=74 |
|---|---|---|---|
| Baseline | 0.97 (0.37) | 1.0 (0.44) | 0.90 (0.35) |
| Month 1 | 8.73 (7.74) | 7.98 (5.07) | 1.03 (0.81) |
| Month 5 | 6.53 (5.12) | 7.47 (4.86) | 1.01 (0.51) |
| Month 9 | 5.36 (5.02) | 6.87 (5.20) | 0.90 (0.32) |
Discussion
QALS is an innovative, two-stage, randomized, double-blind, placebo-controlled, adaptive Phase II trial designed to minimize required sample size and length of follow-up for the individual patient. Features presented in detail elsewhere include the statistical design itself35; and the demonstration that the ALSFRSr is associated with mortality in ALS patients49, a relationship that had previously been reported.33, 34 Since mortality as primary outcome has been successfully used in an ALS trial and may be considered a “gold standard” for Phase III trials we considered it important to use a functional outcome in Phase II that is associated with mortality.50
Our results provide insufficient evidence to justify a Phase III trial of high dose CoQ10 for treatment of ALS. In Stage 1 we selected the dose of 2,700 mg/day as preferable to 1,800 mg/day, given its lower mean decline in 9-month efficacy and lack of safety problems. In Stage 2 we initially encountered somewhat conflicting results on futility. Although we did not reject the primary null hypothesis that CoQ10 is at least 20% superior to placebo, a pre-specified sensitivity test, and additional analyses, did reject this hypothesis, and provided substantial evidence of futility. In addition, pre-specified secondary analyses showed no differences between CoQ10 and placebo. Our interpretation is that if a phase III trial were contemplated with the same scoring of 0 for deceased patients as the primary endpoint in this study, it might not be futile to proceed. However, because of the sensitivity of the results to the choice of scoring for deaths, the wisdom of such a trial could be questioned. In fact, these data as a whole leave us confident in our conclusion that while CoQ10 at 2,700 mg/day is safe and tolerable for ALS, it does not merit Phase III testing. The post hoc slope analysis is consistent with our conclusion declaring futility.
To avoid bias, a score for death must be used in assessment of interventions for ALS that use functional rating scales for living patients. There is no single best or correct score that can be specified a priori. The approach which we employed may merit further use and development, and allow accumulation of a useful body of evidence and experience. A clinically reasonable upper and lower boundary is pre-specified. We used the lowest possible score of zero as the lower bound and the 10th percentile among 9-month survivors as the upper bound—other choices are possible. We studied how these, and all intermediate scores, affected the result, and found this informative.
While we are disappointed by the performance of COQ10 with regards to efficacy, we found its safety profile acceptable with the formulation used and a dose of 2,700mg/day. In particular gastrointestinal side effects were less common than we had anticipated.
The feasibility of our two-stage, adaptive Phase II design for ALS has been demonstrated. QALS includes three innovative features (use of a selection procedure rather than a significance test to identify a preferred dose; use of a one-sided futility test with the major benefit of concurrent placebo controls; and use of the data from cases recruited during Stage 1 in a bias-adjusted analysis after stage 2). As a result, it required only 185 participants to both select a preferred dose and establish that Phase III testing would not be promising. A more conventional approach would have required almost five times as many participants.35 Adaptive Phase II trials can take many forms51. They are not appropriate in all situations, but they can make a strategically important contribution to ALS and other clinical research. We call for their further development and application in order to accelerate the path to effective treatments.
Supplementary Material
Acknowledgments
We thank Robin A Conwit and Janice Cordell of National Institute of Neurological Disorders and Stroke (NINDS) for their support. We thank the members of the DSMB for their guidance and the patients and caregivers for their generous gift of time and effort.
This trial was funded by federal grants NINDS R01 NS48555 (J.L.P.T.) and NINDS R01 NS 48125 (P.K.), K12RR017648 (PK), and additional clinical research support was provided through CTSA Award NIH 1 UL1 RR024156 (Columbia University); MO1-RR01066 (Harvard University); MO1_RR_01346 (University of Texas in San Antonio); M01 RR023940 (University of Kansas); UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (Washington University).
Footnotes
Conflict of Interest Statement: PK, JLPT, GL, RB, BL have no conflict of interest. HM received honoraria for Advisory Board participation from Avanir, Eisai, Knopp, Neuralstem and Otsuka, and also received grants for clinical trials from Avanir and Knopp. EPP received honoraria for Advisory Board participation from Avanir.
The QALS Study Group
Steering committee
P. Kaufmann, MD, MSc, J.L.P. Thompson, PhD, H. Mitsumoto, MD, S. DiMauro, MD, L.P. Rowland, MD, P.H. Gordon, MD.
Clinical Coordinating Center
Columbia University Medical Center, NY, NY: P. Kaufmann, MD, MSc, A.I. Barsdorf, MA, J. Montes MA, PT, D. Vecchio, MS, K. Bednarz, MS, RD, G. Harrington, RN, BSN, J. Marra, BS, V. Battista, RN, BS, P.H. Gordon, MD, J. Andrews, MD,H. Mitsumoto, MD.
Data Coordinating Center
Columbia University Statistical Analysis Center (SAC), NY, NY: J.L.P. Thompson, PhD, G. Levy, MD, MS, B. Levin, PhD, A. Tierney, MPH, R. Arbing, MSc, MPH, R. Buchsbaum.
Participating clinicians—
J. Taft, MPAS, PA-C, M.L. Watson, RRT, M. Grosso, PT, DPT, RPA-C (State University of New York Upstate Medical, Syracuse, NY);
M. E. Cudkowicz, MD, MSc, A. B. DiBernardo MD,B. Traynor, MD, K. Kruczek, A. Caraganis, J. Eckenrode, P. Butsch, D. Pulley (Harvard University, Boston, MA);
R. G. Miller, MD, D. Forshew RN, BSN, D. Scholtz, MD, S. Champion, S. McDade, PT (California Pacific Medical Center, San Francisco, CA);
H. Neville, MD, S. P. Ringel, MD, B. Oskarsson, MD, B. Hammack, PhD, T. Gamage, PT, B. Hewitt, J. Cumming (University of Colorado, Denver, CO);
A.R. Dick, MD, A. McVey, MD, L. Herbelin, M. Walsh (University of Kansas, Kansas City, KS);
P.P. Kittrell, MSN, RN, D.A. Myers, PT (University of Texas Health Science Center);
W. S. David, MD, PhD, C. Heuer, RN, BSN, S. Conn, RN, and S. Swanson, PT (HFA ALSA-Certified ALS Center/Berman Center for Outcomes and Clinical Research, Minneapolis, MN);
D. Forshew, RN, BSN, L. Squire, C. Villierme (University of California at San Francisco, San Francisco, CA);
Elaine Reich, RN (Baystate Medical Center, Springfield, MA);
K. Moxley, BSN, RN, M.C. Jones, MAMC, C. A. Potter, J Gardner, D. Cutura, (University of Vermont, Burlington, VT);
D. Fewell, LPN (University of Arkansas, Little Rock, AR);
R.P. Roos, MD, B. Soliven, MD, E. Shaviers (University of Chicago, Chicago, IL);
P. Casey, MS, S. Manes, DPT (Northwestern University, Chicago, IL);
B. Abrams, RN, J.M. Florence, DPT, J R. Schierbecker, MHS, PT, C. Wulf, B.C. Malkus, PT, MHS (Washington University, St. Louis, MO);
J.M. Goldstein, MD, L. Marshall, A. Toenjes, RN, MSN, APRN (Yale University School of Medicine, New Haven, CT);
Terry Heiman-Patterson, MD, S Feldman, PT, C.Barr, RN (Drexel University-Hahnemann Campus, Philadelphia, PA);
C. Shrestha, MPH, K. Vanderpool, RN, BS, D. G, Taylor, S. Wells. R. Gleason, PT, MS, R.A. English, PT (University of Kentucky, Lexington, KY);
K. Kelly, MSN, CNS, R. Kolb (Cleveland Clinic, Cleveland, OH)
Data and safety monitoring board
R Holloway, Jr. (Chair); C Clark, C Coffey, L Gutmann, T R Holford,
NIH program management
R Conwit, J Cordell, G Wilkom
Safety monitor
D Chad (back-up: S. Scelsa)
References
- 1.Annegers JF, Appel S, Lee JR, Perkins P. Incidence and prevalence of amyotrophic lateral sclerosis in Harris County, Texas, 1985-1988. Arch Neurol. 1991;48:589–593. doi: 10.1001/archneur.1991.00530180041015. [DOI] [PubMed] [Google Scholar]
- 2.Traynor BJ, Codd MB, Corr B, et al. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology. 1999;52:504–509. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 3.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59:280–282. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 4.Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. 1993;118:48–55. doi: 10.1016/0022-510x(93)90245-t. [DOI] [PubMed] [Google Scholar]
- 5.Traynor BJ, Alexander M, Corr B, et al. An outcome study of riluzole in amyotrophic lateral sclerosis--a population-based study in Ireland, 1996-2000. J Neurol. 2003;250:473–479. doi: 10.1007/s00415-003-1026-z. [DOI] [PubMed] [Google Scholar]
- 6.Valdmanis PN, Rouleau GA. Genetics of familial amyotrophic lateral sclerosis. Neurology. 2008;70:144–152. doi: 10.1212/01.wnl.0000296811.19811.db. [DOI] [PubMed] [Google Scholar]
- 7.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 8.Perluigi M, Fai Poon H, Hensley K, et al. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;38:960–968. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Ohtsuka C, Terayama Y. Increased mitochondrial oxidative damage in patients with sporadic amyotrophic lateral sclerosis. J Neurol Sci. 2008;267:66–69. doi: 10.1016/j.jns.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Murata T, Ohtsuka C, Terayama Y. Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radic Res. 2008;42:221–225. doi: 10.1080/10715760701877262. [DOI] [PubMed] [Google Scholar]
- 11.Mitsumoto H, Santella RM, Liu X, et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler. 2008;9:177–183. doi: 10.1080/17482960801933942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson EP, Henry YK, Henkel JS, et al. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- 13.Sohmiya M, Tanaka M, Suzuki Y, et al. An increase of oxidized coenzyme Q-10 occurs in the plasma of sporadic ALS patients. J Neurol Sci. 2005;228:49–53. doi: 10.1016/j.jns.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Cookson MR, Menzies FM, Manning P, et al. Cu/Zn superoxide dismutase (SOD1) mutations associated with familial amyotrophic lateral sclerosis (ALS) affect cellular free radical release in the presence of oxidative stress. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:75–85. doi: 10.1080/146608202760196048. [DOI] [PubMed] [Google Scholar]
- 15.Jung C, Higgins CM, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002;83:535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 16.Wiedemann FR, Manfredi G, Mawrin C, et al. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 17.Lenaz G, Fato R, Formiggini G, Genova ML. The role of Coenzyme Q in mitochondrial electron transport. Mitochondrion. 2007;7(Suppl):S8–33. doi: 10.1016/j.mito.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 19.Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res. 2002;36:455–460. doi: 10.1080/10715760290021315. [DOI] [PubMed] [Google Scholar]
- 20.Lass A, Sohal RS. Effect of coenzyme Q(10) and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. Faseb J. 2000;14:87–94. doi: 10.1096/fasebj.14.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Matthews RT, Yang L, Browne S, et al. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 23.Shults CW, Haas R. Clinical trials of coenzyme Q10 in neurological disorders. Biofactors. 2005;25:117–126. doi: 10.1002/biof.5520250113. [DOI] [PubMed] [Google Scholar]
- 24.Beal MF, Shults CW. Effects of Coenzyme Q10 in Huntington’s disease and early Parkinson’s disease. Biofactors. 2003;18:153–161. doi: 10.1002/biof.5520180218. [DOI] [PubMed] [Google Scholar]
- 25.Ferrante KL, Shefner J, Zhang H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld J, Ellis A. Nutrition and dietary supplements in motor neuron disease. Phys Med Rehabil Clin N Am. 2008;19:573–589. doi: 10.1016/j.pmr.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shults CW, Flint Beal M, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp Neurol. 2004;188:491–494. doi: 10.1016/j.expneurol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Heemskerk J. High throughput drug screening. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(Suppl 1):19–21. doi: 10.1080/17434470410019735. [DOI] [PubMed] [Google Scholar]
- 29.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim WH, Bhagavan HN, Chopra RK, Chow CK. Dietary coenzyme Q10 and vitamin E alter the status of these compounds in rat tissues and mitochondria. J Nutr. 2000;130:2343–2348. doi: 10.1093/jn/130.9.2343. [DOI] [PubMed] [Google Scholar]
- 31.Ascherio A, Weisskopf MG, O’Reilly EJ, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol. 2005;57:104–110. doi: 10.1002/ana.20316. [DOI] [PubMed] [Google Scholar]
- 32.Graf M, Ecker D, Horowski R, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm. 2005;112:649–660. doi: 10.1007/s00702-004-0220-1. [DOI] [PubMed] [Google Scholar]
- 33.Cedarbaum JM, Stambler N. Performance of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152(Suppl 1):S1–9. doi: 10.1016/s0022-510x(97)00237-2. [DOI] [PubMed] [Google Scholar]
- 34.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 35.Levy G, Kaufmann P, Buchsbaum R, et al. A two-stage design for a phase II clinical trial of coenzyme Q10 in ALS. Neurology. 2006;66:660–663. doi: 10.1212/01.wnl.0000201182.60750.66. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons JD, Olkin I, Sobel M. Selecting and Ordering Populations: A New Statistical Methodology. New York: Wiley&Sons; 1977. [Google Scholar]
- 37.Bechofer RE, Santner TJ, Goldsman DM. Design and Analysis of Experiments for Statistical Selection, Screening, and Multiple Comparisons. New YOrk: John Wiley&Sons, Inc; 1995. [Google Scholar]
- 38.Levin B. The utility of futility. Stroke. 2005;36:2331–2332. doi: 10.1161/01.STR.0000185722.99167.56. [DOI] [PubMed] [Google Scholar]
- 39.Palesch YY, Tilley BC, Sackett DL, et al. Applying a phase II futility study design to therapeutic stroke trials. Stroke. 2005;36:2410–2414. doi: 10.1161/01.STR.0000185718.26377.07. [DOI] [PubMed] [Google Scholar]
- 40.Elm JJ, Goetz CG, Ravina B, et al. A responsive outcome for Parkinson’s disease neuroprotection futility studies. Ann Neurol. 2005;57:197–203. doi: 10.1002/ana.20361. [DOI] [PubMed] [Google Scholar]
- 41.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 42.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 44.Bogdanov MB, Beal MF, McCabe DR, et al. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2’-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radic Biol Med. 1999;27:647–666. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 45.CTCAE. http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 46.Thompson JL, Levy G. ALS issues in clinical trials. Missing data. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(Suppl 1):48–51. doi: 10.1080/17434470410019753. [DOI] [PubMed] [Google Scholar]
- 47.Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003;61:456–464. doi: 10.1212/wnl.61.4.456. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann P, Levy G, Montes J, et al. Excellent inter-rater, intra-rater, and telephone-administered reliability of the ALSFRS-R in a multicenter clinical trial. Amyotroph Lateral Scler. 2007;8:42–46. doi: 10.1080/17482960600888156. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann P, Levy G, Thompson JL, et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 50.Lacomblez L, Bensimon G, Leigh PN, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 51.Coffey CS, Kairalla JA. Adaptive clinical trials: progress and challenges. Drugs R D. 2008;9:229–242. doi: 10.2165/00126839-200809040-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.