Abstract
The treatment of metastatic breast cancer is challenging. We recently assisted in the development of targeted therapies (in combination with chemotherapy or as monotherapy) that have improved results for selected groups of patients. Lapatinib is a dual tyrosine kinase inhibitor that has shown efficacy in breast cancer. Consequently, its use has been approved, in combination with capecitabine, for the treatment of disease positive for the human epidermal growth factor receptor. Here, we present a case of complete clinical response to a combination of lapatinib and gemcitabine that was maintained for 1 year.
Keywords: Breast cancer, lapatinib, gemcitabine, complete response
1. INTRODUCTION
Metastatic disease ultimately develops in 20%–30% of all patients with breast cancer. Anthracyclines and taxanes are increasingly being used as adjuvant treatment early in the course of breast cancer, and they are the foundation of treatment for metastatic disease. There is a pressing need for novel therapies, including combination therapies, in this setting of drug resistance or disease progression after anthracycline and taxane therapy. Alternatives to anthracyclines are also greatly needed.
Epidermal growth factor receptors 1 (egfr) and 2 [her2 (ErbB2)] are members of the type i receptor tyrosine kinase family and have been key targets for cancer therapy because of their overexpression in a variety of neoplastic tissues 1. When ligands bind to type i receptors, dimerization occurs, causing a conformational change in the receptor that activates the kinase domain and results in autophosphorylation and initiation of divergent signal transduction cascades 2. Although her2 is generally thought to lack a high-affinity ligand, it participates in signalling by heterodimerization with ligand-bound members of the type i receptor family. Signalling by egfr and her2 is known to occur through the ras pathway, stimulating cell division 3, and through the phosphoinositide 3 kinase (pi3k) pathway, resulting in cell growth and survival 1. Because these effects on cell growth and survival depend on the catalytic activity of egfr and her2, it is thought that inhibition of this activity might provide a therapeutic opportunity for patients with tumours expressing elevated levels of egfr and her2.
Lapatinib is a small-molecule competitive inhibitor of tyrosine kinases that binds reversibly to the cytoplasmic adenosine triphosphate binding site in the kinase domains of egfr and her2, thereby inhibiting receptor phosphorylation—that is, activation 4. Lapatinib induces growth arrest or apoptosis in egfr- and her2-dependent tumour cell lines. This marked inhibition of egfr1 and her2 results in the inhibition of mitogen-activated protein kinase and Akt kinase. In fact, experimental evidence suggests that lapatinib may be superior to egfr-specific tyrosine kinase inhibitors in inhibiting pi3k in vitro and in vivo. Complete inhibition of activated Akt in her2-overexpressing cells correlated with a strong increase in apoptosis 5. These observations were also reproduced in vivo in human tumour xenografts 6,7. Lapatinib’s inhibition of Akt may be of therapeutic interest as monotherapy or as an enhancer of the antitumour activity of chemotherapeutics for which Akt may mediate chemoresistance.
2. CASE REPORT
A 62-year-old woman who had been diagnosed with cancer in the right breast was seen because of pain in her right arm and the presence of a right axillary mass, which she had noticed a month before the consultation, in September 2007.
On physical examination, we found a mass of 30×50 mm in the right breast and a right axillary mass that could not be measured. Homolateral supraclavicular lymph nodes of 40×50 mm were also detected.
Breast echography revealed a 29-mm mass with microcalcifications in the upper external quadrant and a lymph node conglomerate. The breast was biopsied. The pathology study showed a high-grade infiltrating ductal carcinoma, negative for the estrogen and progesterone receptors (er−/pr−), and positive for her2 (+++). Magnetic resonance imaging detected a 28×26×20-mm lesion in the upper exterior quadrant and another lesion of 30×21 mm in the lower exterior quadrant of the right breast, and multiple right axillary lymph nodes. Neither computed tomography (ct) imaging nor a bone scan found lesions suspicious for metastatic dissemination.
The patient received chemotherapy (doxorubicin 60 mg/m2 and yclophosphamide 600 mg/m2) every 21 days, with 4 cycles planned. That treatment was followed by docetaxel 75 mg/m2 with trastuzumab 8 mg/kg (first dose) and 6 mg/kg in subsequent administrations, every 21 days, for a total of 4 cycles. The ct imaging performed after chemotherapy revealed a partial response of the axillary metastases. Physical examination did not reveal any breast tumour.
After treatment completion, this patient was sent to surgery. In January 2008, a mastectomy with lymph node dissection was performed. Pathology findings described a 4-mm infiltrating ductal carcinoma only. Of the 6 nodes resected with extension to the perinodal tissues, metastases were found in 5. The patient continued with 6 mg/kg trastuzumab every 3 weeks. She did not receive adjuvant radiotherapy.
In March 2008, the patient detected a right subscapular tumour and growth of the supraclavicular and right axillary lymph nodes. At that time, she came to our hospital, and we performed a pathology review.
Physical examination revealed a right supraclavicular mass measuring 50×40 mm, a right subscapular mass measuring 30×40 mm, and enlargement of some right axillary lymph nodes measuring up to 20 mm.
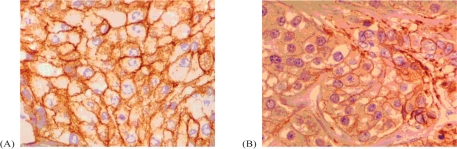
Neck–thorax and abdominal ct imaging revealed right supraclavicular adenopathies up to 14 mm, a subcutaneous periscapular nodule of 14 mm, and two smaller nodules (Figure 1, left panel). The bone scan did not reveal bone dissemination. The patient’s performance status (ps) was established at 2, and a biopsy of the supraclavicular lymph node confirmed metastatic breast dissemination positive for her2 (+++) and moderate (35%) for egfr (Figure 2).
FIGURE 1.
Computed tomography (ct) imaging. Left panel: Time of consultation, March 2008. Right panel: After treatment, in July 2008.
FIGURE 2.
(A) Positivity (+++) for the human epidermal growth factor receptor 2 (her2). (B) Moderate expression (35%) of epidermal growth factor 1.
The patient was considered to have progressive disease with primary resistance to trastuzumab. After discussing treatment options with her, we decided to offer daily lapatinib 1250 mg to be taken orally (5 tablets). When the patient was reluctant to take so many tablets, we instead offered a combination of lapatinib and gemcitabine 1000 mg/m2 on days 1 and 8 every 21 days. A compassionate-use agreement under Spanish rules was requested.
The patient began treatment at the end of March 2008, after authorization had been received. After 2 cycles, we reduced the daily dose of lapatinib to 1000 mg as a result of grade 2 diarrhea, which could not be controlled with codeine. No further relevant side effects were recorded after the dose reduction. At the beginning of every cycle, the patient was examined, and we confirmed a progressive reduction of all the masses that were present at the first physical examination.
Control ct imaging of neck–thorax–abdomen carried out in July 2008 confirmed complete radiologic remission of all of the described lesions [Figure 1 (right panel)]. At that time, the patient had a ps of 1 and treatment was continued.
Imaging by ct in May 2009 (Figure 3) showed continuation of a complete clinical response in the patient, who had a ps of 0 at that time. The complete response continued until October 2009; the patient is now in progression.
FIGURE 3.
Computed tomography (ct) imaging showing more than a year of complete radiologic response. Left panel: September 2008. Right panel: May 2009.
3. DISCUSSION
Many options are currently available for the treatment of metastatic breast cancer. The use of trastuzumab, both combined with chemotherapy and as a maintenance treatment, provides great benefit to patients with tumours positive for her2/neu. Primary resistance to trastuzumab is challenging, and the prognosis for these patients is poor.
Lapatinib is a modern tyrosine kinase inhibitor that is able to inhibit both egfr1 and her2/neu. In a phase iii randomized study, lapatinib combined with capecitabine, as compared with capecitabine alone, improved the response rate and significantly delayed disease progression (p = 0.002) in patients with metastatic her2-overexpressing breast cancer whose disease had progressed following trastuzumab-based therapy 8. Lapatinib is now approved in combination with capecitabine for the treatment of metastatic her2/neu-positive breast cancer. In breast cancer, lapatinib has shown activity independent of egfr expression 9, and such activity would probably be the case in other tumours as well. Currently, many clinical trials are exploring combinations of lapatinib with various chemotherapeutics and hormone treatments.
In the impressive work of Li et al. 10, lapatinib activity was linked to the inhibition of breast cancer stem cells, suggesting that self-renewal in cancer stem cells may be governed by her signalling, which plays an important role in both normal and cancer cell proliferation. Self-renewal, the key property of stem cells, reflects their immortality 11. Lapatinib seems to have no major side effects, illustrating its apparent ability to target cancer stem cells specifically 7. In preclinical studies, lapatinib was not cross-resistant with trastuzumab 12–14.
Gemcitabine is an active drug for the treatment of breast cancer. Gemcitabine is a pyrimidine nucleoside anti-metabolite. It is converted to difluorodeoxycytidine triphosphate, which inhibits dna synthesis by inhibiting dna polymerase and directly incorporating into the dna strand, which in turn leads to premature termination of dna chain elongation. The diphosphate intermediate of gemcitabine also inhibits ribonucleotide reductase and thereby depletes the intracellular pools of deoxyuridine monophosphates required for dna synthesis 15.
Gemcitabine has proven antitumour activity and tolerability in various malignancies, including breast cancer. Monotherapy has led to response rates of 37% in the first line 16, 26% in the second line 17, and 18% in the third line 13. Gemcitabine has a unique mechanism of action and a favourable toxicity profile, thereby limiting the risks of drug resistance related to pretreatment and of overlapping toxicity, making it an excellent agent in combination therapy. Recent phase ii and iii studies combining gemcitabine with taxanes, platinum agents, vinorelbine, anthracyclines, and 5-fluorouracil have shown higher efficacy than was seen with any of those agents alone, especially in pretreated patients. Because of its unique mechanism of action and non-overlapping toxicities, gemcitabine can be added to several chemotherapeutic agents and is not considered to be cross-resistant with taxanes or anthracyclines. Those factors constituted our major reason for choosing gemcitabine when our patient decided that she did not want to take so many pills. No published or ongoing clinical trials are currently testing this particular combination in breast cancer.
Our results raise new questions, given the immediate tumour growth after 2 cycles of maintenance therapy with trastuzumab and the proximity of that growth to surgery, which both reflect a primary resistance to trastuzumab in the tumour.
We cannot correlate the patient’s response with the moderate expression of egfr, as has been reported in the literature 18,19, but such a conclusion is tempting. A complete response was seen in only 1 patient treated with lapatinib and capecitabine in the most important lapatinib clinical trial published to date. That trial was the basis for the approval of lapatinib by the U.S. Food and Drug Administration 9. Although that trial recruited a different subset of patients, our patient, like those in the trial, had been treated with taxanes and anthracyclines, but developed metastases during an early phase of trastuzumab adjuvant therapy. Complete responses have also been reported anecdotally in patients treated with gemcitabine monotherapy.
To our knowledge, and on the basis of a literature search, our patient is the first to be treated with a combination of these two drugs. Considering that she had primary resistance to trastuzumab, this result is very exciting. We cannot exclude the existence of a synergistic effect, but we are not able to investigate it. In view of the current result, we can conclude that the combination of gemcitabine and lapatinib deserves future consideration in the basic and clinical research into breast cancer. This combination is already being investigated for the treatment of pancreatic cancer (BrUOG-PA-209), and the combination of cisplatin, gemcitabine, and lapatinib is being used for the treatment of metastatic urothelial cancer in the study on urothelial cancer by the European Organization for Research and Treatment of Cancer.
4. ACKNOWLEDGMENTS
We thank Francisco Sanchez Garcia for linguistic advice and revision.
5. REFERENCES
- 1.Krasilnikov MA. Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc) 2000;65:59–67. [PubMed] [Google Scholar]
- 2.Moyer JD, Barbacci EG, Iwata KK. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–48. [PubMed] [Google Scholar]
- 3.Ciardiello F, Caputo R, Bianco R. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor–selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–63. [PubMed] [Google Scholar]
- 4.Rusnak DW, Lackey K, Affleck K. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 5.She QB, Chandarlapaty S, Ye Q. Breast tumor cells with pi3k mutation or her2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual egfr/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–53. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 7.Xia W, Mullin RJ, Keith BR. Antitumour activity of GW572016: a dual tyrosine kinase inhibitor blocks egf activation of egfr/ErbB2 and downstream Erk1/2 and Akt pathways. Oncogene. 2002;21:6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 8.Geyer C, Forster J, Lindquist D. Lapatinib plus capecitabine for her-2–positive advanced breast cancer. N Engl J Med. 2006;55:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 9.Spector N, Blackwell K, Hurley J. EGF103009, a phase ii trial of lapatinib monotherapy in patients with relapsed/refractory inflammatory breast cancer (ibc): clinical activity and biologic predictors of response. J Clin Oncol. 2006;24:502. [Google Scholar]
- 10.Li X, Creighton C, Wong H. Decrease in tumorigenic breast cancer stem cells in primary breast cancers with neoadjuvant lapatinib [abstract 82] Breast Cancer Res Treat. 2007;106(suppl 1) [Available online at: www.abstracts2view.com/sabcs07/view.php?nu=SABCS07L_484&terms=; cited December 30, 2009] [Google Scholar]
- 11.Schmidt C. Lapatinib study supports cancer stem cell hypothesis, encourages industry research. J Natl Cancer Inst. 2008;100:694–5. doi: 10.1093/jnci/djn168. [DOI] [PubMed] [Google Scholar]
- 12.Rusnak DW, Lackey K, Affleck K. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 13.Wood ER, Truesdale AT, McDonald OB. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 14.Konecny GE, Pegram MD, Venkatesan N. Activity of the dual kinase inhibitor lapatinib (GW572016) against her-2–overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 15.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on dna synthesis. Cancer Res. 1991;51:6110–17. [PubMed] [Google Scholar]
- 16.Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase ii trial. Oncology. 2002;62:2–8. doi: 10.1159/000048240. [DOI] [PubMed] [Google Scholar]
- 17.Brodowicz T, Kostler WJ, Moslinger R. Single-agent gemcitabine as second- and third-line treatment in metastatic breast cancer. Breast. 2000;9:338–42. doi: 10.1054/brst.2000.0170. [DOI] [PubMed] [Google Scholar]
- 18.Smylie M, Blumenschein GR, Dowlati A. A phase ii multicenter trial comparing two schedules of lapatinib (lap) as first or second line monotherapy in subjects with advanced or metastatic non-small cell lung cancer (nsclc) with either bronchioloalveolar carcinoma (bac) or no smoking history [abstract 7611] Proc Am Soc Clin Oncol. 2007;25 [Available online at: meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/7611; cited August 8, 2009] [Google Scholar]
- 19.Gilmer TM, Cable L, Alligood K. Impact of common epidermal growth factor receptor and her2 variants on receptor activity and inhibition by lapatinib. Cancer Res. 2008;68:71–9. doi: 10.1158/0008-5472.CAN-07-2404. [DOI] [PubMed] [Google Scholar]