Abstract
Historically, first-line treatment of non-small-cell lung cancer (nsclc) has been based on giving a limited number of cycles of chemotherapy to achieve tumour response or stable disease. Patients are then observed without active therapy until disease progresses, at which point, subsequent lines of therapy are given. In recent years, two new concepts have been introduced to the management of nsclc: maintenance therapy and therapy with targeted agents. Maintenance therapy—with either a chemotherapeutic or biologic agent—is given immediately after first-line therapy to patients who have achieved tumour response or stable disease. Choice of therapy may include continuation of the agents included in the induction regimen or introduction of different agents (early second-line treatment) with the aim of preventing progression and prolonging progression-free survival. Targeted agents such as bevacizumab and erlotinib target critical molecular signalling pathways and provide several advantages over chemotherapy, including fewer toxicities and the possibility of a longer duration of therapy. This review examines the treatment options in all lines of therapy for metastatic nsclc, focusing particularly on targeted therapies that have been approved in the United States, Canada, or Europe.
Keywords: Targeted therapies, non-small-cell lung cancer, nsclc, metastatic, maintenance
1. INTRODUCTION
Historically, treatment of non-small-cell lung cancer (nsclc) has involved a finite number of cycles of first-line chemotherapy, after which patients with tumour response or stable disease are observed for evidence of disease progression; progression is followed by second-line therapy in suitable patients. However, the therapeutic efficacy of platinum doublets, the most commonly used first-line regimen 1, has reached a plateau, and the introduction of a third chemotherapeutic agent increases toxicity without improving efficacy. Only about 50% of patients in nsclc clinical trials go on to receive second-line therapy, and only about 50% of those will receive third-line therapy. It is therefore important to ensure that patients receive the best therapeutic option in each line of therapy 2.
In recent years, two new concepts have been introduced to the field of nsclc: maintenance therapy and targeted biologic agents. Maintenance therapy, with either a chemotherapeutic or biologic agent, is given to patients after first-line therapy. Choice of therapy may include drugs included in the induction regimen or different agents (early second-line treatment) with the aim of preventing progression and prolonging progression-free survival (pfs). Targeted agents modulate events in the cancer cells and provide several advantages over chemotherapeutics, including fewer toxicities and the possibility of a longer duration of therapy 3.
Two main groups of targeted agents for nsclc are the inhibitors of epidermal growth factor receptor (egfr) and vascular endothelial growth factor (vegf). Erlotinib and bevacizumab are the respective representatives of these groups, and both are approved for use in the United States, Canada, and Europe based on their safety and efficacy profiles. A wealth of available clinical data supports the use of these agents in the treatment of metastatic nsclc. Other egfr inhibitors include cetuximab, which is not currently approved in the United States, Canada, or Europe for the treatment of nsclc, and gefitinib, which was recently granted marketing authorization by the European Medicines Agency (emea) the United States, and Canada for the treatment of EGFR mutation–positive nsclc.
This review examines the treatment options in all lines of therapy for metastatic nsclc, focusing particularly on targeted therapies that have been approved in the United States, Canada, or Europe.
2. A STEP-BY-STEP APPROACH: CHOOSING THE BEST TREATMENT OPTION FOR EVERY LINE OF THERAPY
2.1. The Beginning: First-Line Treatment
2.1.1. Chemotherapy in First Line
There is currently no universal consensus regarding the accepted standard of care for the first-line treatment of advanced nsclc. For instance, cisplatin–gemcitabine is the standard regimen in Europe and other parts of the world, but carboplatin–paclitaxel is preferred in the United States 4. The inclusion of third-generation chemotherapy agents such as paclitaxel, docetaxel, gemcitabine, vinorelbine, irinotecan, and pemetrexed in platinum-based doublets is more effective in terms of response rates and survival, and also improves tolerability compared with cisplatin alone or older platinum-based combinations 5. The overall benefit obtained by modifying chemotherapy regimens has been small and has yielded no tangible improvement in overall survival (os) 6. Maximum median os reached with chemotherapy plateaus at 8–10 months, even with third-generation chemotherapy agents such as pemetrexed 4,7.
In a large phase iii study comparing platinum doublets, first-line cisplatin–pemetrexed provided efficacy similar to that for cisplatin–gemcitabine, with a median os of 10.3 months for each treatment arm 7. In a pre-specified analysis, the median os was significantly longer for cisplatin–pemetrexed than for cisplatin–gemcitabine in patients with adenocarcinoma histology [n = 847; 12.6 months vs. 10.9 months; hazard ratio (hr): 0.84; p = 0.03] and large-cell carcinoma histology (n = 153; 10.4 months vs. 6.7 months; hr: 0.67; p = 0.03). However, the median survival of patients with squamous histology assigned to cisplatin–pemetrexed (n = 244) was 9.4 months; it was 10.8 months for patients assigned to cisplatin–gemcitabine (n = 229; hr: 1.23; p = 0.05). For patients having nsclc without further subtype classification (n = 252), no significant difference was observed between the two arms 7. These outcomes support the use of cisplatin–pemetrexed in non-squamous tumours only. Carboplatin–pemetrexed demonstrated efficacy similar to that of carboplatin–gemcitabine in the first-line treatment of metastatic nsclc 8. Further trials are required to fully elucidate the role of platinum doublets in nsclc based on histology.
2.1.2. Targeted Therapies in First Line
The anti-vegf monoclonal antibody bevacizumab was the first targeted agent to increase efficacy in first-line nsclc when added to a platinum doublet. The key angiogenic factor vegf plays multiple roles in tumour angiogenesis and has become a target for anticancer drug development. Specifically, vegf has been shown to promote survival 9 and to increase permeability of existing tumour vasculature 10 while stimulating the growth of new tumour vessels 9. In addition to its effects on tumour vasculature, vegf is known to have a direct effect on tumour cells, including survival, migration, and invasion 11.
Anti-vegf therapy such as bevacizumab has been proposed to exert a “dynamic” anti-angiogenic effect on tumour vasculature throughout the course of its use, with important effects observed early and continued later in treatment. Two early effects of anti-vegf therapy include regression of existing tumour microvasculature and normalization of remaining tumour vasculature 10. A third effect is the continued inhibition of new tumour vasculature 12 that may contribute to additional benefits observed over longer periods of time.
Bevacizumab in First Line:
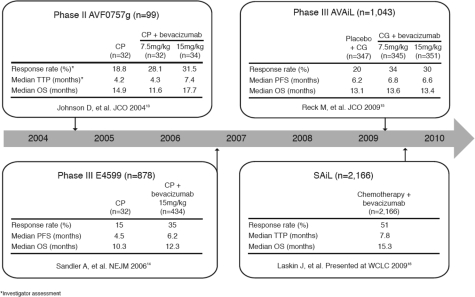
Figure 1 shows data from key first-line bevacizumab trials. In a phase ii trial, addition of bevacizumab to first-line carboplatin–paclitaxel significantly improved response rates and pfs in patients with advanced nsclc 13. The pivotal phase iii trial, E4599, demonstrated significant improvements in median os (12.3 months vs. 10.3 months; hr: 0.79; p = 0.003), median pfs (6.2 months vs. 4.5 months; hr: 0.66; p < 0.001), and response rates (35% vs. 15%, p < 0.001) for bevacizumab in combination with carboplatin–paclitaxel as compared with chemotherapy alone 14. Bevacizumab is the first novel agent combined with chemotherapy to improve survival beyond the historical benchmark of 1 year for non-squamous patients with advanced nsclc. Furthermore, exceptional os benefit (14.2 months vs. 10.3 months for control) has been reported in patients with adenocarcinoma histology treated with bevacizumab in E4599 17.
FIGURE 1.
First-line bevacizumab data in non-small-cell lung cancer. cp = carboplatin–paclitaxel; ttp = time to progression; os = overall survival; jco = J Clin Oncol; cg = cisplatin–gemcitabine; pfs = progression-free survival; nejm = N Engl J Med; wclc = World Conference on Lung Cancer
The phase iii BO17704 (avail) trial is the second large randomized study to confirm the consistent positive efficacy and safety of bevacizumab with platinum doublet chemotherapy (cisplatin–gemcitabine) 15,18. Because the optimal dose of bevacizumab in lung cancer had not been fully explored at the time, avail adopted a three-arm study design. The aim was to determine the efficacy and safety of two doses of bevacizumab in combination with cisplatin–gemcitabine as compared with placebo combined with cisplatin–gemcitabine, although the study was not powered to allow a direct comparison of the two bevacizumab-containing arms. To avoid the risk that the os endpoint might be confounded by the increasing use of the second-line therapies that had become available in the four years since the E4599 trial was initiated, and also by any non-protocol crossover of patients from the placebo arm to bevacizumab, pfs was selected as the primary endpoint of avail. Progression-free survival was significantly prolonged with bevacizumab 7.5 mg/kg plus chemotherapy compared with chemotherapy alone (6.7 months vs. 6.1 months; hr: 0.75; p = 0.003), and an objective response rate of 34.1% was achieved compared with 20.1% for chemotherapy alone (p < 0.0001). Progression-free survival was also significantly improved in patients receiving bevacizumab 15 mg/kg plus chemotherapy as compared with placebo (6.5 months vs. 6.1 months; hr: 0.82; p = 0.03).
A large phase iv study, MO19390 (sail), examined the safety of bevacizumab in the day-to-day clinical setting and confirmed the well-established and favourable safety profile of bevacizumab in a broad patient population 16,19. That trial involved more than 2000 patients, demonstrated a clinical benefit for bevacizumab not only with cisplatin doublet, but also with carboplatin doublet, regimens (approximately half of all patients). Preliminary efficacy data confirmed the clinical benefit of bevacizumab-based therapy, with a median pfs of 7.8 months and os of 15.3 months 16.
A large registry trial (aries) in the United States further confirmed the safety of bevacizumab in patients underrepresented in randomized controlled trials 20. Data suggest that most patients with metastatic nsclc—including elderly patients; those with hypertension, central tumour location, central nervous system (cns) metastases; and those receiving concurrent anticoagulation therapy at baseline—can receive bevacizumab. Preliminary median pfs is reported to be 6.7 months.
All bevacizumab clinical trials to date have demonstrated benefit when this agent is given first line and until disease progression. Other targeted therapies have shown minimal benefit in the first-line setting. Phase iii trials of cetuximab plus taxane–carboplatin (BMS-099) and cetuximab plus cisplatin–vinorelbine (flex) failed to demonstrate a pfs benefit in patients with nsclc (4.4 months vs. 4.2 months and 4.8 months respectively) 21,22. A marginal os benefit was observed in flex (11.3 months vs. 10.0 months); however, the lack of a pfs benefit raises the question of the influence of subsequent therapies or other still unknown factors (possibly biologic differences) on os.
A large phase iii trial (escape) of sorafenib, a multikinase inhibitor, in combination with carboplatin–paclitaxel, showed no benefit in patients with nsclc; moreover, the addition of sorafenib appeared to have a detrimental effect in patients with squamous cell histology. As a result, the trial was stopped prematurely, and the study failed to meet its primary os endpoint 23. The br.24 phase ii/iii study of cediranib (Recentin: AstraZeneca Pharmaceuticals, Wilmington, DE, U.S.A.) in first-line nsclc is another trial that was discontinued because of unacceptable toxicity. Although an improvement in objective response and a trend toward better pfs were observed, the disproportional increase in the death rate in the cediranib arm, even at the reduced dose of 30 mg, was considered enough for the study not to have met the predefined criteria for automatic continuation into phase iii 24. A follow-up randomized phase ii/iii trial (br.29) is currently ongoing, testing cediranib at the lower dose of 20 mg in patients with stage iiib or iv nsclc. Many other randomized trials of targeted therapies combined with chemotherapy have failed to demonstrate clinical benefit.
Regulatory Approval Status of Bevacizumab:
Based on results from the E4599 trial, bevacizumab plus carboplatin–paclitaxel became the Eastern Cooperative Oncology Group (ecog) reference standard and received approval from the U.S. Food and Drug Administration for first-line treatment of non-squamous advanced nsclc. Similsarly, based on avail and E4599, bevacizumab in combination with platinum-doublet chemotherapy received emea approval. In April 2009, bevacizumab in combination with carboplatin–paclitaxel was approved in Canada. A meta-analysis of more than 13,000 bevacizumab-treated patients provided reassurance that the risk of cns bleeding in patients with brain metastases is not unduly increased with the use of bevacizumab 25, which led to an update of the emea label to allow patients with untreated cns metastases to receive the drug 26. The United States has never had any label restriction on bevacizumab for patients with cns metastases; however, new data regarding the safety of bevacizumab in patients with cns metastasis is included in the “warnings and precautions” section of that country’s monograph.
2.1.3. Evidence-Based Medicine: A Rational Approach in First Line
With the availability of a wide range of agents, first-line therapy often presents a challenge for physicians treating patients with nsclc. A number of factors affect the choice of first-line therapy, including available clinical data, patient characteristics (age, disease stage, histology, smoking status, tumour mutation status), patient preference, and physician experience with certain agents.
A wealth of data demonstrate clinical benefit with manageable toxicity for bevacizumab in patients with non-squamous pathology (E4599, avail, sail, aries). Although pemetrexed has demonstrated an os benefit in patients with non-squamous nsclc, that benefit is restricted to a subanalysis of a subgroup of patients and to those eligible for cisplatin 7,27. Patients not eligible for bevacizumab should receive platinum-containing doublet chemotherapy, of which cisplatin–pemetrexed is the most promising for non-squamous histology. Data from phase iii trials will help to determine the role of pemetrexed–cisplatin with bevacizumab in the first-line setting.
Biomarkers are increasingly being used to monitor a patient’s clinical course and response to therapy. Specific markers may predict the likelihood of benefit from a particular therapy, permitting an optimal selection of treatment for the individual patient. EGFR mutations have been implicated as potential biomarkers for nsclc, and evidence suggests that egfr tyrosine kinase inhibitors (tkis) are particularly effective agents in patients with EGFR mutation–positive tumours.
A phase iii, randomized, open-label study (the Iressa Pan-Asia Study) examined the efficacy of gefitinib in first line as compared with carboplatin–paclitaxel in clinically selected patients with nsclc. The results revealed significantly longer pfs times, increased objective response rates, and improved quality of life among EGFR mutation–positive patients who received gefitinib than among those who received carboplatin–paclitaxel. The difference in the rates of objective response with gefitinib was remarkable at 71.2% and 1.1% for EGFR mutation–positive and –negative patients respectively, suggesting that the presence of an EGFR mutation can be a robust predictor of improved efficacy with gefitinib in first-line nsclc 28. Currently, there are no predictive markers for anti-vegf therapy. In mutation-positive patients (exon 19 + 21), egfr tkis are the treatment of choice in the first line for metastatic nsclc. Tests for EGFR mutations within tumours are not readily available in Canada. In a case of unknown mutation status, patients should receive chemotherapy combination treatment 29.
2.2. Continuing the Efficacy Benefit: Maintenance
The optimal treatment duration for nsclc patients remains a matter of discussion. A number of studies have evaluated regimens using either sequential or maintenance chemotherapy as post-first-line treatment for nsclc patients who have not experienced disease progression. A review of those studies suggests that the optimal timing and duration of maintenance therapy (or immediate compared with delayed second-line therapy) remain unclear 2,30.
2.2.1. Chemotherapy in Maintenance
A recent phase iii trial 31 compared the efficacy and safety of docetaxel administered to patients either immediately after first-line gemcitabine–carboplatin or at the time of disease progression. The study showed a statistically significant improvement in pfs of 3 months for patients receiving immediate docetaxel therapy and a nonsignificant trend toward an improved os. A notable observation from the trial was that, although 95% of patients in the immediate-therapy arm received docetaxel, only 63% of patients in the delayed-therapy arm received docetaxel. When os was compared for patients in the safety population (that is, only patients that received docetaxel), os was 12.5 months in both arms.
The jmen trial evaluated maintenance pemetrexed plus best supportive care (bsc) against placebo plus bsc. With maintenance pemetrexed, the pfs in the overall patient population was 4.0 months as compared with 2.0 months for placebo 32; however, patients with squamous histology did not benefit from pemetrexed therapy. The trial excluded patients who had previously received pemetrexed with cisplatin, and it was therefore similar to the immediate-docetaxel arm of the trial by Fidias and colleagues: the lack of a delayed pemetrexed arm means that it is difficult to ascertain the true benefit of immediate compared with second-line pemetrexed. It should also be noted that only patients who responded to initial chemotherapy (stable disease or better) were randomized to pemetrexed or placebo, and therefore the trial assessed pemetrexed only in patients who were known to have chemosensitive tumours. Furthermore, only 19% of patients in the placebo arm received pemetrexed in the second line, raising a question concerning whether the observed survival benefit would have been maintained if more patients had received second-line pemetrexed.
Myelosuppression is a known dose-limiting toxicity of pemetrexed therapy. Patients are required to continuously take prophylactic folic acid and vitamin B12 to reduce treatment-related toxicities, especially hematologic toxicities. The most common adverse events related to pemetrexed therapy include neutropenia and fatigue.
2.2.2. Targeted Therapies in Maintenance
All bevacizumab clinical trials to date have demonstrated efficacy when bevacizumab is administered in combination with first-line chemotherapy, followed by bevacizumab monotherapy as maintenance. In the maintenance phase of avail, there was a significant increase in pfs for patients in the bevacizumab arm as compared with the placebo arm 33 (4.6 months vs. 3.2 months; Table i). Furthermore, the atlas trial confirmed the efficacy of maintenance bevacizumab and demonstrated that the benefit is further improved with the addition of erlotinib (4.76 months vs. 3.75 months; hr: 0.722) 35. A large proportion of patients (66%) received maintenance therapy in avail, suggesting that more patients can benefit from maintenance therapy after having received first-line bevacizumab-based therapy. In patients who receive first-line chemotherapy without bevacizumab, erlotinib maintenance therapy can still deliver a significant efficacy benefit: in the saturn trial, a 41% improvement in pfs was observed for erlotinib as compared with placebo 34. In addition, saturn demonstrated a significant survival benefit for maintenance erlotinib over placebo—a benefit that extended to all patient subgroups, including squamous tumour pathology, which is especially important because this benefit was independent of EGFR mutation status 36.
TABLE I.
Efficacy outcome of trials in the maintenance setting in patients with non-small-cell lung cancer
| Reference | Trial short name | Treatment | Patients (n) | Median pfs (months) | hr |
|---|---|---|---|---|---|
| Belani et al. 200932 | jmen | Pemetrexed | 441 | 4.0 | 0.5 |
| Placebo | 222 | 2.0 | (p<0.0001) | ||
| Cappuzzo et al. 200934 | saturn | Erlotinib | 437 | nr | 0.71 |
| Placebo | 447 | nr | (p<0.0001) | ||
| Mezger et al. 200933 | avail | Placebo | 41 | 3.2 | nr |
| Bevacizumab 7.5 mg/kg | 174 | 4.6 | |||
| Bevacizumab 15 mg/kg | 162 | 4.6 | |||
| Miller et al. 200935 | atlas | Bevacizumab + erlotinib | 370 | 4.76 | 0.722 |
| Bevacizumab + placebo | 373 | 3.75 | (p=0.0012) |
pfs = progression-free survival; hr = hazard ratio; nr = not reported.
2.2.3. Combining Agents to Maximize Benefit: Future Directions in Maintenance Therapy
In a phase ii trial, Patel et al. 37 evaluated the efficacy and safety of first-line pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab in non-squamous nsclc patients. An overall response rate of 55% was achieved, with a median pfs of 7.8 months and an os of 14.1 months. Another phase ii trial demonstrated that bevacizumab plus pemetrexed and oxaliplatin followed by bevacizumab maintenance delivered a median pfs of 7.8 months and a median os of 16.7 months 38. These data suggest that consistent clinical benefit is achieved when bevacizumab is combined with different agents and continued in the maintenance setting. Phase iii trials of bevacizumab with pemetrexed maintenance therapy in nsclc are pending.
2.2.4. Evidence-Based Medicine: A Rational Approach in Maintenance
Clinical trial data in colorectal cancer suggest that patients eligible for bevacizumab therapy should continue bevacizumab to keep vegf levels down and to deliver continued clinical benefits 39. Patients who are not eligible for bevacizumab may receive pemetrexed or erlotinib. Erlotinib treatment is well tolerated and is associated with manageable adverse events such as rash or diarrhea. Furthermore, oral administration of erlotinib means fewer hospital visits for the patient; erlotinib therefore remains the agent of choice in patients with advanced nsclc who are not eligible for bevacizumab and who prefer more convenient treatment. Unlike pemetrexed, erlotinib therapy is also effective in patients with squamous histology 34.
2.3. A Step Further: Second Line
2.3.1. Chemotherapy in Second Line
Patients with advanced nsclc eventually relapse or become refractory to first-line treatment. Acceptable toxicity and improved quality of life are especially important for those patients (although efficacy remains the main goal of therapy). Several chemotherapy agents, including docetaxel and pemetrexed, have demonstrated efficacy and have been approved by the U.S. Food and Drug Administration for second-line treatment of patients with locally advanced or metastatic nsclc 40–43. In Canada, approved second-line chemotherapy agents are intravenous docetaxel and intravenous pemetrexed, the latter for non-squamous histology only. Docetaxel has been reported to achieve response rates of 15%–20% 44,45, an os of 8.3 months 42, and 1-year survival rates of up to 37% 40. However, docetaxel is associated with serious toxicities. Pemetrexed offers a similar median os of 7.9 months, but with a milder toxicity profile than is seen with docetaxel 42.
2.3.2. Targeted Therapies in Second Line
Erlotinib is an egfr tki that suppresses intracellular signalling pathways, which normally promote cell growth and proliferation 46,47. Erlotinib is the only approved targeted biologic agent for nsclc in the second-line setting in Canada. Unlike chemotherapy, erlotinib has no cumulative hematologic toxicities, allowing for a longer treatment duration. In contrast, the toxicities associated with chemotherapy allow for only a limited number of cycles (approximately 4 cycles median). Table ii compares clinical data for erlotinib, docetaxel, and pemetrexed.
TABLE II.
Efficacy data in the second-line setting
| Outcome | Erlotinib43(150 mg daily) | Docetaxel40–42,48(75 mg/m2 every 3 weeks) | Pemetrexed42(500 mg/m2 every 3 weeks) |
|---|---|---|---|
| Risk ratio (%) | 8.9 | 6.7–8.8 | 9.1 |
| Median duration of response (months) | 7.9 | 5.3–9.1 | 4.6 |
| Median pfs (months) | 2.2 | 2.7–6 | 2.9 |
| Median os (months) | 6.7 | 5.7–7.9 | 8.3 |
| 1-Year survival (%) | 31 | 30–37 | 30 |
| 2-Year survival (%) | 13 | 0 | 0 |
| Median os (months)a | 9.4 | 9.1 | 9.4 |
In patients with a performance status of 0 or 1 with 1 prior regimen.
pfs = progression-free survival; os = overall survival.
In a randomized, placebo-controlled study (br.21), erlotinib demonstrated improvement in median os (6.7 months vs. 4.7 months) and quality of life across all subgroups 43,49. The safety and efficacy of erlotinib was confirmed in a phase iv trial (trust) in a broad patient population 50, where median os was 8.1 months, with a 1-year survival rate of 38.6%. Another egfr tki, gefitinib, failed to demonstrate a survival advantage in the overall population of a phase iii trial (isel). A phase ii study of single-agent sorafenib in second line suggests that this agent has modest benefits and specific toxicity 51, but that finding has yet to be demonstrated in a phase iii trial.
Vandetanib (Zactima: AstraZeneca) is a monotherapy agent that combines targeting of vegf receptor–dependent tumour angiogenesis and egfr-dependent tumour cell proliferation and survival. Phase iii trials of second-line single-agent vandetanib have demonstrated only modest benefit to date 52–54. In zest, vandetanib monotherapy showed efficacy equivalent to that with erlotinib, but with additional toxicity in second-line treatment of nsclc. The zeal trial showed no statistical benefit for pemetrexed plus vandetanib compared with pemetrexed alone in previously-treated nsclc patients. The zodiac trial assessed the efficacy of docetaxel in combination with vandetanib compared with docetaxel and placebo in nsclc patients who progressed after treatment with standard chemotherapy. Combination therapy demonstrated improved pfs and quality of life as compared with docetaxel alone.
2.3.3. Evidence-Based Medicine: A Rational Approach in Second Line
The selection of second-line therapy often depends on the first-line therapy used to treat the patient. A good response to first-line chemotherapy may warrant further chemotherapy in second line. The benefit of single-agent versus doublet chemotherapy was evaluated in a meta-analysis, and the improvements in response rate achieved with doublet chemotherapy were not found to translate into a pfs or os benefit; moreover, they were associated with additional toxicity 55.
For patients who did not respond to first-line chemotherapy or who tolerated it poorly, an egfr inhibitor may be the preferred choice in second line. Non-inferiority in terms of os for gefitinib, an egfr tki, compared with docetaxel was demonstrated in a phase iii trial (interest). Non-inferiority was shown regardless of a patient’s egfr protein expression, EGFR gene mutation, or k-ras gene mutation status. Survival results were significantly different only for patients who received third-line treatment, with a longer survival observed for those receiving docetaxel compared with those receiving gefitinib. Given the lack of difference in clinical benefit relating to the sequence of chemotherapy versus egfr tki in the second and third lines (interest), as well as lesser toxicity and easy oral administration, egfr tki agents are preferred second-line agents for nsclc. Obtaining mutation status (egfr exon 19 + 21) of the tumour for second-line nsclc treatment is not a necessity. When available in future, k-ras mutations could also facilitate the decision on choice of treatment.
2.4. Looking Further Down the Line: Third Line
A retrospective practice review found that second-line erlotinib treatment is efficacious and well-tolerated, and does not diminish the benefit of third-line chemotherapy 56. Furthermore, erlotinib and docetaxel can be given for third-line treatment of nsclc. Pemetrexed is approved for use in second line in the United States and Canada, and it remains a strong candidate for patients who experience disease progression after bevacizumab and erlotinib in previous lines of therapy.
A number of trials are investigating the role of anticancer therapies in the third- or fourth-line setting. BIBW 2992, a dual inhibitor of egfr (Erb1) and her2, was evaluated in a phase iib/iii trial of bsc versus placebo and bsc in nsclc patients who had failed 1–2 lines of chemotherapy and erlotinib or gefitinib (LUX-Lung 1). Interim results have been reported, and the trial completed accrual in August 2009 57. Two ongoing trials (phase ii SUN-1058 and phase iii SUN-1087) are exploring the combination of sunitinib with erlotinib as second- and third-line therapy. Results from the phase iii zephyr trial will help define the role of vandetanib in the third- and fourth-line settings after egfr tki failure. Results are expected in 2010. A phase iii trial of sorafenib versus placebo as third- or fourth-line therapy is currently recruiting patients; data are expected in April 2011. Combining an insulin-like growth factor inhibitor with erlotinib after progression of disease in second line to try to reverse resistance to erlotinib is also under investigation. Sufficient tumour biopsies will be essential to guide decisions for treatment with targeted agents.
2.4.1. Evidence-Based Medicine: A Rational Approach in Third Line
Clinical data are suggestive of improved third-line options for nsclc.
The use of erlotinib as third-line therapy is supported by the br.21 study, in which approximately half the patients had failed two previous lines of chemotherapy. In addition to an acceptable safety profile, erlotinib demonstrated clinical benefit in terms of response and os in patients with a good or poor ecog performance status. Erlotinib should therefore be considered a viable third-line treatment option for patients who have not yet received this agent.
The non-inferiority of the egfr tki gefitinib over docetaxel in terms of os (interest), together with its improved safety profile and preferred oral administration over a longer period of time, makes egfr tkis prime candidates for second-line rather than third-line treatment in patients with nsclc. Educating physicians about the importance of re-biopsying the tumour at the time of progression to help guide treatment decisions in the future (and about optimal biopsy techniques) will be of utmost importance.
3. FUTURE STEPS
The main goal is to provide the best possible treatment in terms of both efficacy and safety in each line of therapy. The striking improvements in outcomes demonstrated in both first- and second-line settings with targeted therapies provide a rationale for their use. The targeting of multiple pathways using a wide range of new drugs and combinations of agents is currently under investigation. The mode of action of bevacizumab suggests that its continued use may prevent recurrence of tumour angiogenesis. The advantage of continuing bevacizumab therapy beyond progression has been demonstrated in an observational study (brite) in patients with colorectal cancer 39. Compared with alternative or no post-progression treatment, bevacizumab given to patients post progression resulted in superior improvement in os. However, these data have yet to be confirmed in a prospective phase iii trial in patients with nsclc.
As compared with chemotherapeutic agents, targeted agents may offer reduced toxicity, especially with prolonged use. Combinations of targeted agents may also have potential as novel treatment paradigms, perhaps even representing an alternative to chemotherapy. Bevacizumab and erlotinib combination therapy has shown promise in phase i/ii 58 and phase ii trials 59 in patients with recurrent nsclc, with reduced toxicity as compared with chemotherapy. In the phase iii trial BETA-Lung, the addition of erlotinib to second-line bevacizumab led to an improvement in pfs, but did not translate into a longer os 60. Strict criteria for patient selection resulted in the recruitment of healthier patients in each treatment arm, and these patients went on to receive multiple (up to 5) lines of post-progression treatment, which may have confounded the os result. However, this combination has demonstrated significant benefit when used as maintenance therapy post first line (atlas). Table iii summarizes ongoing and future trials of bevacizumab and erlotinib.
TABLE III.
Selected ongoing and future trials of erlotinib and bevacizumab
| Study short name | Phase | Pts (n) | Eligibility | Regimen | Line of therapy | Primary endpoint |
|---|---|---|---|---|---|---|
| passport (AVF3752g) | ii | 110 | Previously treated or untreated non-squamous nsclc with treated cns metastases | Chemotherapy or erlotinib followed by bevacizumab | First/second | Grade 2 or greater symptomatic cns hemorrhage |
| brain (AVF21823) | ii | 115 | Stage iv non-squamous nsclc with asymptomatic brain metastases in first and second line | First line: bevacizumab + carboplatin/paclitaxel Second line: bevacizumab + erlotinib |
First/second | pfs |
| eagles (ML21896) | ii | 78 | Patients older than 70 years without important comorbidities | Bevacizumab + gemcitabine OR bevacizumab + gemcitabine/cisplatin |
First | pfs at 6 months |
| ii | ∼250 | Patients 65 years or age or older with advanced metastatic or recurrent non-squamous nsclc | Bevacizumab + pemetrexed OR bevacizumab + pemetrexed/carboplatin |
First | Proof of non-inferiority of bevacizumab + pemetrexed | |
| bridge (AVF2744g) | i/ii | 40 | Previously untreated squamous nsclc | Bevacizumab + carboplatin/paclitaxel | First | Grade 3 or greater pulmonary haemorrhage |
| abigail (BO21015) | ii | ∼300 | Locally advanced, metastatic or recurrent non-squamous nsclc | Bevacizumab + carboplatin/gemcitabine OR carboplatin/paclitaxel |
First | Correlation of biomarkers with response |
| mimeb (ML21803) | ii | 40 | Histologically confirmed advanced non-squamous nsclc stage iiib/iv | Bevacizumab + erlotinib | First | Accuracy of fdg- or flt-pet and dce-mri for early prediction of non-progression |
| eurtac | iii | 146 | egfr mutation-positive nsclc | Erlotinib | First | pfs in patients with mutation |
| saturn (BO18192) | iii | 1949 | Previously untreated advanced nsclc | Erlotinib | First | pfs in all patients and in patients with EGFRihc-positive tumours |
| radiant | 945 | Advanced nsclc | Erlotinib vs. placebo | Adjuvant | dfs | |
| fastact-2 | iii | 450 | Asian patients with previously untreated advanced nsclc | Erlotinib + chemotherapy vs. placebo + chemotherapy |
First line | pfs |
| atlas (AVF3671f) | iii | 1150 | Previously untreated advanced nsclc | Bevacizumab + carboplatin/paclitaxel OR + gemcitabine/cisplatin OR + carboplatin/docetaxel Non-progressing pts randomized (1:1) to bevacizumab + erlotinib OR bevacizumab + placebo |
First line maintenance | pfs |
| target | ii | 428 | EGFR mutation–positive nsclc | Erlotinib | First | pfs |
| torch | iii | 900 | Previously untreated advanced nsclc | First-line erlotinib, second-line gemcitabine/cisplatin vs. first-line gemcitabine/cisplatin, second-line erlotinib | First/second | os |
Pts = patients; nsclc = non-small-cell lung cancer; cns = central nervous system; pfs = progression-free survival; fdg = 18F-2-fluorodeoxy-d-glucose; flt = 18F-fluorodeoxythymidine; pet = positron-emission tomography; dce-mri = dynamic contrast-enhanced magnetic resonance imaging; EGFR = gene for the epidermal growth factor receptor; ihc = immunohistochemistry; dfs = disease-free survival; os = overall survival.
3.1. Tailoring Therapy
Predictors of response may help to guide individual treatment decisions; however, for most drugs, clinically validated markers have yet to be identified. Until reliable biomarkers for response and resistance (old or newly developed) are identified, differences in toxicity between chemotherapeutic and targeted agents may provide the best guide for individual treatment decisions, in view of similar efficacy.
Gefitinib recently received emea and Canadian approval for treatment of patients with EGFR mutation–positive disease. EGFR mutations can be viewed as predictive markers of high clinical benefit with egfr tki therapy, especially for first-line treatment in eligible patients.
Histology appears to be an important consideration for optimal outcomes with both pemetrexed and bevacizumab. A trial (bridge) in patients with predominantly squamous-cell histology (who are currently excluded from bevacizumab clinical trials) is ongoing to determine ways in which to safely integrate bevacizumab into the treatment of these patients. A personalized targeted approach is the future of treatment in all lines, but tumour re-biopsy will be required for analysis of biomarkers, including not only newly developed markers of resistance to egfr tki, but also sensitivity to agents such as BIBW 2992 (T790M mutation and c-Met amplification). Analysis of circulating tumour cells and blood biomarkers to define predictors of tumour response and treatment benefit is a great need for the future.
4. SUMMARY
Given the plateau reached with chemotherapy, and the toxicity associated with prolonged chemotherapy, there is a need to improve outcomes in every line of therapy of advanced nsclc. Targeted biologic agents are effective and well tolerated in nsclc, either combined with chemotherapy or as monotherapy, with bevacizumab (first line) and erlotinib (second and third line) having gained approval in Europe, the United States, and Canada. Novel targeted therapies and their combinations with chemotherapeutic agents are also being explored. Future challenges involve identifying predictors of response and efficacy for targeted nsclc therapies and selecting the optimal therapy for maximum survival benefit in each line of treatment from among currently approved agents.
5. CONFLICT OF INTEREST DISCLOSURE
The author acknowledges medical writing support from Tania Kotsokechagia at Gardiner–Caldwell Communications; this support was funded by F. Hoffmann–La Roche Ltd.
6. REFERENCES
- 1.Schiller JH. Small cell lung cancer: defining a role for emerging platinum drugs. Oncology. 2002;63:105–14. doi: 10.1159/000063807. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Socinski MA. Treatment paradigms for advanced stage non-small cell lung cancer in the era of multiple lines of therapy. J Thorac Oncol. 2009;4:243–50. doi: 10.1097/JTO.0b013e31819516a6. [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam S, Belani CP. Recent advances in targeted therapy for non-small cell lung cancer. Expert Opin Ther Targets. 2007;11:245–57. doi: 10.1517/14728222.11.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, on behalf of the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, Socinski MA. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol. 2007;2:845–53. doi: 10.1097/JTO.0b013e31814617a2. [DOI] [PubMed] [Google Scholar]
- 6.Abratt RP, Hart GJ. 10-Year update on chemotherapy for non-small cell lung cancer. Ann Oncol. 2006;17(suppl 5):v33–6. doi: 10.1093/annonc/mdj947. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 8.Grønberg BH, Bremnes RM, Fløtten O. Phase iii study by the Norwegian Lung Cancer Study Group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–24. doi: 10.1200/JCO.2008.20.9114. [DOI] [PubMed] [Google Scholar]
- 9.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LM, Hicklin DJ. vegf-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 12.Inai T, Mancuso M, Hashizume H. Inhibition of vascular endothelial growth factor (vegf) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DH, Fehrenbacher L, Novotny WF. Randomized phase ii trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Reck M, von Pawel J, Zatloukal P. Phase iii trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: avail. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 16.Laskin J, Crinò L, Tsai C. MO19390 (sail): first-line bevacizumab-based therapy in advanced non-small cell lung cancer (nsclc)—outcome by chemotherapy regimen [abstract C2.5] J Thorac Oncol. 2009;4(Suppl 1):S359. [Available online from: journals.lww.com/jto/toc/2009/09001; cited February 12, 2010] [Google Scholar]
- 17.Sandler A, Kong G, Strickland D, Johnson D. Treatment outcomes by tumor histology in Eastern Cooperative Group (ecog) Study E4599 of bevacizumab (bv) with paclitaxel/carboplatin (pc) for advanced non-small cell lung cancer (nsclc) [abstract 133] J Thorac Oncol. 2008;3(Suppl 4):S283. doi: 10.1097/JTO.0b013e3181da36f4. [Available online from: journals.lww.com/jto/toc/2008/11001; cited February 12, 2010] [DOI] [PubMed] [Google Scholar]
- 18.Hirsh V. Emerging safety data for bevacizumab in advanced non–small-cell lung cancer. Clin Lung Cancer. 2008;9(suppl 2):S62–70. doi: 10.3816/CLC.2008.s.010. [Available online at: cigjournals.metapress.com/content/d43408q34506078m/; cited February 19, 2010] [DOI] [PubMed] [Google Scholar]
- 19.Crinò L, Mezger J, Griesinger F, Zhou C, Reck MM. MO19390 (sail): safety and efficacy of first-line bevacizumab (Bv)-based therapy in advanced non-small cell lung cancer (nsclc) [abstract 8043] Proc Am Soc Clin Oncol. 2009;27:417s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=30979; cited February 12, 2010] [Google Scholar]
- 20.Fischbach N, Spigel D, Brahmer J, on behalf of the aries Investigators Preliminary safety and effectiveness of bevacizumab (bv) based treatment in subpopulations of patients (pts) with non-small cell lung cancer (nsclc) from the aries study: a bevacizumab (bv) treatment observational cohort study (ocs) [abstract 8040] Proc Am Soc Clin Oncol. 2009;27:416s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=30542; cited February 12, 2010] [Google Scholar]
- 21.Lynch T, Patel T, Dreisbach L. A randomized multi-center phase iii study of cetuximab (Erbitux) in combination with taxane/carboplatin versus taxane/carboplatin alone as first-line treatment for patients with advanced/metastatic non-small cell lung cancer (nsclc): B3-03. J Thorac Oncol. 2007;2(suppl 4):S340–1. [Google Scholar]
- 22.Pirker R, Pereira JR, Szczesna A. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (flex): an open-label randomised phase iii trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti GV, von Pawel J, Reck M. Sorafenib plus carboplatin/paclitaxel in chemonaïve patients with stage iiib–iv non-small-cell lung cancer (nsclc): interim analysis (ia) results from the phase iii, randomized, double-blind, placebo-controlled, escape (Evaluation of Sorafenib, Carboplatin, and Paclitaxel Efficacy in nsclc) trial. J Thor Oncol. 2008;3:S97–8. [Google Scholar]
- 24.Krupitskaya Y, Wakelee H. Vascular endothelial growth factor pathway. J Thor Oncol. 2009;4(suppl 3):S1071–3. doi: 10.1097/01.JTO.0000361755.32660.a1. [DOI] [PubMed] [Google Scholar]
- 25.Rohr U, Augustus S, Lasserre S, Compton P, Huang J. Safety of bevacizumab in patients with metastases to the central nervous system [abstract 2007] Proc Am Soc Clin Oncol. 2009;27:88s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=30766; cited February 12, 2010] [Google Scholar]
- 26.European Medicines Agency (emea), Evaluation of Medicines for Human Use . Assessment Report for Avastin. International Nonproprietary Name/Common Name: Bevacizumab. Procedure no. EMEA/H/C/582/II/0025. London, U.K: EMEA; 2008. [Available online at: www.ema.europa.eu/humandocs/PDFs/EPAR/avastin/Avastin-H-582-II-25-AR.pdf; cited February 12, 2010] [Google Scholar]
- 27.Scagliotti G, Hanna N, Fossella F. The differential efficacy of pemetrexed according to nsclc histology: a review of two phase iii studies. Oncologist. 2009;14:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 28.Mok TS, Wu YL, Thongprasert S. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Eng J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 29.Mok T, To KF, Srimunimimit V. Clinical outcomes of patients with epidermal growth factor receptor (EGFR) mutations in ipass (Iressa Pan Asia Study) [abstract B9.5] J Thorac Oncol. 2009;4(suppl 1):S351. [Available online from: journals.lww.com/jto/toc/2009/09001; cited February 12, 2010] [Google Scholar]
- 30.Gridelli C, Maione P, Rossi A. Potential treatment options after first-line chemotherapy for advanced nsclc: maintenance treatment or early second-line? Oncologist. 2009;14:137–47. doi: 10.1634/theoncologist.2008-0152. [DOI] [PubMed] [Google Scholar]
- 31.Fidias P, Dakhil S, Lyss A. Phase iii study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 32.Belani C, Brodowicz T, Ciuleanu T. Maintenance pemetrexed (Pem) plus best supportive care (bsc) versus placebo (Plac) plus bsc: a randomized phase iii study in advanced non-small cell lung cancer (nsclc) [abstract CRA8000] Proc Am Soc Clin Oncol. 2009;27:806s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=33019; cited February 12, 2010] [Google Scholar]
- 33.Mezger J, von Pawel J, Reck M. Bevacizumab (Bv) single-agent maintenance following Bv-based chemotherapy in patients with advanced non-small cell lung cancer (nsclc): results from an exploratory analysis of the avail study [abstract e19001] Proc Am Soc Clin Oncol. 2009;27:e19001. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=32977; cited February 12, 2010] [Google Scholar]
- 34.Cappuzzo F, Ciuleanu T, Stelmakh L. saturn: a double-blind, randomized, phase iii study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced nsclc [abstract 8001] Proc Am Soc Clin Oncol. 2009;27:407s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=32756; cited February 12, 2010] [Google Scholar]
- 35.Miller V, O’Connor P, Soh C, Kabbinavar F, on behalf of the atlas Investigators A randomized, double-blind, placebo-controlled, phase iiib trial (atlas) comparing bevacizumab (b) therapy with or without erlotinib (e) after completion of chemotherapy with b for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (nsclc) [abstract LBA8002] Proc Am Soc Clin Oncol. 2009;27:799s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=30819; cited February 12, 2010] [Google Scholar]
- 36.Cappuzzo F, Coudert B, Wierzbicki R. Efficacy and safety of erlotinib as first-line maintenance in nsclc following non-progression with chemotherapy: results from the phase iii saturn study [abstract A2.1] J Thorac Oncol. 2009;4(suppl 1):S289. [Available online from: journals.lww.com/jto/toc/2009/09001; cited February 12, 2010] [Google Scholar]
- 37.Patel J, Bonomi P, Socinski M. Treatment rationale and study design for the PointBreak Study: a randomized, open-label phase iii study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage iiib or iv nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2009;10:252–6. doi: 10.3816/CLC.2009.n.035. [DOI] [PubMed] [Google Scholar]
- 38.Waples J, Auerbach M, Steis R, Boccia R, Wiggans R. A phase ii study of oxaliplatin and pemetrexed plus bevacizumab in advanced non-squamous non-small cell lung cancer (an International Oncology Network study, #I-04-015) [abstract 19018] Proc Am Soc Clin Oncol. 2008;26:707s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=32137; cited February 12, 2010] [Google Scholar]
- 39.Grothey A, Sugrue MM, Purdie DM. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (brite) J Clin Oncol. 2008;26:5326–34. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd FA, Dancey J, Ramlau R. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 41.Fossella FV. Second-line chemotherapy for non-small-cell lung cancer. Curr Oncol Rep. 2000;2:96–101. doi: 10.1007/s11912-000-0016-x. [DOI] [PubMed] [Google Scholar]
- 42.Hanna N, Shepherd FA, Fossella FV. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd FA, Rodrigues Pereira J, Tan EH. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 44.Novello S, Le Chevalier T. Chemotherapy for non-small cell-lung cancer. Part 2: advanced disease. Oncology. 2003;17:457–71. [PubMed] [Google Scholar]
- 45.Haura E. Treatment of advanced non-small-cell lung cancer: a review of current randomized clinical trials and an examination of emerging therapies. Cancer Control. 2001;8:326–36. doi: 10.1177/107327480100800404. [DOI] [PubMed] [Google Scholar]
- 46.Moyer JD, Barbacci EG, Iwata KK. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–48. [PubMed] [Google Scholar]
- 47.Pollack VA, Savage DM, Baker DA. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumour effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–48. [PubMed] [Google Scholar]
- 48.Kim E, Hirsh V, Mok T. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 49.Bezjak A, Tu D, Seymour L. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study br.21. J Clin Oncol. 2006;24:3831–7. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 50.Reck M, Mali P, Arrieta O, Gottfried M, Van Meerbeeck J. Global efficacy and safety results from the trust study of erlotinib monotherapy in > 7,000 patients with non-small-cell lung cancer (nsclc) [abstract 262P] Ann Oncol. 2008;19(suppl 8):viii100. [Google Scholar]
- 51.Gutierrez M, Kummar S, Allen D. A phase ii study of multikinase inhibitor sorafenib in patients with relapsed non-small cell lung cancer (nsclc) [abstract 19084] Proc Am Soc Clin Oncol. 2008;26:712s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=36323; cited February 12, 2010] [Google Scholar]
- 52.De Boer R, Arrieta Ó, Gottfried M. Vandetanib plus pemetrexed vs pemetrexed as second-line therapy in patients with advanced non-small-cell lung cancer (nsclc): a randomized, double-blind phase iii trial (zeal) [abstract 8010] Proc Am Soc Clin Oncol. 2009;27:409s. doi: 10.1200/JCO.2010.29.5717. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=31867; cited February 12, 2010] [DOI] [PubMed] [Google Scholar]
- 53.Natale R, Thongprasert S, Greco F. Vandetanib versus erlotinib in patients with advanced non-small cell lung cancer (nsclc) after failure of at least one prior cytotoxic chemotherapy: a randomized, double-blind phase iii trial (zest) [abstract 8009] Proc Am Soc Clin Oncol. 2009;27:409s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=31610; cited February 12, 2010] [Google Scholar]
- 54.Herbst R, Sun Y, Korfee S. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small cell lung cancer (nsclc): a randomized, double-blind phase iii trial (zodiac) [abstract CRA8003] Proc Am Soc Clin Oncol. 2009;27:807s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=31495; cited February 12, 2010] [Google Scholar]
- 55.Di Maio M, Chiodini P, Georgoulias V. Single agent vs combination chemotherapy (ct) as second-line treatment of advanced non-small-cell lung cancer (nsclc): a meta-analysis of individual data of five randomized trials [abstract 8052] Proc Am Soc Clin Oncol. 2008;26:436s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=31189; cited February 12, 2010] [Google Scholar]
- 56.Melosky B, Agulnik J, Assi H. Retrospective practice review of metastatic non-small cell lung cancer treatment with second-line erlotinib. Curr Oncol. 2008;15:279–85. doi: 10.3747/co.v15i6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C, Shih J, Chao T. Use of BIBW 2992, a novel irreversible egfr/her2 tki, to induce regression in patients with adenocarcinoma of the lung and activating EGFR mutations: preliminary results of a single-arm phase ii clinical trial [abstract 8026] Proc Am Soc Clin Oncol. 2008;26:430s. [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=31779; cited February 12, 2010] [Google Scholar]
- 58.Herbst RS, Johnson DH, Mininberg E. Phase i/ii trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the her-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–55. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 59.Herbst RS, O’Neill VJ, Fehrenbacher L. Phase ii study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol. 2007;25:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 60.Herbst R, Stern H, Amler L. Biomarker evaluation in the phase iii, placebo-controlled, randomized BeTa Trial of bevacizumab and erlotinib for patients with advanced non-small cell lung cancer (nsclc) after failure of standard 1st-line chemotherapy: correlation with treatment outcomes [abstract B2.1] J Thorac Oncol. 2009;4(suppl 1):S323. [Available online from: journals.lww.com/jto/toc/2009/09001; cited February 12, 2010] [Google Scholar]