Abstract
Hepatocellular carcinoma (hcc) is an uncommon tumour, but its incidence is increasing in Canada and elsewhere. Currently, there are no Canadian recommendations for diagnosis and treatment of hcc, and possible options may have regional limitations. A consensus symposium was held in the Ottawa region to consider current diagnostic and management options for hcc. These recommendations were developed:
Diagnosis—with adequate imaging, a biopsy is not required pre-surgery, but is required before the start of systemic therapy; lesions smaller than 1 cm should be followed and not biopsied; repeat biopsies should be core tissue biopsies; magnetic resonance imaging is preferred, but triphasic computed tomography imaging can be useful
Resection—recommended for localized hcc
Radiofrequency ablation—recommended for unresectable or non-transplantable hcc; should not be performed in the presence of ascites
Trans-arterial chemoembolization (tace)—doxorubicin with lipiodol is the agent of choice; trans-catheter embolization is an alternative for patients if tace is not tolerated or is contraindicated
Medical management—first-line sorafenib should be considered the standard of care
Transplantation—suitable patients meeting Milan criteria should be assessed for a graft regardless of other treatments offered
The authors feel that the recommendations from this consensus symposium may be of interest to other regions in Canada.
Keywords: hcc, hepatoma, treatment, regional consensus guideline
1. INTRODUCTION
A consensus symposium on hepatocellular carcinoma (hcc) was held in Ottawa, Ontario, in September 2008 to consider current diagnostic and therapeutic options for hcc. The framework of these deliberations is likely to be of interest in other parts of Canada where variability in access to imaging, pathology testing, and treatment expertise make it helpful to develop a regional approach. An approach to standards of cancer care is considered appropriate if it is in the context of local resources and needs.
2. EPIDEMIOLOGY AND PATHOGENESIS
Worldwide, hcc is the sixth most common cancer and the third leading cause of cancer-related death 1. The incidence of hcc varies widely geographically because of differences in the major causative factors. The most dominant risk factor for development of hcc is underlying liver cirrhosis 2. Regardless of cirrhotic causation, hcc develops 80%–90% of the time in patients who have cirrhosis 2. Primary viral infection with the hepatitis B or C virus and high alcohol intake are associated with the highest risk of developing cirrhosis and thus hcc 3. Most recently, non-alcoholic fatty liver disease (nafld)—and associated obesity and diabetes—has emerged as a risk factor for hcc 4,5.
Asia and sub-Saharan Africa have the highest incidences of hcc—attributable to the high rate of chronic hepatitis B viral infection 6. North America, Western Europe, and Australia are considered low-incidence regions, although their incidence of hcc is rising. In the United States, the rate of hcc has doubled since the start of the 1990s, with the age-specific incidence progressively shifting toward a younger population 4,7. This increase in the rate of hcc has been attributed to the large pool of people with longstanding chronic hepatitis C infection contracted either domestically or in areas in which hepatitis C is endemic, and to the increased prevalence of nafld 4,8–10.
If diagnosed in its early stages, hcc is amenable to potentially curative treatment with surgery (resection with partial hepatectomy or liver transplantation) and locoregional therapy [radiofrequency ablation, trans-catheter embolization (tae), trans-arterial chemoembolization (tace)]. However, for patients with advanced—that is, unresectable—disease, the goal has been palliative treatment to prolong survival and control symptoms.
3. PATHOLOGY DIAGNOSIS OF HCC
A high level of serum alpha fetoprotein (>400 ng/mL) in the presence of a solid hypervascular liver lesion is highly suggestive of hcc. Although differentiating regenerative nodules from other nodules in the liver remains a challenge, advances in cross-sectional imaging have improved the characterization of focal liver lesions, greatly reducing the requirement for a liver biopsy. Still, cross-sectional imaging remains inaccurate for diagnosing small malignant tumours, especially tumours less than 2 cm in diameter. If the diagnosis remains in question, biopsy may be required to make a definitive diagnosis. In such cases, positive results of a fine-needle aspiration (fna) biopsy are helpful; however, negative fna results should be interpreted with caution. Patients with negative fna biopsy should undergo a second biopsy or repeated computed tomography (ct) or magnetic resonance imaging (mri) investigations, or both.
Compared with fna, core biopsy may provide more pathologic information because, in addition to cytologic features, architecture can be evaluated. The cells of hcc grow in a pattern that mimics normal liver, most often producing a trabecular pattern with thickened cords (more than 3 cells) separated by vascular sinusoids. Less common patterns are pseudoglandular, solid, scirrhous, and hcc with clear cells. Fibrolamellar hcc occurs mostly in young people and shows abundant intercellular fibrosis and pleomorphic nuclei. Grading of hcc can be done on the basis of nuclear features alone, from grade 1 (well differentiated) to grade 4 (poorly differentiated).
On fna and small core biopsy, a diagnosis of high-grade hcc is usually straightforward; however, well-differentiated hcc is difficult to differentiate from adenoma or regenerative and dysplastic nodules. The cells of well-differentiated hcc are very similar to those of normal liver, showing only minimal nuclear irregularity, a slightly higher N:C ratio, and abundant eosinophilic cytoplasm. Numerous currently available immunostains provide valuable diagnostic information to assist pathologists in the diagnosis of difficult cases. These include, but are not limited to, hepatocyte paraffin 1 (which stains the cytoplasm of hepatocytes in 90% of hccs), alpha fetoprotein (positive in 40%–50% of hccs), CD34 and CD10 (which stain diffusely the endothelial cells surrounding the trabeculae of hccs), and polyclonal carcinoembryonic antigen (which stains the bile canaliculi, in both normal liver and hcc). The most recent antibody added to the panel is glypican-3 (gpc3), a membrane-bound proteoglycan that is overexpressed in hcc, but undetectable in normal liver or other primary benign or malignant hepatic lesions. In a recent study, gpc3 analysis in cytology material demonstrated a sensitivity of 83% and a specificity of 96% for detecting hcc 11 as compared with adenoma.
Selected immunostains, in addition to the morphologic and clinical features, can be very helpful in establishing the diagnosis of hcc in difficult cases.
3.1. Recommendations
If a patient presents with classical imaging and is a candidate for surgery, then biopsy is not required.
If a patient has unresectable hcc and is being considered for systemic therapy, then biopsy is required.
A lesion smaller than 1 cm should not be biopsied, but should be followed at 3-month intervals (changing to 6-month intervals if the lesion remains stable).
Repeat biopsies should be core tissue biopsies, rather than fnas.
4. DIAGNOSTIC IMAGING IN HCC
Imaging has numerous roles in hcc, including screening, diagnosis, staging, and follow-up. A recently published systematic review used a computerized decision-analytic model to compare various surveillance strategies for early diagnosis of hcc 12. Based on the assumptions used in the model, the most effective surveillance strategy is a combination of testing for alpha fetoprotein and ultrasound imaging performed every 6 months. Compared with no surveillance, this strategy was estimated to more than triple the number of people with operable hcc tumours at time of diagnosis and to almost halve the number of deaths from hcc. The cost-effectiveness of various surveillance strategies for hcc varies depending on the causation involved. Screening for hcc with triphasic ct is a cost-effective strategy in transplant-eligible patients with cirrhosis secondary to chronic hepatitis C viral infection 13.
Diagnosis of hcc depends heavily on imaging characteristics; biopsy is generally not required before surgery. Ultrasound plays a key role in the detection of hcc, being widely available, relatively inexpensive and easy to perform, but of low sensitivity for identifying additional small nodules 14. A 2006 systematic review and meta-analysis pooled data from fourteen ultrasound studies, and found sensitivity to be 60%, and specificity to be 97% 15. In the same systematic review, pooled data from ten helical ct studies indicated that ct has better sensitivity than ultrasound (68% vs. 60%) and similar specificity (93%). Of three imaging techniques, mri had the best sensitivity at 81% and a similar specificity of 85% (pooled data from nine mri studies) 15.
In addition to assisting with the detection of hepatic metastases, intraoperative ultrasound (ious) has evolved into a valuable tool for a final evaluation of operability during surgical exploration. In a study by Silberhumer et al. that compared liver imaging with histopathology results, the sensitivity of ious was 92%–99%, and surgical strategy was changed based on the ious results in 10% of cases 16. Thus, ious is highly sensitive and remains a mandatory tool for evaluating patients undergoing liver surgery.
4.1. Recommendations
The ideal imaging modality is mri, which should be performed as early in the disease course as possible.
Triphasic ct scanning can be useful for preoperative planning.
5. STAGING
To date, no single staging system for hcc has been universally accepted. The most widely accepted staging systems include tumour–node–metastasis (TNM), Okuda, the Cancer of the Liver Italian Program score, and the Barcelona Clinic Liver Cancer (bclc) staging classification.
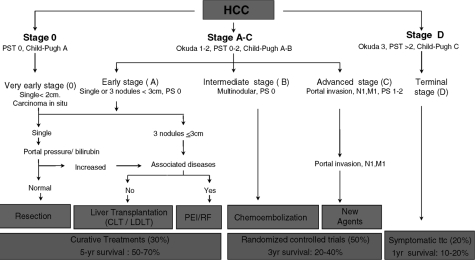
Most of these models describe the severity of the underlying liver disease, the size of the tumour, extension of the tumour into adjacent structures, and the presence of metastases. The bclc classification uses parameters related to tumour stage, liver function status, physical status, and cancer-related symptoms, and it brings those four parameters into a treatment algorithm (Figure 1). In comparative studies, bclc was shown to be a more superior prognostic model in patients undergoing surgical therapy and the best suited for treatment guidance in patients who could benefit from curative therapies 18–22.
FIGURE 1.
Barcelona Clinic Liver Cancer staging and treatment schedule. pst = performance status test. Adapted from Llovet et al. 200317.
5.1. Recommendations
Multiple staging classification systems for hcc are accepted.
The bclc classification has been shown to be a superior for predicting prognosis and for guiding therapy in early-stage disease.
6. ROLE OF RESECTION IN HCC
Hepatic resection is widely accepted as the primary treatment for patients with localized hcc, even in selected patients with cirrhosis. Accordingly, estimates suggest that only 15%–30% of patients with hcc are candidates for resection at the time of presentation 23. As a result of advances in patient selection and surgical resection procedures, particularly in cirrhotic patients, the perioperative mortality in hcc is below 3%, and 5-year survival rates are at least 50% 24,25. This 5-year survival represents a significant improvement from earlier years, when 5-year survival for patients undergoing hepatectomy for hcc was 12%–20% 26. Long-term survival remains unsatisfactory, however, because of the high incidence of tumour recurrence (the main cause of poor prognosis), which ranges from 60% to 100% at 5 years 27.
Numerous studies have evaluated the factors that could improve surgical outcomes and disease-free survival. Specifically, these studies retrospectively analyzed patients with liver resection for hcc to glean information on the prognostic values of clinical, surgical, pathologic, and biologic factors affecting long-term outcome and intrahepatic recurrence 26–31. For example, in a retrospective analysis, Ramacciato et al. reported that viral cause of cirrhosis, presence of multiple nodules, and vascular invasion negatively affected recurrence rate and long-term survival 30. Similarly, for 320 patients with hcc who underwent hepatectomy in Japan, Taniai et al. 31 reported an overall 5-year disease-free survival rate of 45.0% in the group with smaller hccs and 33.6% in the group with hccs of diameter 10 cm or greater. This finding led the authors to suggest that cirrhotic patients with huge hccs (that is, ≥10 cm) and both macrovascular invasion and multiple tumours may not be appropriate candidates for hepatic resection 31. In general, the conclusion of the various studies has been that selection of ideal candidates for surgical resection involves adequate assessment of a number of factors, including liver function (that is, Child–Pugh stage) and level of tumour extension.
6.1. Recommendation
Hepatic resection should be considered the primary treatment for patients with localized hcc.
7. ROLE OF LIVER TRANSPLANT IN HCC
By resection of the whole liver, liver transplantation has the advantage of treating the underlying liver disease and removing any undiagnosed hcc. Recent studies have demonstrated excellent outcomes in carefully selected patients with limited hcc disease who were treated with liver transplantation. In a landmark study, Mazzaferro et al. found that patients with small, unresectable tumours had recurrence-free survival rates similar to those in patients undergoing liver transplantation for non-malignant liver disease 32. Their results formed the basis of the Milan criteria for liver transplantation, which advocates liver grafts for potential recipients who have 1 lesion 5 cm or smaller, or up to 3 lesions each 3 cm or smaller.
No prospective randomized controlled trials (rcts) have compared hepatic resection and liver transplantation for patients with hcc. In a 2002 review, Wong summarized the results of trials that retrospectively compared liver resection with transplantation in patients with hcc 33. In eight trials published between 1995 and 2001, the 5-year survival was 35%–51% for liver resection, compared with 60%–72% for liver transplantation. In nine studies published between 1991 and 2001, the recurrence rates were 19%–65% for liver resection, compared with 0%–43% for liver transplantation. Although the studies varied with regard to length of follow-up, each individual study demonstrated a lower recurrence rate with transplantation.
7.1. Recommendation
Patients with hcc who meet the Milan criteria and who are otherwise deemed candidates for transplantation should be assessed for transplantation regardless of other treatments offered.
8. ROLE OF RADIOFREQUENCY ABLATION IN HCC
Radiofrequency ablation (rfa) has rapidly become one of the treatments of choice for patients with hcc who are not candidates for resection or transplantation. In patients with hcc awaiting liver transplantation, rfa has also been evaluated as bridge therapy, and in patients with early, non-surgical hcc, rfa is considered more effective than percutaneous ethanol injection with respect to local recurrence and overall and disease-free survival 34,35. A recent rct compared rfa and surgical resection in patients with a solitary resectable hcc ≤5 cm and found similar overall and disease-free survival rates, suggesting that rfa may be as effective as resection in this patient cohort 36. Percutaneous ethanol injections are also a local control option that may be safer than rfa in tumours near major blood vessels 37.
Unfortunately, local recurrence at the treatment site after rfa is not an uncommon observation. In a retrospective study, Lam et al. found that, at 2 years’ median follow-up, risk of local recurrence was 13%, and tumour size greater than 2.5 cm was the only independent risk factor for local recurrence. No notable difference in overall survival was evident between patients with and without local recurrence 38.
Major complications with rfa are thought to be rare and mostly self-limiting. In a recent study of 218 patients treated with rfa for a single small hcc, the rate of major complications was 1.8% 39.
8.1. Recommendations
In the presence of ascites, rfa increases the risk of seeding and should not be performed. Consider drainage of the ascites before rfa.
Needle biopsy before rfa increases the risk of seeding and should be avoided; however, it may be performed as part of the rfa procedure.
Imaging by ct post rfa must be labelled as such and assigned to an appropriate (rfa-experienced) radiologist.
Note: In Ottawa, the first follow-up ct imaging is done 28 days following the rfa procedure to avoid interpretation errors from immediate post-rfa inflammatory changes.
9. ROLE OF REGIONAL CHEMOTHERAPY IN THE MANAGEMENT OF HCC
The most widely used primary treatment for patients with unresectable hcc is tae to induce tumour necrosis. Materials commonly used to induce tumour-vessel embolization include microspheres and Gelfoam (Pfizer, New York, NY, U.S.A.) particles. The tace procedure combines selective embolization of tumour vessels with administration of chemotherapeutic drugs including doxorubicin, cisplatin, and mitomycin, alone or in combination. Lipiodol is often also added to the chemoembolization region to enhance the antitumour effect of the drugs by prolonging their contact with tumour cells. For patients with unresectable primary liver cancer, tace is intended as a palliative treatment; for patients awaiting liver transplantation, it is a bridging therapy. More recently, small trials have investigated the utility of using doxorubicin beads in the tace procedure 40.
Two meta-analyses demonstrated that tace significantly improves overall survival in individuals with unresectable hepatocarcinoma 41,42, although treatment with tamoxifen did not modify the survival of patients with advanced disease 42. A third meta-analysis failed to demonstrate a significant survival advantage for therapeutic embolization over supportive care alone in patients with unresectable hcc 43. Geschwind and colleagues concluded that existing survival data from rcts are of poor quality, and the low numbers of patients in these trials eliminate the possibility of drawing meaningful conclusions regarding the effect of tace on patient survival 43.
The role of tace remains controversial, with uncertain efficacy when compared with tae alone in patients with inoperable disease. Marelli et al. performed a systematic review to evaluate whether embolization alone confers a survival advantage over tace in patients with hcc. Although a meta-analysis of nine rcts confirmed that tace improves survival, a meta-analysis of three rcts comparing tace with tae (n = 412) failed to demonstrate a survival difference between the two techniques 44. Marelli and colleagues concluded that further trials comparing these treatments are needed to determine unequivocally if tace provides a survival advantage over tae alone 44.
9.1. Recommendations
If tace is used, doxorubicin (with lipiodol) is the chemotherapeutic agent of choice.
Bland embolization may be the treatment modality of choice, especially when tace is contraindicated or not tolerated.
The role of embolization should not depend on sorafenib (localized versus systemic therapy).
Most patients with hcc present with advanced disease; only 10%–20% of patients are candidates for curative surgery. For patients with advanced disease, the prognosis is poor, with a median survival of only 4 months 45. Patients classified bclc stage C are candidates for systemic therapy; those classified stage D, for palliative care only 20.
In patients not eligible for surgery or declining liver surgery, systemic treatment options are available, but have traditionally been limited and minimally effective 46. In 2007, however, the antitumour agent sorafenib, widely used for the treatment of primary kidney carcinoma 47, was found to produce a clinically meaningful improvement in survival in patients with advanced hcc 48.
Sorafenib is an orally active multikinase inhibitor with activity against tyrosine and serine/threonine kinases, key components in hepatocarcinogenesis 49,50. It also exerts a direct antitumour effect by raf kinase inhibition and an anti-angiogenic effect by vascular endothelial growth factor receptor inhibition.
In the randomized double-blind placebo-controlled phase iii sharp trial, 400 mg of oral sorafenib administered twice daily significantly improved survival by 3 months in patients with advanced hcc (n = 299; placebo group, n = 303). In addition, the median time to disease progression was significantly longer in patients receiving sorafenib than in those receiving placebo (5.5 months vs. 2.8 months). Treatment with sorafenib twice daily was generally well tolerated with a manageable adverse event profile 48. The sharp trial was stopped at the second planned interim analysis because of the survival advantage favouring the sorafenib arm. Sorafenib was also shown to be effective in patients from the Asia–Pacific region 51. Sorafenib was subsequently approved by both the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of patients with advanced hcc 46. Current U.S. treatment guidelines recommend sorafenib as a first-line treatment option in patients with unresectable hcc who are classified Child–Pugh A or B 46.
Recently, combination therapy with sorafenib and doxorubicin has shown promise for the treatment of patients with advanced hcc. The randomized double-blind phase ii clinical trial of this therapy (n = 96) demonstrated that oral sorafenib (400 mg) twice daily combined with intravenous doxorubicin 60 mg/m2 every 21 days has potential in patients with advanced hcc 52.
9.2. Recommendation
First-line therapy with sorafenib should be considered the standard-of-care treatment for advanced hcc.
Patients should still be enrolled in clinical trials, where available.
10. SUMMARY
Hepatocellular carcinoma is a malignancy that affects a large number of patients, and the incidence of this disease can be expected to increase in Canada and throughout the world. Treatment and diagnostic changes have improved prognosis for many patients with hcc. As seen in the present paper, the optimal method for improving the care of these patients is multidisciplinary collaboration. Improvements in therapies such as hepatic resection and hepatic transplantation have improved the cure rate of this disease. As well, advances in the medical treatment of hcc and in interventional techniques such as rfa have significantly improved the palliative treatment of hcc.
11. CONFLICT OF INTEREST DISCLOSURE
This consensus meeting was funded by an unrestricted educational grant from Bayer.
12. ACKNOWLEDGMENTS
The authors thank Isabella Steffensen for editorial assistance.
13. REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Davis GL, Dempster J, Meler JD. Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent) 2008;21:266–80. doi: 10.1080/08998280.2008.11928410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005;3:27. doi: 10.1186/1477-7819-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Ohki T, Tateishi R, Sato T. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–64. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB. Epidemiology of hepatocellular carcinoma in U.S.A. Hepatol Res. 2007;37(suppl 2):S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 8.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–9. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Roberts SK, Kemp W. Hepatocellular carcinoma in an Australian tertiary referral hospital 1975–2002: change in epidemiology and clinical presentation. J Gastroenterol Hepatol. 2007;22:191–6. doi: 10.1111/j.1440-1746.2006.04459.x. [DOI] [PubMed] [Google Scholar]
- 11.Ligato S, Mandich D, Cartun RW. Utility of glypican-3 in differentiating hepatocellular carcinoma from other primary and metastatic lesions in fna of the liver: an immunocytochemical study. Mod Pathol. 2008;21:626–31. doi: 10.1038/modpathol.2008.26. [DOI] [PubMed] [Google Scholar]
- 12.Thompson Coon J, Rogers G, Hewson P. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1–206. doi: 10.3310/hta11340. [DOI] [PubMed] [Google Scholar]
- 13.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost–utility analysis. Am J Gastroenterol. 2003;98:679–90. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:75–80. doi: 10.2214/ajr.179.1.1790075. [DOI] [PubMed] [Google Scholar]
- 15.Colli A, Fraquelli M, Casazza G. Accuracy of ultrasonography, spiral ct, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–23. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 16.Silberhumer GR, Steininger R, Laengle F, Muehlbacher F, Zacherl J, Pokieser P. Intraoperative ultrasonography in patients who undergo liver resection or transplantation for hepatocellular carcinoma. Surg Technol Int. 2004;12:145–51. [PubMed] [Google Scholar]
- 17.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 18.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrero JA, Fontana RJ, Barrat A. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 20.Cillo U, Vitale A, Grigoletto F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–31. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 22.Cillo U, Bassanello M, Vitale A. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124–31. doi: 10.1016/j.jhep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Emond JC, Samstein B, Renz JF. A critical evaluation of hepatic resection in cirrhosis: optimizing patient selection and outcomes. World J Surg. 2005;29:124–30. doi: 10.1007/s00268-004-7633-8. [DOI] [PubMed] [Google Scholar]
- 24.Grazi GL, Ercolani G, Pierangeli F. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–8. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 26.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon’s role in long-term survival. Arch Surg. 1999;134:1124–30. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 27.Ercolani G, Grazi GL, Ravaioli M. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–43. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998;82:1028–36. doi: 10.1002/(sici)1097-0142(19980315)82:6<1028::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Benzoni E, Lorenzin D, Favero A. Liver resection for hepatocellular carcinoma: a multivariate analysis of factors associated with improved prognosis. The role of clinical, pathological and surgical related factors. Tumori. 2007;93:264–8. doi: 10.1177/030089160709300306. [DOI] [PubMed] [Google Scholar]
- 30.Ramacciato G, Mercantini P, Corigliano N. Hepatic resections for hepatocellular carcinoma (hcc): short and long-term results on 106 cirrhotic patients. J Exp Clin Cancer Res. 2003;22:233–41. [PubMed] [Google Scholar]
- 31.Taniai N, Yoshida H, Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:410–16. doi: 10.1007/s00534-007-1317-3. [DOI] [PubMed] [Google Scholar]
- 32.Mazzaferro V, Regalia E, Doci R. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 33.Wong LL. Current status of liver transplantation for hepatocellular cancer. Am J Surg. 2002;183:309–16. doi: 10.1016/s0002-9610(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 34.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–6. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doffoel M, Bonnetain F, Bouche O. Multicentre randomised phase iii trial comparing tamoxifen alone or with transarterial lipiodol chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Federation Francophone de Cancerologie Digestive 9402) Eur J Cancer. 2008;44:528–38. doi: 10.1016/j.ejca.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Chen MS, Li JQ, Zheng Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20–4. [PubMed] [Google Scholar]
- 38.Lam VW, Ng KK, Chok KS. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–9. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Livraghi T, Meloni F, Di Stasi M. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–9. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 40.Malagari K. Drug-eluting particles in the treatment of hcc: chemoembolization with doxorubicin-loaded DC Bead. Expert Rev Anticancer Ther. 2008;8:1643–50. doi: 10.1586/14737140.8.10.1643. [DOI] [PubMed] [Google Scholar]
- 41.Camma C, Schepis F, Orlando A. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 42.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 43.Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26:344–9. doi: 10.1097/01.COC.0000020588.20717.BB. [DOI] [PubMed] [Google Scholar]
- 44.Marelli L, Stigliano R, Triantos C. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 45.Yeo W, Mok TS, Zee B. A randomized phase iii study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (piaf) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–8. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 46.Simpson D, Keating GM. Sorafenib: in hepatocellular carcinoma. Drugs. 2008;68:251–8. doi: 10.2165/00003495-200868020-00007. [DOI] [PubMed] [Google Scholar]
- 47.Bayer Inc. Product Monograph: Nexavar (Sorafenib) Toronto, ON: Bayer; Sep 16, 2007. [Google Scholar]
- 48.Llovet JM, Ricci S, Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Cao Y, Chen C. Sorafenib blocks the Raf/Mek/Erk pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm S, Carter C, Lynch M. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 51.Cheng AL, Kang YK, Chen Z. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 52.Abou-Alfa GK, Johnson P, Knox J. Final results from a phase ii (phii), randomized, double-blind study of sorafenib plus doxorubicin (s+d) versus placebo plus doxorubicin (p+d) in patients (pts) with advanced hepatocellular carcinoma (ahcc) [abstract 128]. Proc Am Soc Clin Oncol Gastrointest Cancer Symp 2008; [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=53&abstractID=10215; cited March 10, 2010] [Google Scholar]