Abstract
Cell penetrating peptides (CPPs) have been used to deliver the anti-apoptotic Bcl-xL-derived BH4 peptide to prevent injury-induced apoptosis both in vitro and in vivo. Here we demonstrate that the nuclear localization sequence (NLS) from the SV40 large T antigen has favorable properties for BH4 domain delivery to lymphocytes compared to sequences based on the HIV-1 TAT sequence. While both TAT-BH4 and NLS-BH4 protected primary human mononuclear cells from radiation-induced apoptotic cell death, TAT-BH4 caused persistent membrane damage and even cell death at the highest concentrations tested (5–10 μM) and correlated with in vivo toxicity as intravenous administration of TAT-BH4 caused rapid death. The NLS-BH4 peptide has significantly attenuated toxicity compared to TAT-BH4 and we established a dosing regimen of NLS-BH4 that conferred a significant survival advantage in a post-exposure treatment model of LD90 total body irradiation.
Keywords: cell penetrating motif, NLS, TAT, Bcl-2, BH4, cell death, apoptosis, necrosis, total body irradiation
Introduction
Total body irradiation (TBI) produces a consistent, dose-dependent pattern of organ damage, primarily in tissues undergoing rapid proliferation [1]. With a threshold of approximately 2 Gray TBI, there is a rapid drop in circulating blood cells that persists until surviving hematopoietic stem cells proliferate sufficiently to reconstitute the periphery. During this period of leukopenia, patients are susceptible to opportunistic infection and hemorrhage. At higher radiation doses, radio-sensitive stem cells in the small intestine crypt are also killed causing intestinal barrier failure and allowing bacteria to invade from the gut [2] placing further demands on the already compromised immune system.
Existing radiation treatments (such as granulocyte-macrophage colony stimulating factor or granulocyte colony stimulating factor) stimulate hematopoietic stem cell proliferation, reduce the extent and duration of leukopenia, and increase platelet and hematocrit values in irradiated mice [3,4] and primates [5]. This approach is dependent upon survival of essential stem cells following radiation exposure and becomes less successful as the fraction of surviving cells decreases.
We and others have taken a complementary approach based on the molecular biology of radiation-induced apoptosis. For example, the p53 tumor suppressor protein is activated following radiation-induced DNA damage [6,7] and triggers apoptotic cell death, in part by increasing the expression of pro-apoptotic genes such as Bax, Noxa, and Puma [8]. These gene products trigger the formation of Bax/Bak oligomeric channels in mitochondrial membranes [9, 10], resulting in loss of mitochondrial membrane potential, cleavage of caspase-9 and apoptosis. Recently, a small molecule inhibitor of p53, pifithrin-μ, was shown to protect thymocytes from radiation-induced apoptosis and mice from radiation-induced death when administered prior to TBI [11].
Transgenic mice that over-express Bcl-2 in the hematopoietic compartment are protected from radiation-induced lymphocyte apoptosis have a significant survival advantage in lethal TBI [12]. We and others have shown that TAT peptide-mediated intracellular delivery of the obligate anti-apoptotic BH4 domain of Bcl-2 is remarkably versatile in preventing injury-induced apoptosis in radiation injury, sepsis, fulminant hepatitis, cardiac ischemia-reperfusion injury, spinal cord injury, pancreatic β-cell death and amyloid toxicity [13–19]. However, while TAT-BH4 confers remarkable protection in acute studies, it has not been reported to ameliorate primary end points in vivo. In fact, Cittelly, et al. showed that a 7-day course of intrathecal TAT-BH4 exacerbated spinal cord injury in rats, shifting injury-induced cell death from apoptosis to necrosis [20].
We now demonstrate a cellular basis for that toxicity, namely that the TAT-BH4 peptide caused persistent damage to cell membranes and eventual permeabilization. Furthermore, we show that an alternative CPP, the nuclear localization sequence (NLS) of the SV40 large T antigen [21], when fused to the BH4 domain of Bcl-xL (NLS-BH4) had similar radioprotective activity as the TAT-BH4 peptide. In vitro treatment with NLS-BH4 did not cause persistent cellular damage or permeabilization. We also established a post-irradiation dosing regimen of NLS-BH4 that significantly enhanced survival following lethal radiation injury.
Materials and Methods
Peptide Synthesis
Peptides were synthesized using standard Fmoc/HOBt chemistry, cleaved from the resin and purified using preparative scale HPLC to >95% (Tufts University Core Facility, Waltham, MA) [22]. Peptide sequences (Table 1) were confirmed using mass spectrometry.
Table 1.
Peptides used in study.
| Compound | Sequence |
|---|---|
| TAT | (d)1-Ac-RKKRR-Orn-RRR-COOH |
| NLS | Ac-KKKRKV-COOH |
| TAT-BH4 | (d)1-Ac-RKKRR-Orn-RRR-β-A-(l)1-SNRELVVDFLSYKLSQKGYS-COOH |
| TAT-BH4i | (d)1-Ac-RKKRR-Orn-RRR-β-A-(l)1-SNRELVVDFLSDKLSQKGDS-COOH |
| NLS-BH4 | Ac-KKKRKV-β-A-(l)1-SNRELVVDFLSYKLSQKGYS-COOH |
| NLS-BH4i | Ac-KKKRKV-β-A-(l)1-SNRELVVDFLSDKLSQKGDS-COOH |
| FITC-TAT-BH4 | (d)1-Ac-C(FM) RKKRR-Orn-RRR-β-A-(l)1-SNRELVVDFLSYKLSQKGYS-COOH |
| FITC-NLS-BH4 | (d)1-Ac-C(FM) KKKRKV-β-A-(l)1-SNRELVVDFLSYKLSQKGYS-COOH |
(d) indicates that the subsequent amino acids are D-amino acids while (l) indicates that the subsequent amino acids are L-amino acids. Unmarked sequences are composed entirely of L-amino acids.
Isolation of primary human lymphocytes
Peripheral blood mononuclear cells (PBMCs) were obtained from fresh whole blood of healthy human volunteers after differential migration over Ficol Paque Plus. Cells were cultured in RPMI 1640 supplemented with 10% FBS, 0.1% 2-mercaptoethanol, and 1% non-essential amino acids, and incubated at 37 °C in 5% CO2 at a density of 1×106 cells/mL unless otherwise noted. Informed consent was obtained from healthy volunteers for the collection of blood specimens. This study was approved by the Washington University Human Research Protection Office.
In vitro studies in PBMCs
In one study cohort, PBMCs from 5 donors were subjected to 20 Gy gamma radiation using a Cobalt-60 γ-ray source (J.L. Shepherd and Associates), cultured for 20h, and the extent of apoptosis was measured by flow cytometry as described below. The TAT-BH4 or NLS-BH4 peptides were added to culture within 30 minutes following radiation at the concentrations indicated. In a second study cohort, human PBMCs were treated with either TAT-BH4 or NLS-BH4 and the concentrations indicated, and at the times indicated cells were assessed for changes in morphology, permeability and extracellular phosphatidyl serine by flow cytometry as described below. Two technical replicates were done for each biological replicate. In additional study cohorts, human PBMCs were treated with FITC-labeled peptides (TAT-BH4 or NLS-BH4) to determine the extent of peptide internalization and subcellular distribution of internalized peptide (as described below).
Flow cytometric assessment of PBMCs
Apoptosis was determined using the APO-BrdU kit (Phoenix Flow Systems) according to manufacturer’s instructions. Fluorescently-labeled mAbs identified CD3 T cells (BD Pharmingen). Cell permeability and extracellular phosphatidyl serine were measured simultaneously by staining cells with Annexin V-FITC (BioVision) and 7AAD (BD Pharmingen) according to manufacturer’s instructions. Flow cytometry (25,000–50,000 events/sample) was performed on FACScan (BD Biosciences).
In vitro assessment of cellular uptake and subcellular distribution
Primary human lymphocytes were treated with 5 μM FITC-labeled TAT-BH4 or NLS-BH4 and incubated for 1 h in either RPMI with 10% FBS or sterile-filtered, serum-free PBS, washed twice with PBS, and assessed by flow cytometry. A second identically prepared cohort of cells was treated with Alexa 595-conjugated Wheat Germ Agglutinin (WGA) (Invitrogen) according to manufacturer’s instructions to visualize the plasma membrane. Cells were washed again, resuspended in RPMI with 10% FBS, and added to coverslip-bottomed 35 mm culture dishes (MatTek) for imaging. Images (400X) were taken sequentially to prevent fluorochrome cross-talk. Gain and photomultipliers were the same for all samples. In fluorescence microscopy studies, PBMCs were treated as above with the fluorescently-labeled peptides, omitting the WGA labeling. Cells were deposited on slides for imaging (600X, Nikon Eclipse E600).
Animal studies
Both CD1 and C57Bl/6J male mice (Jackson Laboratories) weighing 18–26 grams and 8–12 weeks of age were used for in vivo experiments. Animals were allowed free access to food and water.
A toxicity study comparing intravenous (i.v.) via tail vein and intraperitoneal (i.p.) TAT-BH4 and NLS-BH4 was conducted where mice were anesthetized with halothane (5% induction and 2% maintenance) and injected with the appropriate compounds dissolved in 200 microliters of PBS. Mice received graded doses of compounds. Survival was recorded for 24 hrs.
In two additional cohorts of male C57Bl/6 animals, a post-exposure dosing regimen of NLS-BH4 was established to determine the effects of the daily peptide treatment on survival following lethal radiation injury. Briefly, mice received total body irradiation to a dose of 7.5 Gy (AECL Gammacell 40 irradiator). Within 30 minutes of injury, mice were administered NLS-BH4, NLS-BH4i (1 mg in 200 microliters of saline) or saline alone and again daily for a total of 5 doses. Survival was recorded for 30 days. The Animal Studies Committee at Washington University School of Medicine approved all animal studies.
Statistical Analysis
Data were analyzed with one-way ANOVA with Tukey’s multiple comparison test (Prism, GraphPad). Data reported are means +/− SEM. Thirty day survival data was analyzed using the Kaplan-Meier (product-limit) method. Tests of equivalence of survival were performed by the generalized Wilcoxon log-rank test. Significance was accepted at p < 0.05.
Results and Discussion
Dose limiting toxicity of TAT-BH4
Mice that received TAT-BH4 (2 mg in 200 μL saline) via tail-vein injection died within 3–5 minutes following injection (N=6). The deaths were characterized by agonal gasping respirations. To determine how the domains of the TAT-BH4 construct contributed to the observed toxicity, i.v. injections were repeated with both TAT and BH4 peptides, similar to the TAT-BH4, all mice died within minutes of the injection (N = 2 each). Likewise, i.v. administration of the polyarginine peptide R7 gave similar results, no control mice died (200 μL saline i.v.).
An i.p. injection study was conducted as a dose escalation trial. All mice receiving 2 or 5 mgs of TAT-BH4 survived but only 1 of the 3 mice that received 30 mgs of TAT-BH4 survived (Table 2). While the mice that received 2 mg TAT-BH4 appeared normal the following day, the surviving mice that received 5 mg or 20 mg TAT-BH4 were characterized by piloerection and distended abdomens. Necropsy of these mice revealed distended and edematous bowel and small necrotic foci.
Table 2.
Mouse survival study. Compounds were delivered by intravenous tail vein injection (i.v.) or intraperitoneal injection (i.p.).
| Compound | Treatmenta | N | Percent Surviving |
|---|---|---|---|
| BH4 | |||
| 2 mg – i.v. | 3 | 33 | |
| 10 mg – i.p. | 1 | 100 | |
| TAT | |||
| 2 mg – i.v. | 6 | 0 | |
| 2 mg – i.p. | 10 | 100 | |
| 5 mg – i.p. | 2 | 0 | |
| TAT-BH4 | |||
| 2 mg – i.v. | 7 | 14 | |
| 2 mg – i.p. | 4 | 100 | |
| 5 mg – i.p. | 4 | 75 | |
| 20 mg – i.p | 4 | 25 | |
| NLS | |||
| 2 mg – i.v. | 6 | 100 | |
| NLS-BH4 | |||
| 2 mg – i.v. | 6 | 100 | |
| 2 mg – i.p. | 3 | 100 | |
| 10 mg – i.p. | 3 | 100 | |
| 30 mg – i.p. | 3 | 100 |
Intravenous injections were performed in C57Bl/6J mice. Intraperitoneal injections were performed in both C57Bl/6J and CD1 mice. Results were similar for both strains and data from the CD1 mice is presented.
Selection of an alternative CPP
A panel of alternative CPPs was screened to identify an alternative to the TAT peptide that had been used in our acute lymphoprotection study [13] (Supplemental Table 1). These studies demonstrated that the nuclear localization sequence (NLS) from the SV40 large T antigen best met the criteria of being relatively non-toxic and effectively delivering cargo into lymphocytes. We then performed head-to-head comparisons between the TAT-BH4 and NLS-BH4 peptides to determine relative efficacy of our second generation peptide.
Anti-apoptotic efficacy of TAT-BH4 versus NLS-BH4
Lymphocyte apoptosis increased from a baseline level of 4.5 ± 0.7% in the control group to 17.7 ± 2.4% in the group subjected to 20 Gy radiation (p < 0.001, n = 5). There was a dose-dependent reduction in the percentage of TUNEL positive T-cells treated with either TAT-BH4 or NLS-BH4 (Figure 1). The NLS-BH4 peptide provided cellular protection at lower concentrations than TAT-BH4, though at the highest dose tested (5 μM) the peptides provided equivalent protection. The extent of cellular protection plateaued between 2 and 5 μM peptide with approximately 8% of cells being TUNEL positive for both TAT-BH4 and NLS-BH4 treatment. As previously reported, exogenous BH4 peptide only reduced injury-induced apoptosis by ~50% [23].
Figure 1.
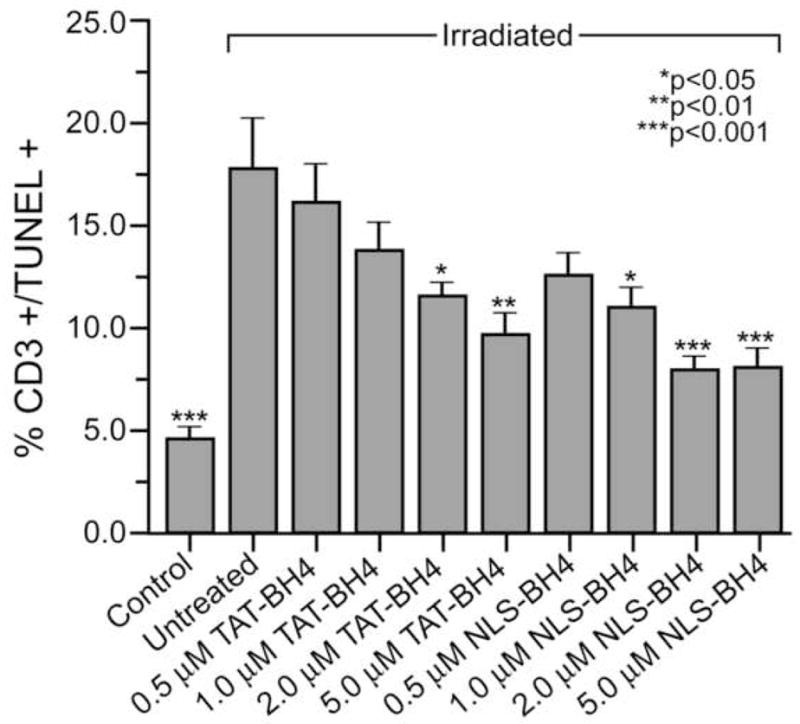
Both TAT-BH4 and NLS-BH4 reduce radiation-induced apoptosis in CD3+ human PBMCs in vitro. Neither TAT-BH4i nor NLS-BH4i had any appreciable effect on radiation-induced apoptosis. Statistical significance marked is for the comparison with the untreated, irradiated PBMCs exposed to 20 Gy radiation.
In vitro effects of TAT-BH4 and NLS-BH4 on lymphocytes
We observed atypical forward and side scatter profiles of lymphocytes treated with 5 μM TAT-BH4 and sought to better characterize this phenomenon. Both NLS-BH4 and TAT-BH4 treated lymphocytes exhibited rapid changes in size (FSC) and granularity (SSC). These differences were most pronounced at the highest dose of peptide tested (10 μM), though the effects were consistently seen at 5 μM. NLS-BH4-treated PBMCs cultured for 20 hours returned to pretreatment size and granularity while TAT-BH4-treated PBMCs underwent profound structural changes based on their FACS profiles. The majority of TAT-BH4 treated cells became compacted within 30 minutes. Keeping these cells in culture for 20 hours resulted in two distinct populations of cells. The minority of cells in culture fell within the typical lymphocyte gate (see figure legend); while the majority of TAT-BH4 treated cells became more compact and granular as judged by the decreased forward and side scatter, respectively (Figure 2, Panel A).
Figure 2.
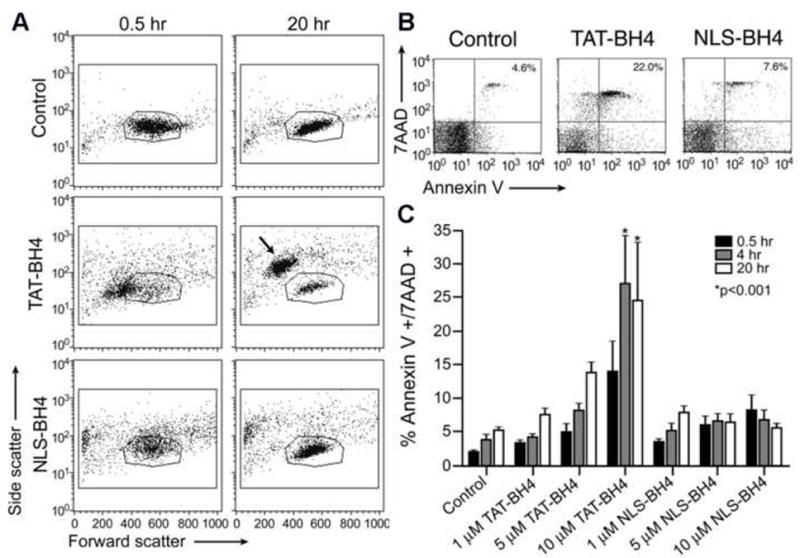
Flow cytometric assessment of PBMCs treated with CPP-BH4 conjugates. (A) Morphological changes in human PBMCs following treatment with PTD-conjugated BH4 peptides. These data show the entire population of isolated PBMCs from one representative donor (no gating was done). The outlined region (polygon) indicates the typical FSC/SSC region where unmanipulated lymphocytes are observed. (B and C) Morphological changes correlate with phosphatidylserine exteriorization and loss of membrane integrity following peptide treatment. (B) Representative FACS plots 4 h after the administration of saline, NLS-BH4, or TAT-BH4 (10 μM). These data show the entire population of isolated PBMCs from one representative donor (no gating was done). (C) The time and dose dependence of Annexin V and 7AAD staining of PBMCs following peptide exposure. These data are calculated from the percentage of cells in the upper right quadrant (AnxV+/7AAD+) of FACS diagrams as shown in panel A (N=5). Statistical significance marked is compared to untreated (control) cells at the same time point.
Following treatment with either TAT-BH4 or NLS-BH4 (10 μM), cells were stained using both Annexin-V and 7-AAD. Annexin V is a measure of membrane injury that is used as an early marker of apoptosis, though cells that are annexin V positive are not necessarily committed to apoptosis. 7AAD is a membrane impermeant dye that is used to determine cell membrane integrity; cells that are 7AAD positive have lost membrane integrity and are no longer viable. Within 30 minutes, there was a profound increase in Annexin-V+ cells that were treated with either TAT-BH4 (46.5%) or NLS-BH4 (22.1%) compared to untreated cells (7.9%). TAT-BH4, but not NLS-BH4, caused an increase in the percentage of 7AAD positive cells (6.7%) compared to the control (2.6% Figure 2, Panel B).
Further characterization of these groups identified time-, agent- and concentration-dependencies on the percentage of Annexin-V+/7-AAD+ cells. In untreated cells, there was a slight time-dependent increase in Annexin-V+/7-AAD+ cells from 2% to 5% over the course of 20h in culture. Treatment with either TAT-BH4 or NLS-BH4 increased the percentage of Annexin-V+/7-AAD+ cells at all times. In addition to a time-dependent increase in apoptotic cells, TAT-BH4 treatment resulted in a dose-dependent increase in the percentage of apoptotic cells with approximately 25% of PBMCs incubated with 10μM TAT-BH4 becoming apoptotic within 4h post-treatment. This result is consistent with recent reports of CPPs such as R9 causing macroscopic tubulation and destruction of giant unilamellar vesicles [24], suggesting that some CPPs, especially polyarginine containing peptides, can cause irreparable membrane damage. There was no significant effect of the concentration of NLS-BH4 on the percentage of double positive cells (Figure 2, Panel C).
Cellular uptake of TAT-BH4 versus NLS-BH4
Fluorescein labeled peptides were used to demonstrate uptake by PBMCs. Serum reduced the amount of either peptide internalized, however NLS-BH4 was less dependent on the presence of serum (Figure 3, Panels A and B). Under all conditions the fluorescence intensity of TAT-BH4-FITC treated cells was greater than that of cells treated with NLS-BH4-FITC. Laser scanning confocal microscopy confirmed that the peptides were internalized, not merely associated with the external cell membrane, and corroborated the findings from FACS analysis that uptake of TAT-BH4 was superior to NLS-BH4. Both peptides were found diffusely throughout the cytoplasm while TAT-BH4 was over-represented in the nucleolus (Figure 3, Panel C); NLS-BH4 exhibited little or no nuclear staining. The increased cytoplasmic concentration of NLS peptide may partially compensate for decreased internalization, relative to TAT-BH4, since the BH4 domain of our construct has a mitochondrial binding site (Supplemental Figure). This binding site may include FKBP38, a protein known to target Bcl-2 family members to mitochondria in a BH4 domain-dependent fashion [25, 26].
Figure 3.
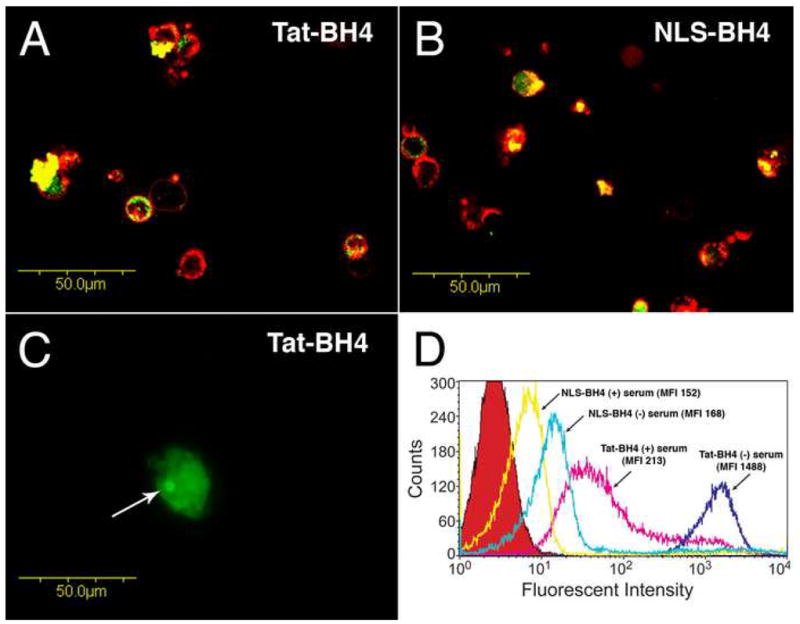
Uptake of fluorescein-conjugated Tat-BH4 and NLS-BH4 in human peripheral mononuclear cells. Panels A and B show, by confocal microscopy, representative images (400X) of the intratracellular localization of both fluorescein-labeled Tat-BH4 and fluorescein-labeled NLS-BH4 as indicated. Cells were incubated with the fluorescein-labeled peptides in the absence of serum (5μM, 1h, green color) and then treated with Alexa 594-conjugated Wheat Germ Agglutinin (red color) to distinguish the plasma membrane. Gain and photomultiplier settings were held constant across samples. Panel C shows, by fluorescence microscopy, a representative image (600X) of the nucleolar accumulation (arrow) of internalized fluorescein-labeled Tat-BH4 (5μM, 1h, serum-free). Panel D shows, by flow cytometry, the fluorescent intensities of cells treated with fluorescein-conjugated Tat-BH4 and NLS-BH4 (5μM) in both the presence and absence of serum as well as untreated cells (red peak).
A non-toxic dosing regimen of NLS-BH4 protects mice from lethal radiation exposure
To determine whether NLS-BH4 was toxic in vivo, a similar study to the TAT-BH4 study was performed. None of the mice (N=6) that received NLS-BH4 (2 mg in 200 μL saline) via tail-vein injection died (p < 0.05 compared to the TAT-BH4 administered animals). The mice receiving NLS-BH4 tolerated the injections without apparent ill effects. All mice receiving 2, 5, or 0 mg NLS-BH4 i.p. tolerated the injections without any apparent ill effects (N = 3 mice per group). These mice appeared normal the following day without piloerection or abdominal distention. Survival data are summarized in Table 2.
In vivo efficacy studies
In a pilot in vivo efficacy study, we found that a 1 mg dose of NLS-BH4 significantly attenuated radiation-induced lymphocyte apoptosis (data not shown). To determine whether intracellular delivery of the BH4 domain of Bcl-xL confers a survival advantage in lethal radiation injury, we gave a course of 5 daily NLS-BH4 injections (1 mg in 200 μL normal saline i.p.) beginning within 30 minutes of lethal TBI (7.5 Gy). This course of treatment improved survival from 15% in untreated animals to 75% in animals receiving NLS-BH4 (p<0.01) and mice treated with the NLS-BH4 inactive peptide had 40% overall survival (Figure 5). Significantly, post-exposure treatment conferred the survival advantage and there is an immediate need for therapeutics to treat patients as they are identified following accidental or deliberate exposure to radiation.
Conclusion
As the field of anti-apoptotic therapy for acute and degenerative disease matures, it will be necessary to determine whether supplementation with exogenous BH4 peptide is sufficient to alter primary end points in disease models. Our study exemplifies a rational experimental approach to this question and together with the recent head-to-head comparison of the biological properties of 22 diverse CPPs [27], we believe that there is now a clear path toward the development of effective, non-necrosis-inducing BH4-based therapies in diverse disease models. Studies of the combined effects of anti-apoptotic and stem cell proliferative therapies are indicated.
Figure 4.
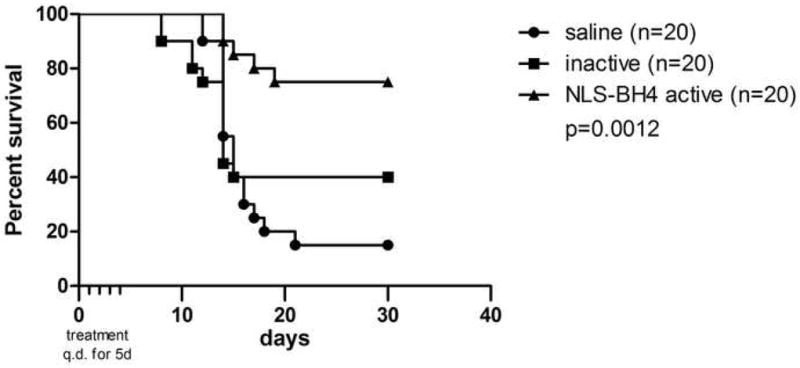
A five-day course of systemic NLS-BH4 (1 mg q.d.) beginning after LD90 TBI conferred a significant survival advantage to mice (7.5 Gy).
Acknowledgments
This work was supported by National Institutes of Health Grants GM055194, GM044118, K12HD0085020, CA104457, CA094056 and CA082841; Defense Threat Reduction Agency Contract HDTRA1-06-C0037; the Alan A. and Edith L. Wolff Foundation; and the Fred Hutchinson Cancer Research Center/University of Washington (FHCRC/UW) Center for Medical Countermeasures Against Radiation (CMCR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 2.Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002 Oct;4(5):361–5. doi: 10.1007/s11894-002-0005-3. [DOI] [PubMed] [Google Scholar]
- 3.Tanikawa S, Nose M, Aoki Y, Tsuineoka K, Shikita M, Nara N. Effect of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:554–449. [PubMed] [Google Scholar]
- 4.Hosoi Y, Kurishita A, Ono T, Sakamoto K. Effect of recombinant human granulocyte colony-stimulating factor on survival of lethally irradiated mice. Acta Oncol. 1992;31:59–63. doi: 10.3109/02841869209088267. [DOI] [PubMed] [Google Scholar]
- 5.Farese AM, Hunt P, Grab LB, MacVittie TJ. Combined administration of recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor enhances multilineage hematopoietic reconstitution in nonhuman primates after radiation-induced marrow aplasia. J Clin Invest. 1996;97:2145–2151. doi: 10.1172/JCI118652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe SW, Schmitt EM, Smith SW, Osborne BAT. Jacks. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362. 1993:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 7.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 8.Michalak E, Villunger A, Erlacher M, Strasser A. Death squads enlisted by the tumour suppressor p53. Biochem Biophys Res Commun. 2005;331:786–798. doi: 10.1016/j.bbrc.2005.03.183. [DOI] [PubMed] [Google Scholar]
- 9.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by Bcl-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 11.Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska L, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 12.Domen J, Gandy KL, Weissman IL. Systemic overexpression of Bcl-2 in the hematopopietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 13.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 14.Sugioka R, Shimizu S, Funatsu T, Tamagawa H, Sawa Y, Kawakami T, Tsujimoto Y. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- 15.McConnell KW, Muenzer JT, Chang KC, Davis CG, McDunn JE, Coopersmith CM, Hilliard CA, Hotchkiss RS, Grigsby PW, Hunt CR. Anti-apoptotic peptides protect against radiation-induced cell death. Biochem Biophys Res Commun. 2007;355:501–507. doi: 10.1016/j.bbrc.2007.01.180. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 17.Nesic-Taylor O, Cittelly D, Ye Z, Xu GY, Unabia G, Lee JC, Svrakic NM, Liu XH, Youle RJ, Wood TG, McAdoo D, Westlund KN, Hulsebosch CE, Perez-Polo JR. Exogenous Bcl-xL fusion protein spares neurons after spinal cord injury. J Neurosci Res. 2005;79:628–637. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- 18.Klein D, Ribeiro MM, Mendoz V, Jayaraman S, Kenyon NS, Pileggi A, Molano RD, Inverardi L, Ricordi C, Pastori RL. Delivery of Bcl-xL or its BH4 domain by protein transduction inhibits apoptosis in human islets. Biochem Biophys Res Commun. 2004;323:473–478. doi: 10.1016/j.bbrc.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 19.Cantara S, Donnini S, Giachetti A, Thorpe PE, Ziche M. Exogenous BH4/Bcl-2 peptide reverts coronary endothelial cell apoptosis induced by oxidative stress. J Vasc Res. 2004;41:202–207. doi: 10.1159/000077408. [DOI] [PubMed] [Google Scholar]
- 20.Cittelly DM, Nesic O, Johnson K, Hulsebosch C, Perez-Polo JR. Detrimental effects of antiapoptotic treatments in spinal cord injury. Exp Neurol. 2008;210:295–307. doi: 10.1016/j.expneurol.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragin AD, Morgan RA, Chmielewski J. Cellular import mediated by nuclear localization peptide sequences. Chem Biol. 2002;9:943–948. doi: 10.1016/s1074-5521(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 22.Bullok KE, Dyszlewski M, Prior JL, Pica CM, Sharma V, Piwnica-Worms D. Characterization of novel histidine-tagged TAT-peptide complexes dual-labeled with (99m)Tc-tricarbonyl and fluorescein for scintigraphy and fluorescence microscopy. Bioconjug Chem. 2002;13:1226–1237. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamaziere A, Burlina F, Wolf C, Chassaing G, Trugnan G, Ayala-Sanmartin J. Non-metabolic membrane tubulation and permeability induced by bioactive peptides. PLoS One. 2007;2:e201. doi: 10.1371/journal.pone.0000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat Cell Biol. 2003;5:28–37. doi: 10.1038/ncb894. [DOI] [PubMed] [Google Scholar]
- 26.Portier BP, Taglialatela G. Bcl-2 localized at the nuclear compartment induces apoptosis after transient overexpression. J Biol Chem. 2006;281:40493–40502. doi: 10.1074/jbc.M606181200. [DOI] [PubMed] [Google Scholar]
- 27.Mueller J, Kretzschmar I, Volkmer R, Boisguerin P. Comparison of cellular uptake of 22 CPPs in 4 different cell lines. Bioconjug Chem. 2008;19:2363–2374. doi: 10.1021/bc800194e. [DOI] [PubMed] [Google Scholar]


