Summary
p63 is a transcription factor that is required for normal epidermal development and differentiation. Due to the complexity of these processes, p63 is expected to regulate a myriad of target genes. This has provided the impetus to many laboratories to identify these genes. To date, p63 target genes have been shown to encode a diverse group of proteins including structural proteins, proteins that control cell cycle withdrawal, and proteins that regulate the epidermal differentiation program. In this issue Antonini et al describe a novel p63 target gene, whose evolutionary conservation suggests a critical role for this gene in the epidermis.
Epidermal morphogenesis initiates during embryonic development when cells of the single-layered surface ectoderm, which initially covers the developing embryo, adopt an epidermal fate and differentiate into actively proliferating basal keratinocytes (Reviewed in Koster and Roop, 2007). The first morphological sign of stratification occurs when these basal keratinocytes give rise to a suprabasal cell layer. Cells in this so-called intermediate layer undergo a few rounds of cell division before they permanently withdraw from the cell cycle and become spinous keratinocytes. During subsequent stages of epidermal morphogenesis, spinous keratinocytes differentiate into cells of the granular and cornified cell layers, the latter of which is primarily responsible for the barrier function of the epidermis. The endpoint of the terminal keratinocyte differentiation program is the shedding of the dead and enucleated keratinocytes of the cornified cell layers into the environment. The embryonic differentiation program described above is maintained, with few modifications, during postnatal development. This program is required to continuously replace cells that are lost and to maintain epidermal tissue architecture. One striking difference between the terminal differentiation programs executed during embryogenesis and postnatally, is the mechanism by which the spinous layer is formed. Whereas this layer develops from intermediate keratinocytes during embryonic skin development, basal keratinocytes directly differentiate into spinous keratinocytes in postnatal epidermis. In addition to generating the epidermis, basal keratinocytes also produce and secrete crucial components of the basement membrane, the dermal-epidermal interface which anchors epidermal keratinocytes to the dermis. Finally, basal keratinocytes participate in epithelial-mesenchymal interactions that are required for the formation of epithelial appendages, including teeth, hair follicles, mammary glands, and limbs.
A key role for the transcription factor p63 in the above-described processes was first inferred from the phenotype of p63 deficient mice. These mice do not develop stratified epithelia or epithelial appendages (Yang et al., 1999; Mills et al., 1999). Instead of an epidermis, these mice are covered by a single layered epithelium that resembles the surface ectoderm which covers the early embryo, suggesting that loss of p63 leads to a block of keratinocyte commitment and differentiation, and thus aborted skin development. As a consequence, p63 deficient mice die shortly after birth due to uncontrolled water loss caused by a lack of skin barrier function.
The p63 gene encodes six different proteins, each of which can function as a transcriptional activator or transcriptional repressor. Since p63-deficient mice lack expression of all six proteins, it is not possible to determine which isoform(s) is/are required for normal skin and appendage development using this animal model. In late embryonic and postnatal epidermis, ΔNp63α is the predominantly expressed p63 isoform, whereas expression of the other isoforms is barely detectable (Yang et al., 1998). To determine the contribution of ΔNp63α to epidermal development and differentiation, two different groups downregulated ΔNp63 expression in the epidermis using different approaches. We generated transgenic mice which express a hairpin sequence (shRNA) consisting of sequences unique to the ΔNp63 isoforms using a epidermalspecific inducible promoter. The hairpin was designed to be processed into short interfering RNA (siRNA) sequences which subsequently degrade endogenous ΔNp63 transcripts (Koster et al., 2007). Truong and colleagues took an in vitro approach by downregulating ΔNp63 expression in human primary keratinocytes using an siRNA specific for ΔNp63. These keratinocytes were then placed into organotypic cultures to allow them to regenerate epidermis (Truong et al., 2006). In both the transgenic mouse epidermis and the human skin equivalents, the spinous layer failed to develop properly as demonstrated by a delay in the expression of a marker of the spinous layer, keratin 1 (K1). As a consequence, subsequent stages of terminal differentiation failed to proceed normally. The data summarized above clearly highlight the importance of ΔNp63α in epidermal differentiation.
How does ΔNp63α regulate epidermal development and differentiation? One approach to obtain insight into the p63 regulatory network is to identify genes whose expression is controlled by p63. Although several groups have performed microarray or ChIP-on-chip experiments to address this question, the use of cell lines rather than primary cultures or tissue extracts to perform these high-throughput experiments has limited their biological significance. Nevertheless, several downstream effectors of ΔNp63α have been identified.
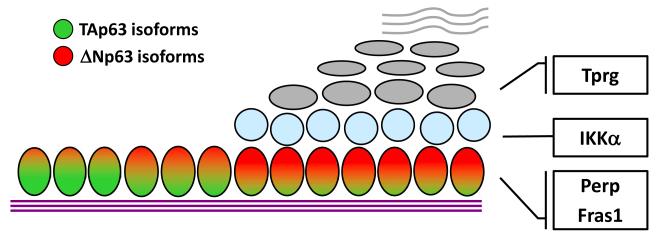
Most recently, Antonini and colleagues identified a novel target gene of ΔNp63, which they have named Tprg (Transformation related protein 63 regulated; Antonini et al., 2008; Figure 1). Unlike ΔNp63, Tprg is predominantly expressed in the suprabasal layers of the epidermis. Although the role of Tprg in epidermal development and differentiation remains unknown, the expression pattern of Tprg suggests a role for this protein in epidermal differentiation. In further support of this hypothesis, Tprg transcripts are first detected at the developmental stage when terminal differentiation of the epidermis initiates. Although a role for Tprg in epidermal terminal differentiation appears plausible, neither the structure nor the subcellular localization of Tprg provide any clear hints into its precise function. The extensive variety of processes that need to occur during terminal differentiation, leave the possibilities open. For example, Tprg may be involved in cell cycle withdrawal, the regulation of structural proteins, and/or barrier formation. Finally, the evolutionary conservation of Tprg suggests a critical role for this protein in the epidermis. However, future experimental approaches, such as the germline deletion of Tprg, will be required to reveal its exact function.
Figure 1. ΔNp63α regulates the expression of a myriad of target genes.
These target genes encode proteins that have different functions in the epidermis. For example, within the basal layer, ΔNp63α induces expression of Fras1, a critical component of the basement membrane. Another structural protein induced by ΔNp63α is Perp, a desmosomal protein which is essential for keratinocyte adhesion. In addition to structural proteins, ΔNp63α induces the expression of IKKα, a protein that is required for cell cycle withdrawal during spinous layer development. Finally, a novel ΔNp63α target gene, Tprg, is expressed in the suprabasal layers of the epidermis and may have a role in epidermal terminal differentiation.
In addition to Tprg, ΔNp63α also regulates the expression of other genes involved in epidermal terminal differentiation. One of these genes is IKKα, which is a direct transcriptional target of ΔNp63α in the developing epidermis (Koster et al., 2007; Figure 1). The importance of IKKα in epidermal morphogenesis is highlighted by the phenotype of mice lacking IKKα. In IKKα-deficient mice, the intermediate cell layer forms normally. However, in the absence of IKKα intermediate keratinocytes fail to withdraw from the cell cycle and therefore, the spinous layer fails to develop. The consequent failure of the granular and cornified cell layers to develop results in compromised barrier function and early postnatal lethality. In addition to the defects in epidermal morphogenesis, development of epithelial appendages, including teeth and limbs, is aborted in IKKα deficient mice. These defects are a direct consequence of the failure of the underdeveloped epidermis to participate in the epithelial-mesenchymal interactions required for appendage development (Sil et al., 2004). Whereas IKKα is primarily responsible for cell cycle withdrawal during keratinocyte terminal differentiation, ΔNp63α also induces markers of terminal differentiation, such as K1. For example, ΔNp63α was found to synergize with Notch signaling components to initiate K1 expression (Nguyen et al., 2006).
In addition to the induction of genes that are required for epidermal differentiation, ΔNp63α also directly induces the expression of genes required for maintaining the structural integrity of the skin. This includes Fras1, a basement membrane component, and Perp, a desmosomal component which is important for cell-cell adhesion (Ihrie et al., 2005; Koster et al., 2007; Figure 1). Thus, it comes as no surprise that mice with reduced ΔNp63 expression in the epidermis develop fragile skin which is easily inured, resulting in large skin erosions (Koster et al., 2007).
Together, the data summarized above establish an emerging network of signaling pathways controlled by ΔNp63α. Due to the complexity of the p63 signaling network, high-throughput approaches may prove to be less useful in identifying components of the pathway that are only active at a certain stage of epidermal development or differentiation. That being said, ongoing efforts to identify novel ΔNp63α target genes are needed to further clarify the various complex roles of this intriguing transcription factor in the epidermis.
Acknowledgements
Work in our laboratories is supported by NIH grants to MIK (AR054696) and DRR (HD25479, CA52607, AR47898) and a research grant from the National Foundation for Ectodermal Dysplasias (NFED) to MIK and DRR. We would like to thank Dr. Peter J. Koch for his constructive comments on this manuscript.
Footnotes
Conflicts of Interest The authors state no conflict of interest
References
- Antonini D, Dentice M, Mahtani P, De Rosa L, Gatta GD, Mandinova A, et al. Tprg, a Gene Predominantly Expressed in Skin, Is a Direct Target of the Transcription Factor p63. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.12. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, et al. Perp is a p63-regulated gene essential for epithelial integrity. Cell. 2005;120:843–856. doi: 10.1016/j.cell.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, et al. p63 induces key target genes required for epidermal morphogenesis. PNAS. 2007;104:3255–3260. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms Regulating Epithelial Stratification. Annual Review of Cell and Developmental Biology. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della GG, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]