Abstract
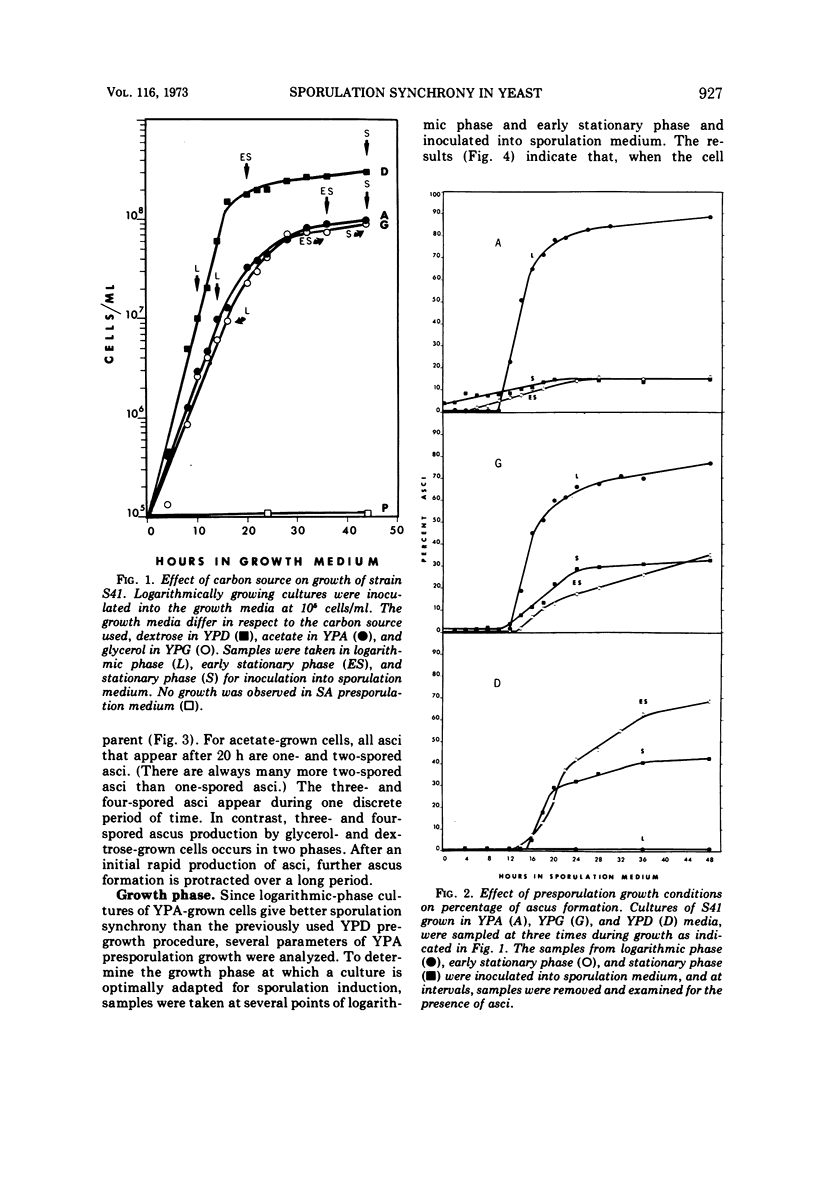
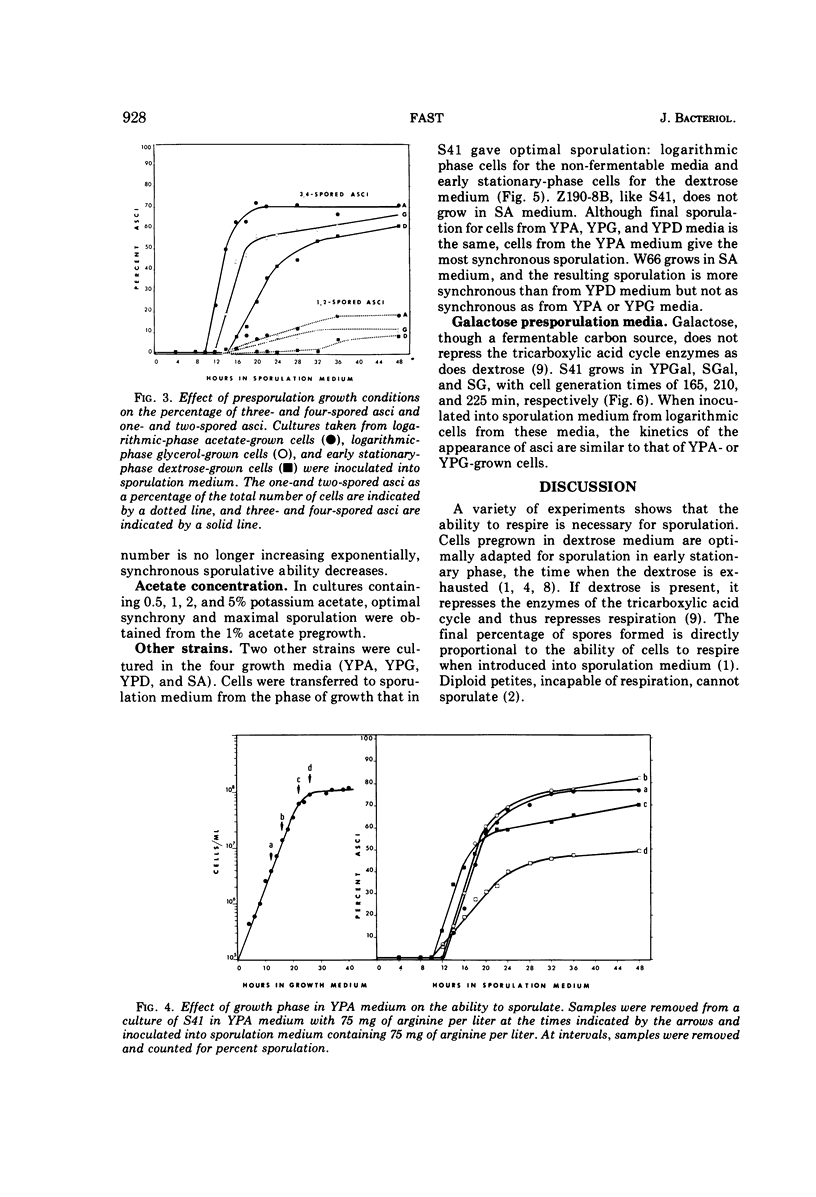
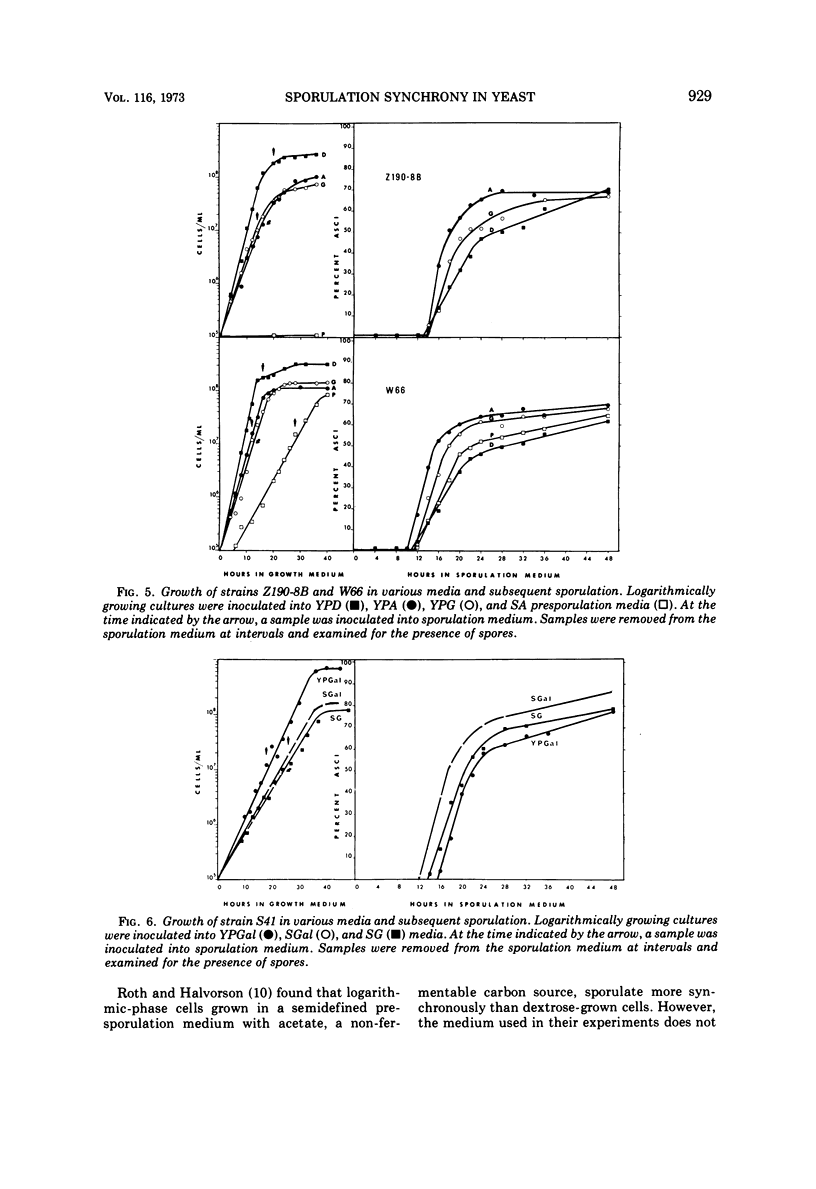
Sporulation of several strains of Saccharomyces cerevisiae grown in a variety of carbon sources that do not repress the tricarboxylic acid cycle enzymes was more synchronous than the sporulation of cells grown in medium containing dextrose which does repress those enzymes. Dextrose-grown cells showed optimal sporulation synchrony when inoculated into sporulation medium from early stationary phase when the dextrose in the medium is exhausted. Logarithmic-phase cells grown in either non-fermentable carbon sources (acetate and glycerol) or a fermentable carbon source that does not repress tricarboxylic acid cycle enzymes (galactose) sporulated more synchronously than the early stationary-phase dextrose cells. Attempts were made to sporulate cells taken from both complex and semidefined media. The semidefined acetate medium failed to support the growth of a number of strains. However, cells grown in the complex acetate medium, as well as both complex and semidefined glycerol and galactose media, sporulated with better synchrony than did the dextrose-grown cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. The genetic control of sporulation in Saccharomyces. I. The isolation of temperature-sensitive sporulation-deficient mutants. Genetics. 1969 Jan;61(1):79–89. doi: 10.1093/genetics/61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Cell cycle dependency of sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Mar;109(3):1027–1033. doi: 10.1128/jb.109.3.1027-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach-Keup G., Ehrenberg M. Eingluss des Phasenstatus der Vorkulturzellen auf die Ascosporenbildung von Saccharomyces cerevisiae. Arch Mikrobiol. 1971;78(1):17–24. [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G., Piñon R., Salts Y. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res. 1972 Nov;75(1):207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]
- Takano I., Oshima Y. Allelism tests among various homothallism-controlling genes and gene systems in Saccharomyces. Genetics. 1970 Feb;64(2):229–238. doi: 10.1093/genetics/64.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]



