Nitric oxide (NO) is a major effector molecule in cancer prevention. A number of studies have demonstrated that NO prodrug JS-K (O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate) induces apoptotic cell death in vitro and in vivo, indicating that it is a promising new therapeutic for cancer. However, the mechanism of its tumor-killing activity remains unclear. Ubiquitin plays an important role in regulation of tumorigenesis and cell apoptosis. Our previous report has shown that inactivation of the ubiquitin system through blocking E1 (ubiquitin-activating enzyme) activity preferentially induces apoptosis in p53-expressing transformed cells. Since E1 has an active cysteine residue that could potentially interact with NO, we hypothesized that JS-K could inactivate E1 activity. E1 activity was evaluated by detection of ubiquitin~E1 conjugates through immunoblotting. JS-K strikingly inhibits ubiquitin~E1 thioester formation in cells in a dose-dependent manner with an IC50 of approximately 2 μM, whereas a JS-K analog that cannot release NO does not affect these levels in cells. Moreover, JS-K decreases total ubiquitylated proteins and increases p53 levels, which is mainly regulated by ubiquitin and proteasomal degradation. Furthermore, JS-K preferentially induces cell apoptosis in p53-expressing transformed cells. These findings indicate that JS-K inhibits E1 activity and kills transformed cells harboring wild type p53.
Nitric oxide (NO) plays a critical role in numerous signaling pathways and induces a wide variety of biological effects. Several reports have demonstrated that NO induces cell apoptosis and inhibits tumorigenesis (Ying and Hofseth, 2007). However, NO has limited solubility in water and is unstable in the presence of various oxidants. Therefore, utilizing chemical agents with stable NO release is an effective approach to analyze the function of NO. One such effective NO prodrug is JS-K.
JS-K (O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate) (Figure 1a) is a diazeniumdiolate prodrug designed to release NO when metabolized by glutathione S-transferase (GST). Several studies have shown that JS-K inhibits cell growth and invasion and induces apoptosis in a variety of tumors and leukemias in vitro (Kiziltepe et al., 2007; Liu et al., 2004; Ren et al., 2003; Shami et al., 2006; Shami et al., 2003; Simeone et al., 2008). In addition, administration of JS-K inhibits the growth of tumors, including leukemias, in animal models and significantly increases the survival of these animals (Kiziltepe et al., 2007; Shami et al., 2006; Shami et al., 2003). These findings suggest that JS-K could be a lead compound for anti-tumor therapeutics. However the mechanism of its tumor-killing activity remains unclear.
Figure 1.
JS-K inhibits ubiquitin~E1 thioester in cells
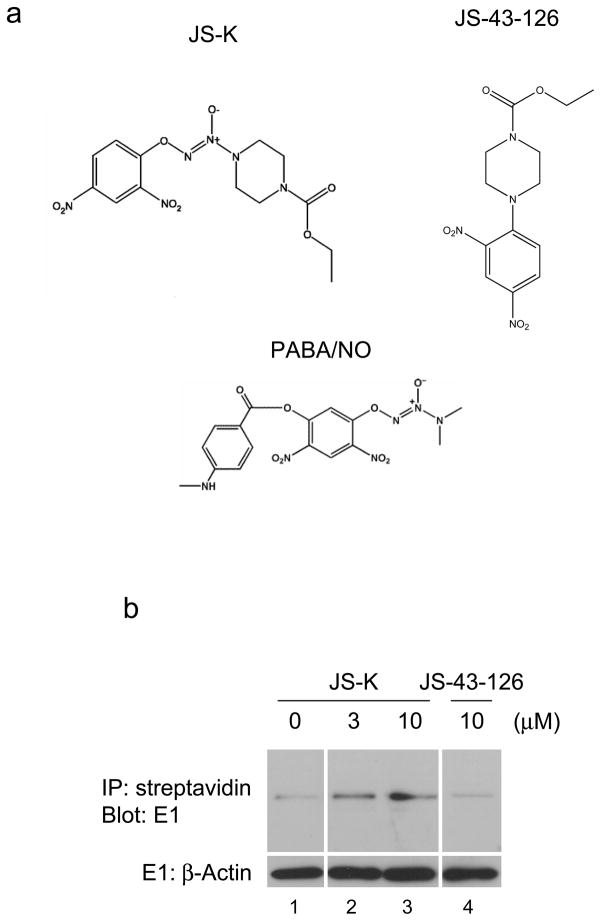
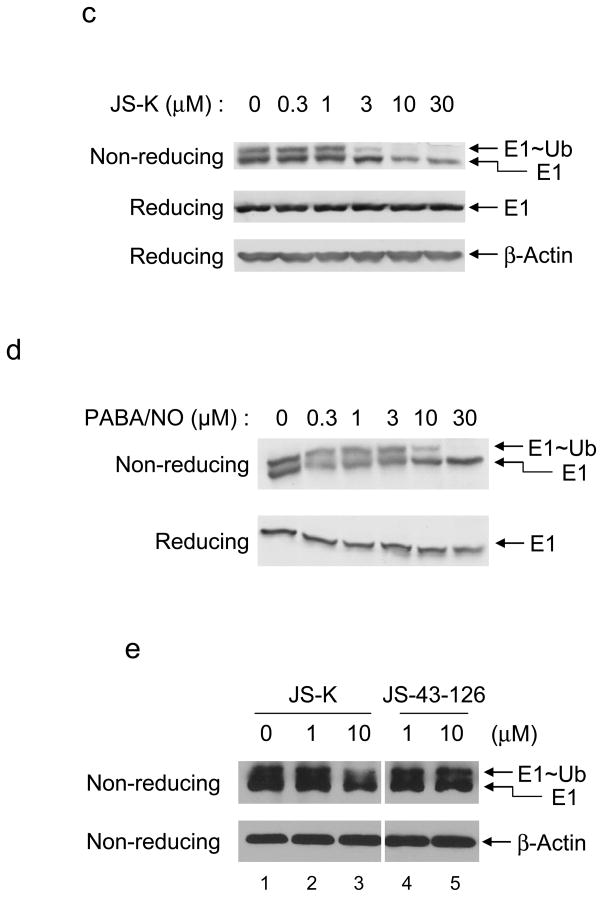
(a) Structure of JS-K, JS-43-126, and PABA/NO. (b) RPE cells were cultured as described previously (Yang et al., 2005) and then treated with JS-K or JS-43-126 for 30 min as indicated. A biotin switch assay was performed according to manufacturer’s instructions (Cayman, Ann Arbor, MI). In the top blot, the biotinylated proteins were then precipitated by streptavidin-agarose resin (Pierce, Rockford, IL). The resultant resin was washed and resolved by SDS-PAGE and immunoblotted with anti-E1 antibody (Calbiochem, La Jolla, CA). In the bottom blot, equal amounts of lysate were immunoblotted with anti-β-Actin antibody (Sigma, St. Louis, MO). (c) RPE cells were incubated with 0.3–30 μM JS-K for 30 min, and cell lysates were prepared in a urea-containing buffer (Jahngen-Hodge et al., 1997). In the top blot, samples were heated with SDS-PAGE sample buffer without DTT (non-reducing) and immunoblotted with E1 antibody. In the middle and bottom blot, samples were heated with SDS-PAGE sample buffer with DTT (reducing) and immunoblotted with E1 (middle blot) and β-Actin (bottom blot) antibodies respectively. (d) RPE cells were treated with 0.3–30 μM PABA/NO for 4 h. Total cellular E1 levels were assessed by immunoblotting. (e) RPE cells were treated for 30 min as indicated. Cell lysates were evaluated by immunoblotting.
Ubiquitin is a highly conserved 76-amino-acid protein. Modification of a target substrate with ubiquitin is catalyzed in sequential steps of three enzymes. In the first step, ubiquitin-activating enzyme (E1) forms a ubiquitin adenylate intermediate that serves as a donor of ubiquitin to an active site cysteine of theE1 in a thioester linkage. Subsequently, ubiquitin is transferred to ubiquitin-conjugating enzyme (E2), and then to ubiquitin ligase (E3). The substrates that are recognized by the 26S proteasome are usually conjugated to a lysine-48 (K48) -linked polyubiquitin chain. Ubiquitin-mediated degradation of target proteins through the proteasome pathway plays an important role in the control of numerous functions, including signal transduction, transcriptional regulation, stabilization of short-lived protein, and cell apoptosis. Abnormalities in ubiquitin-mediated processes have been observed in some pathological conditions, including malignant transformation (Hershko and Ciechanover, 1998). Moreover, our previous study has demonstrated that inactivation of the ubiquitin system through blocking E1 activity causes accumulation of p53 and preferential cell apoptosis in transformed cells and cells harboring wild type p53 (Yang et al., 2007). Therefore, controlling the ubiquitin system by blocking E1 activity could be an effective strategy for cancer therapy.
Several studies have determined that NO modifies active cysteine residues (Lipton, 1999). Since E1 has such a residue, we hypothesized that the NO prodrug JS-K could inactivate E1 activity through interaction with this residue. To investigate our hypothesis, we first examined S-nitrosylation of E1. Tert-immortalized human retinal pigment epithelial (RPE) cells were treated with JS-K or a JS-K analog JS-43-126 that cannot release NO (Figure 1a) (Simeone et al., 2008). Following this incubation, S-nitrosylation of E1 was assessed by a biotin switch assay (Jaffrey et al., 2001). As a result, E1 was readily S-nitrosylated by JS-K but not JS-43-126 (Figure 1b compare lanes 3 and 4). Next, E1 activity following JS-K treatment was evaluated by detection of ubiquitin~E1 thioesters through immunoblotting under either reducing (with DTT) or non-reducing (without DTT) conditions. JS-K markedly reduced ubiquitin~E1 thioesters in a concentration-dependent manner (Figure 1c). The IC50 of JS-K on inhibition of ubiquitin~E1 thioesters was approximately 2 μM. To confirm that NO released by JS-K inhibits E1 activity, a different NO prodrug, PABA/NO, was utilized (Saavedra et al., 2006) (Figure 1a). PABA/NO also decreased the level of ubiquitin~E1 conjugation in cells in a concentration-dependent manner (Figure 1d). To further evaluate the effect of NO on ubiquitin~E1 thioesters in cells, JS-43-126 was employed. JS-K reduced ubiquitin~E1 thioesters, whereas JS-43-126 had no effect on these levels (Figure 1e, compare lanes 3 and 5). These data indicate that JS-K blocks E1 activity in a concentration-dependent manner.
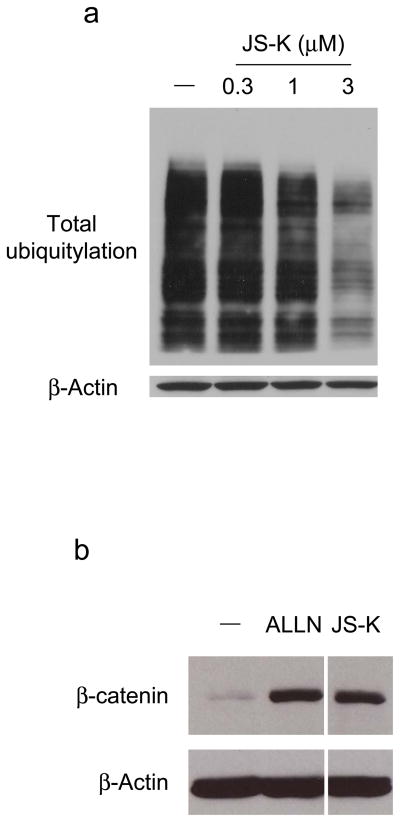
Next, the effect of JS-K on accumulation of total cellular ubiquitylated proteins was analyzed. RPE cells were exposed to JS-K, and total ubiquitylation was assessed. JS-K clearly decreased accumulation of ubiquitylated proteins in cells in a concentration-dependent manner (Figure 2a).
Figure 2.
JS-K inhibits ubiquitylation
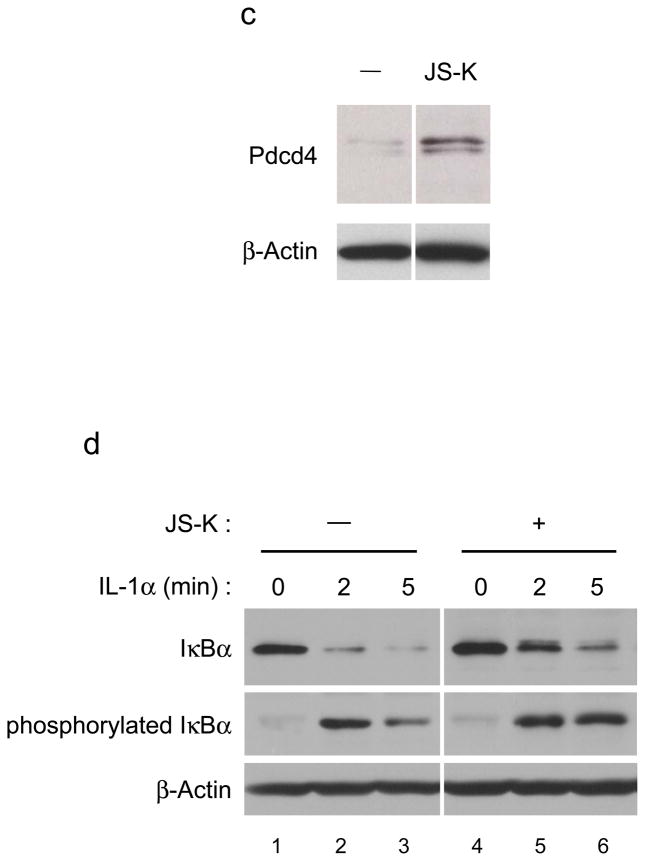
(a) JS-K decreases accumulation of total ubiquitylated proteins. RPE cells were incubated with JS-K as indicated for 24 h and lysed in RIPA buffer as described previously (Yang et al., 2005). Total ubiquitylation in cells and β-Actin were immunoblotted using anti-ubiquitin (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-β-Actin antibodies. (b) JS-K increases cellular β-catenin levels. HEK293 cells were serum starved for 17 h. After this, the cells were exposed to 20 μM ALLN (Calbiochem) or 10 μM JS-K for 2 h. Cell lysates were prepared as described previously (Giarre et al., 1998). Samples were assessed by immunoblotting using β-catenin (Santa Cruz Biotechnology) and β-Actin antibodies. (c) JS-K increases Pdcd4 levels in cells. A549 cells were treated with 10 μM JS-K for 6 h. Cell lysates were immunoblotted as indicated (Jansen et al., 2005). (d) JS-K delays degradation of IL-1α-induced IκBα and phosphorylated IκBα. HeLa cells were incubated with 10 μM JS-K for 30 min prior to treatment with 10 ng/ml IL-1α (R&D systems, Minneapolis, MN) for 2 or 5 min. Cell lysates were immunoblotted using IκBα (Santa Cruz Biotechnology), phosphorylated IκBα (Cell Signaling Technology, Danvers, MA), and β-Actin antibodies.
To further analyze the inactivation of E1 by JS-K, the degradation of β-catenin was assessed. β-catenin controls both cadherin-mediated cell adhesion and activation of Wnt target genes. In the absence of Wnt signals, β-catenin is ubiquitylated, after phosphorylation by glycogen synthase kinase 3β (GSK-3β) and casein kinase 1α (CK1α), and degraded through the proteasome pathway (Kimelman and Xu, 2006). To investigate the effect of JS-K on cellular β-catenin levels, HEK293 cells which express wild type β-catenin were employed. The cells were exposed to JS-K, and β-catenin levels in cells were analyzed by immunoblotting. JS-K accumulated cellular β-catenin similar to that seen with proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal (ALLN) (Adams, 2004) (Figure 2b).
Programmed Cell Death 4 (Pdcd4) is a tumor suppressor protein that inhibits transformation in vitro and tumor formation in vivo (Jansen et al., 2005). Recent studies have shown that Pdcd4 is ubiquitylated through S6K1- and βTRCP-mediated proteasomal degradation (Dorrello et al., 2006). To analyze whether JS-K causes the accumulation of Pdcd4, A549 cells whose Pdcd4 levels are relatively low were treated with JS-K and Pdcd4 levels in cells was assessed. JS-K increased cellular Pdcd4 levels (Figure 2c).
Nuclear factor-κB (NF-κB) is a transcriptional factor that plays an important role in inflammation and tumorigenesis. IκBα is an inhibitor of NF-κB that acts by binding to its nuclear localization sequence. In the presence of an activator of NF-κB such as IL-1α, IκBα is ubiquitylated, after phosphorylation by IκB kinase (Ikk) family proteins, and degraded through proteasome degradation (Chen, 2005). To demonstrate that JS-K reduces degradation of IκBα, HeLa cells which express wild type IκBα were incubated with JS-K prior to treatment with IL-1α. JS-K strikingly delayed IL-1α-induced degradation of IκBα and phosphorylated IκBα levels in cells (Figure 2d, compare lanes 3 and 6). These data clearly support the notion that JS-K blocks the activity of ubiquitylation and degradation of proteins.
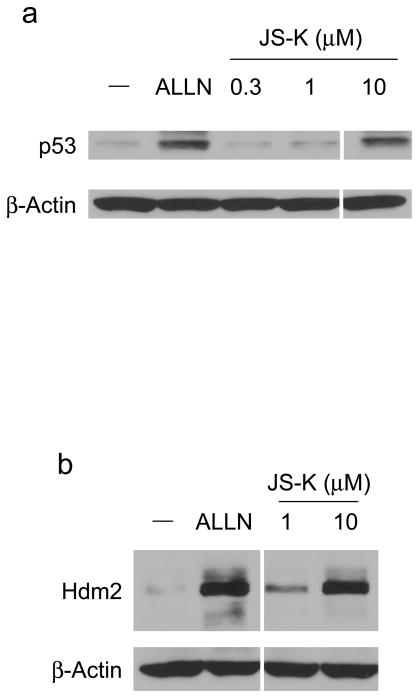
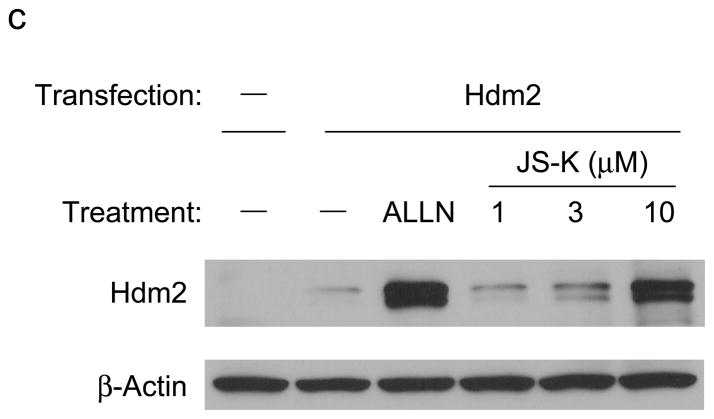
The tumor suppressor p53 mainly functions as a transcription factor to control the expression of a variety of target genes, leading to growth arrest, senescence, and apoptosis. p53 selectively kills transformed cells by inducing apoptosis. The cellular level of p53 is tightly controlled through proteasomal degradation and is very low in normal cells. One of the essential proteins involved in regulating cellular p53 levels is mouse double minute-2 (Mdm2, human ortholog is named Hdm2). Hdm2 is an E3 that ubiquitylates p53 and auto-ubiquitylates itself, resulting in proteasomal degradation of both proteins (Yang et al., 2004). We predicted that JS-K would inhibit Hdm2-mediated p53 ubiquitylation, leading to accumulation of p53. To determine the ability of JS-K to increase p53 and Hdm2, RPE cells were incubated with JS-K, and cellular p53 and Hdm2 levels were assessed. Characteristic of a proteasome inhibitor, ALLN increased cellular p53 and Hdm2 levels. JS-K also increased p53 and Hdm2 levels in cells in a concentration-dependent manner as observed for ALLN (Figure 3a and b). Next, to ensure the increase in Hdm2 by JS-K is independent of p53 transactivation, p53−/− mdm2−/− mouse embryo fibroblasts (MEFs) were transiently transfected with an Hdm2 plasmid under the control of a p53-independent CMV promoter. Following this incubation, the cells were exposed to JS-K, and cellular Hdm2 levels were evaluated. Treatment of JS-K stabilized Hdm2 in cells in a dose-dependent manner similar to that which was seen with ALLN (Fig 3c). These data suggest that JS-K stabilizes p53 and Hdm2 through inhibition of E3 activity of Hdm2.
Figure 3.
JS-K increases p53 and Hdm2 levels in RPE cells and accumulates Hdm2 levels in p53−/− mdm2−/− mouse embryo fibroblasts (MEFs)
(a) RPE cells were treated with 50 μM ALLN or JS-K for 4 h as indicated. Cell lysates were analyzed by immunoblotting using p53 (Santa Cruz Biotechnology) and β-Actin antibodies. (b) RPE cells were incubated with 50 μM ALLN or 1 or 10 μM JS-K for 6 h. The resultant lysates were immunoblotted with antibodies specific for Hdm2 (Oncogene, Boston, MA) and β-Actin. (c) Fibroblasts from p53−/− mdm2−/− mice were transiently transfected with Hdm2 plasmid (Chen et al., 1995). Twenty h after transfection, cells were treated with 50 μM ALLN or 1–10 μM JS-K for 8 h. Cellular Hdm2 and β-Actin was determined by immunoblotting.
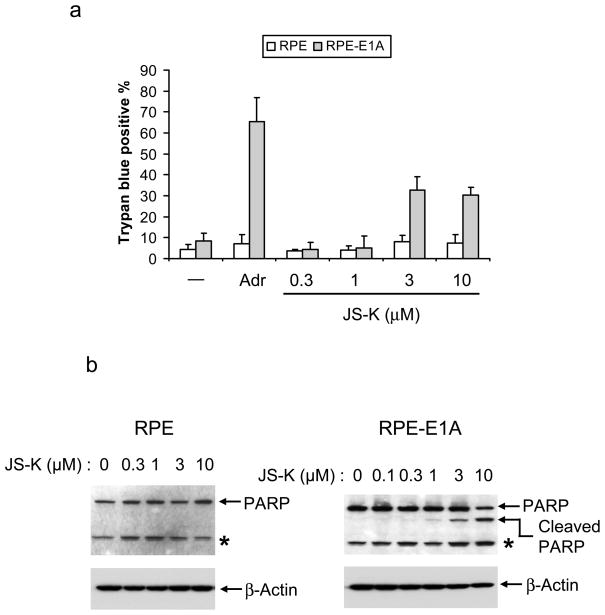
Previous studies have shown that transformed cells are sensitive to apoptosis in response to p53. To evaluate whether JS-K induces differential killing of transformed cells, we compared parental RPE cells and RPE cells transformed with adenovirus E1A (RPE-E1A) which interacts with the retinoblastoma tumor suppressor gene product but not p53 (Bandara and La Thangue, 1991; Yang et al., 2005). Both cell types were treated with JS-K, and cell death was assessed by trypan blue exclusion. JS-K resulted in cell death in RPE-E1A cells, but had no measurable effect on parental RPE cells, similar to Adriamycin, a DNA-damaging chemotherapeutic agent known to induce p53 accumulation (Figure 4a). To determine whether JS-K preferentially kills RPE-E1A cells by apoptosis, we assessed the cleavage of caspase substrate poly (ADP-ribose) polymerase (PARP) in JS-K-treated cells. JS-K induced PARP cleavage in RPE-E1A cells in a dose-dependent manner but not in RPE cells (Figure 4b). These results indicate that JS-K preferentially induces cell apoptosis in transformed cells.
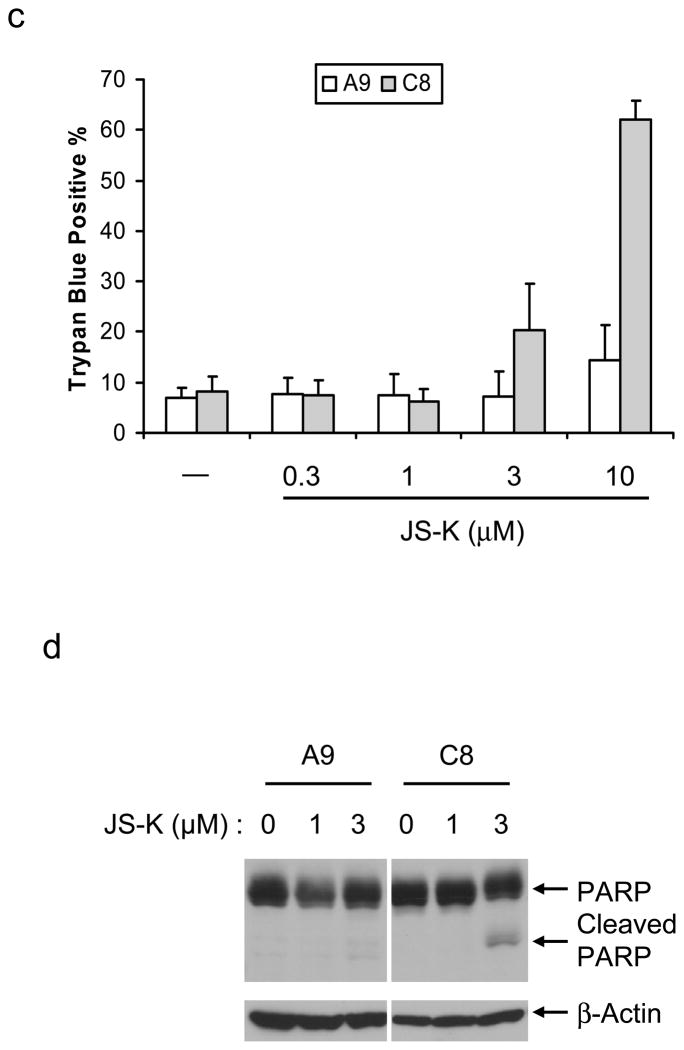
Figure 4.
JS-K selectively kills p53-expressing transformed cells
(a) Parental RPE cells and E1A-transformed RPE (RPE-E1A) cells were incubated with 1 μg/ml Adriamycin (Sigma) or 0.3–10 μM JS-K for 20 h. Cell death was assessed by trypan blue exclusion using 0.4% Trypan Blue Stain (Cambrex Bio Science, Walkersville, MD). Data represent an average and standard deviation of three different experiments. (b) RPE and RPE-E1A cells were treated with JS-K for 20 h as indicated. Cell lysates were immunoblotted using PARP (Santa Cruz Biotechnology) and β-Actin antibodies. Asterisk indicates non-specific band. (c) Wild type (C8) or p53-deficient (A9) MEFs were grown as described previously (Yang et al., 2005). These cells were treated with 0.3–10 μM JS-K for 18 h. Cell death was measured by trypan blue exclusion. Data represent an average and standard deviation of three different experiments. (d) C8 and A9 cells were exposed to 1 or 3 μM JS-K for 16 h. Cell lysates were immunoblotted as indicated.
The ability of p53 to induce apoptosis is important in tumor suppression (Lowe et al., 1993a). To evaluate whether the selective cell death of transformed cells correlates with p53, we used adenovirus E1A and Ha-ras transformed MEFs from p53+/+ mice (C8) or p53−/− mice (A9) (Lowe et al., 1993b). Both cells were treated with JS-K, and cell viability was assessed. While JS-K increased cell death in C8 cells in a concentration-dependent manner, JS-K did not induce cell death in A9 cells (Figure 4c). JS-K also increased PARP cleavage in C8 cells but not A9 cells (Figure 4d), indicating that JS-K induces apoptotic cell death in a p53-dependent manner.
Cancer recurrence and chemotherapeutic resistance remain the major limitation to chemotherapy. Cisplatin is one of the most widely used chemotherapeutic drugs for cancer treatment (Turchi, 2006). Recently JS-K has been shown to induce cell death synergistically with cisplatin (Liu et al., 2004). Proteasome inhibitor Bortezomib is also an anti-tumor drug for multiple myeloma cells (Adams, 2004). JS-K has been reported to have a synergistic effect with Bortezomib on cell killing in multiple myeloma cells (Kiziltepe et al., 2007). These findings indicate that JS-K, alone or in combination with other drugs, could be a useful compound for anti-tumor therapy.
The mechanism of cytotoxicity by O-arylated diazeniumdiolates such as JS-K and PABA/NO appears to involve at least two major pathways of electrophilic attack on cellular constituents (Shami et al., 2006): (a) irreversible transfer of the drugs’ dinitrophenyl rings to thiol residues in cellular proteins and peptides in the initial metabolic activation step; and (b) subsequent NO release leading under oxidizing conditions to the potentially reversible formation of S-nitros(yl)ating agents that mask the thiol residues, at least temporarily.
With this in mind, it may not be surprising that JS-K and PABA/NO differ in their effects on the pathways examined here. In the case of JS-K, the dinitrophenyl ring is transferred as such to the nucleophilic center, while PABA/NO’s aryl ring also bears an oxygen substituent that fundamentally changes the size and physicochemical properties of the molecular modification that results. With regard to NO generation, nucleophilic attack on JS-K produces a labile diazeniumdiolate ion that releases NO with simple, first-order kinetics. PABA/NO, on the other hand, is attacked by nucleophiles in two different positions; one leads to straightforward NO release as with JS-K, while the other, competing pathway cleaves the ester group to produce a phenolate intermediate whose capacity for NO generation is severely compromised (Saavedra et al., 2006).
We conclude that E1 is a target of JS-K. JS-K also leads to a decrease in total cellular ubiquitylation and an increase in cellular p53 levels. Moreover, JS-K selectively induces cell apoptosis in transformed cells expressing wild type p53. Therefore, inhibition of E1 by JS-K could be responsible for the therapeutic effect of JS-K that was observed in vitro and in animal models of tumor. These studies support the notion that targeting the ubiquitin system may represent a novel approach to the selective elimination of tumor cells retaining wild type p53.
Acknowledgments
We are grateful to the NIH Fellows Editorial Board for revisions of this manuscript. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by National Cancer Institute Contract No. NOI-CO-12400 to SAIC, Inc. J.K. was a fellow of the Japanese Society for the Promotion of Science.
Abbreviations
- NO
Nitric oxide
- E1
ubiquitin-activating enzyme
- RPE
Tert-immortalized human retinal pigment epithelial cells
References
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Chen J, Lin J, Levine AJ. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med. 1995;1:142–152. [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Giarre M, Semenov MV, Brown AM. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann N Y Acad Sci. 1998;857:43–55. doi: 10.1111/j.1749-6632.1998.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR, Jr, Gong J, et al. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Neuronal protection and destruction by NO. Cell Death Differ. 1999;6:943–951. doi: 10.1038/sj.cdd.4400580. [DOI] [PubMed] [Google Scholar]
- Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS, et al. Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol Cancer Ther. 2004;3:709–714. [PubMed] [Google Scholar]
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993a;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993b;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI. JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways. J Cell Physiol. 2003;197:426–434. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, et al. PABA/NO as an anticancer lead: analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem. 2006;49:1157–1164. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, et al. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, et al. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Ther. 2003;2:409–417. [PubMed] [Google Scholar]
- Simeone AM, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM, et al. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi JJ. Nitric oxide and cisplatin resistance: NO easy answers. Proc Natl Acad Sci U S A. 2006;103:4337–4338. doi: 10.1073/pnas.0601001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL, et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li CC, Weissman AM. Regulating the p53 system through ubiquitination. Oncogene. 2004;23:2096–2106. doi: 10.1038/sj.onc.1207411. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV, et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–1410. doi: 10.1158/0008-5472.CAN-06-2149. [DOI] [PubMed] [Google Scholar]