Abstract
Nervous system growth factor gene delivery can promote axonal growth and prevent cell death in animal models of CNS trauma and neurodegenerative diseases. The ability to regulate growth factor expression or signaling pathways downstream from growth factor receptors remains a desirable goal for in vivo gene transfer. To achieve precise pharmacological modulation of neurotrophin activity, we have generated a chimeric trkA receptor (ItrkA) by fusing the entire intracellular domain of the trkA high-affinity NGF receptor to two intracellular, modified FK506 binding domains for the synthetic small molecule dimerization ligand, AP20187. Rat PC12 cells were transduced with lentiviral vectors containing ItrkA and GFP (via an internal ribosome entry site). Treatment of ItrkA-expressing PC12 cells with AP20187 induced neurite outgrowth and differentiation in a time- and dose-dependent fashion, with a half-maximal response at a concentration of 1 nM AP20187. 70% of cells responded to AP20187 by day 3. Western blots demonstrated that AP20187 treatment resulted in phosphorylation of Erk1/2 and Akt in ItrkA-transduced PC12 cells, but not in non-transduced, naive cells. Phosphorylation levels were comparable to levels obtained with 50ng/ml NGF. In addition, ItrkA lentiviral transduction of primary E15 dorsal root ganglion neurons significantly increased neurite growth 3–4-fold in the presence of AP20187 compared to control GFP transduced and naïve neurons. These results demonstrate that small ligand-induced dimerization of the intracellular domain of trkA can efficiently simulate the biological activity of NGF and provide a means to regulate intracellular neurotrophin receptor signaling.
Keywords: gene therapy, neurotrophin, NGF, trkA, AP20187, regeneration
INTRODUCTION
Neurotrophic factors have been shown to augment neuronal survival, function and axon growth in numerous animal models of neuronal degeneration and nervous system injury. However, clinical translation of neurotrophic factor therapy has been hampered by the inability of these proteins to traverse the blood-brain-barrier and reach target areas of the CNS, and by dose-limiting adverse effects from broad exposure of non-targeted structures in the CNS and PNS.
Over the last decade, improvements in viral vectors and gene therapy have provided a novel means for targeted, spatially restricted delivery of trophic factors in the CNS. This has led to several clinical trials delivering trophic factors by ex vivo and in vivo gene therapy in neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease (Tuszynski et al. 2005). Despite promising results from these trials, regulation of neurotrophin expression or regulated neurotrophin signaling remains highly desirable to optimize the dose of neurotrophin delivered and to increase the safety of growth factor gene therapy.
Several studies have demonstrated that neurotrophin gene expression can be efficiently modulated in MLV-based retroviral and in lentiviral vectors using tetracycline regulated systems. However, these systems require the expression of a fusion protein with bacterial and viral components - notably, the tetracycline transactivator or reversed tetracycline transactivator- to bind to a regulatable promoter, and can therefore lead to immunological responses (Favre et al. 2002; Latta-Mahieu et al. 2002). Other regulated systems that are based on chimeric transcription factors to regulate promoter activity such as antibiotic-based systems (Fussenegger et al. 2000; Weber et al. 2002), ecdysone (No et al. 1996), or other stereoid-based systems (Delort and Capecchi 1996) are potentially prone to similar complications.
Tight control of receptor activation that is independent of growth factor binding could provide an alternative approach to regulating the amount of growth factor expression. The activity of transmembrane tyrosine kinase receptors such as trkA is induced by receptor dimerization following ligand binding to the extracellular domain. A system that would allow regulated dimerization of the intracellular receptor domains might therefore initiate similar phosphorylation and subsequent downstream signaling as ligand binding. Previous studies have shown that a system based on the FK506 binding protein and a synthetic ligand consisting of 2 chemically linked molecules of the natural ligand FK506 can be used to induce protein homodimerization (Spencer et al. 1993). Further developments of this system including remodeling of the FKBP-ligand interface and generation of a small molecule ligand (AP20187) lacking the immunosuppressive properties of FK506 have enhanced the specificity and affinity allowing for more potent dimerization (Clackson et al. 1998).
Using this homodimerization system, several studies have shown that cell proliferation signals in response to fibroblast growth factor (FGF) receptor and glial cell line-derived neurotrophic factor receptor (RET) activation can be mimicked by expressing a fusion of a modified human FK506 binding protein (FKBP) (Clackson et al. 1998) to intracellular tyrosine kinase domains. Upon administration of the small modified FKBP dimerizing, membrane permeable ligand AP20187, intracellular signal transduction can be initiated (Allocca et al. 2007; Freche et al. 2005; Pownall et al. 2003; Welm et al. 2002).
Whether such a system would also be suitable to control neuronal survival and/or neurite outgrowth in conjunction with neurotrophic factor receptors such as trkA, has not been investigated to date.
To control neurotrophin signal transduction, we have constructed a novel, pharmacologically inducible NGF receptor. The inducible trkA receptor (ItrkA) exploits the inherent dimerization-induced activation of receptor tyrosine kinases to trigger trkA signaling in response to a small molecule ligand. Using lentiviral gene delivery, we now report that ItrkA activation can efficiently stimulate trkA signaling pathways leading to neuronal differentiation and neurite outgrowth. These results demonstrate the ability of ItrkA to regulate trkA signaling and biological responses, in the absence of NGF.
METHODS
Cloning and viral vector construction
The rat trkA intracellular domain (1410–2458; Accession: NM_021589) was amplified by PCR using the rat trkA cDNA as template (gift of L. Reichardt, University of California, San Francisco) and the following primers: 5′ ACTTCTAGAAATTTGGGATCAACCGCCCTG-3′ and 5′-CTGTCTAGAGCCCAGAACGTCCAGGTAACT-3′. The 1048 base-pair PCR product was then subcloned into the XbaI site of plasmid pC4M-FV2E (Fan et al. 1999) (ARIAD Pharmaceuticals, Cambridge, MA) to generate an inducible TrkA chimeric protein (ItrkA) consisting of the rat trkA intracellular domain fused to an amino-terminal myristoylation signal, two tandem FK 506 binding domains (FKBP36v) and a c-terminal hemagglutinin tag. The FKBP36V domain is based on the human protein FKBP12, engineered to bind only a synthetic small ligand for dimerization (AP20187), and not endogenous FKBP ligand. (Clackson et al. 1998). A 2418 bp BamHI/NheI ItrkA fragment was then excised after the insertion of an EcoRI/NheI/AgeI linker (5′-AATTGGATCCGCTAGCG-3′ & 5′-AATTCGCTAGCGGATCC-3′) and cloned 3′ of a CAG promoter (Niwa et al. 1991) into a self-inactivating lentivirus vector based on pRRL (Follenzi et al. 2000; Zufferey et al. 1998). An internal-ribosomal entry site (IRES) for GFP reporter gene expression was also included to generate the plasmid p156sinRRL-ItrkA-IRES-GFP. The same lentiviral plasmid expressing only GFP was used as control.
Production of lentiviral vectors
Third generation lentiviral vector plasmids with a split genome packaging system were used for the production of HIV vectors. VSV-G pseudotyped lentivirus was generated by transient co-transfection of a vector construct (15 μg) with the VSV-G expressing construct pMDG (5μg) and the packaging construct (10 μg) into 293T cells, as previously described (Blesch 2004).
PC12 cell culture and transduction
PC12 cells were maintained in RPMI 1640 containing 10% heat inactivated horse serum and 5% fetal bovine serum and Penicillin-Streptomycin-Glutamine (complete medium; Invitrogen, Carlsbad, CA) at 37°C and 5% CO2 as described (Greene and Tischler 1976). Cells were seeded in complete medium and switched to RPMI with 2% horse serum for neurite extension and to cell culture medium without serum for western blot assays. PC12 cells were transduced with ItrkA lentivirus at an MOI of 1 to minimize transgene copy number. Cells were cultivated for 1 week to maximize GFP expression and all GFP positive cells irrespective of their GFP expression level were selected by fluorescent activated cell sorting.
PC12 Neurite Outgrowth Assays
For neurite outgrowth assays, cells were plated in complete RPMI medium at a density of 5×103 cells/ml on collagen (50 μg/ml) coated 12-well tissue culture plates. For each treatment group, cells were seeded in triplicates or quadruplicates. 24 hours after seeding, cells were switched to low serum medium (2% horse serum) and treated as indicated. AP20187 stock solutions (molecular mass 1428.8 Da; Ariad Pharmaceuticals) were prepared according to the manufacturer’s instructions in 100% ethanol and cells were treated at a final ethanol concentration of 0.1%. Controls received only 0.1% ethanol. NGF (gift of Genentech, San Francisco, CA) were diluted in an appropriate volume of PBS and cells were treated at a final concentration of 50 ng/ml NGF.
For time series experiments, neurite extension was quantified daily for 4 days and cell culture medium with or without treatment was exchanged every 2 days. Neurite outgrowth was quantified by counting neurite bearing cell clumps in 3 non-overlapping random fields of view (top, middle, bottom) per well using an inverted microscope at 100X magnification using phase contrast illumination. All quantifications were made by an observer blinded to treatment group. Clumps of 2–5 cells extending neurites at least twice the diameter of the cell soma were scored as positive. All data are expressed as a percentage of neurite bearing clumps relative to the total number of cell clumps counted. For dose-response experiments, cells were incubated for 72 hours without changing the culture medium. Neurite extension was quantified after 72 h as described above.
Western Blot
PC12 cells were plated on collagen coated 6-well tissue culture dishes and maintained in complete medium until they reached about 50% confluency. 12 hours prior to protein extraction, cells were replenished with serum-free RPMI containing 1% Penicillin-Streptomycin-Glutamine. Cells were treated for 40 minutes with NGF (50ng/ml), 1.25 μM AP20187 (diluted in ethanol) and ethanol alone as control as indicated. Following treatment, cells were washed 3 times with ice-cold PBS and placed on ice. 500 μl heated 1× loading buffer (2% SDS, 15% glycerol, 50 mM Tris/HCl, 0.1% bromophenol blue, 0.1M DTT) was added per well and extracts were passed 5 times through a 28-G 1cc syringe. 14 μl protein extract/well were separated on 7% Tris-Acetate polyacrylamide gel (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes for immunoblotting. Membranes were probed with the following primary antibodies: HA (1:500; Santa Cruz Biotech., Santa Cruz, CA), Erk1/2 (1:1000; Cell Signaling Tech.), pErk1/2 (1:1000; Cell Signaling Tech.), Akt (1:1000; Cell Signaling Tech.) and pAkt (at 1:1000; Cell Signaling Tech.). Peroxidase conjugated anti-rabbit IgG secondary antibodies (Jackson Immunoresearch Labs, West Grove, PA) were used at 1:100,000 dilution and chemiluminescent detection was performed according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL).
E15 dorsal root ganglion (DRG) neuron cultures
DRGs were dissected from E15 rat embryos, tissue was digested in 0.125% trypsin for 30 min, and after addition of 500 μl soybean trypsin inhibitor (100μg/ml) and DNAse (80μg/ml), cells were triturated and washed in culture medium (D’MEM/Ham’s F12 with N2 supplement). Cells were plated (30,000 cells per dish) under low neurotrophin (0.2 ng/ml NGF) conditions in 35 mm tissue culture plates coated with poly-L-lysine (20 μg/ml) and laminin (10 μg/ml). Cells were immediately transduced with GFP or ItrkA lentivirus at a multiplicity of infection of 50 and treated with AP20187 at a concentration of 125 nM (1:100,000 dilution in PBS from a 12.5 mM stock solution in 100% ethanol). Controls were treated with 0.001% ethanol in PBS. After 72 hours, cells were treated with Calceine live cell stain (Invitrogen, Carlsbad, CA) and photographed at 100x magnification. The longest neurite of 100 cells per dish was measured using ImageJ using the NeuronJ plugin.
Statistical analysis
Data are presented as mean ± standard error of the mean. Groups were compared using analysis of variance (ANOVA) or repeated measures ANOVA for time course studies followed by Fischer’s posthoc analysis. Data were considered significant at p<0.05.
RESULTS
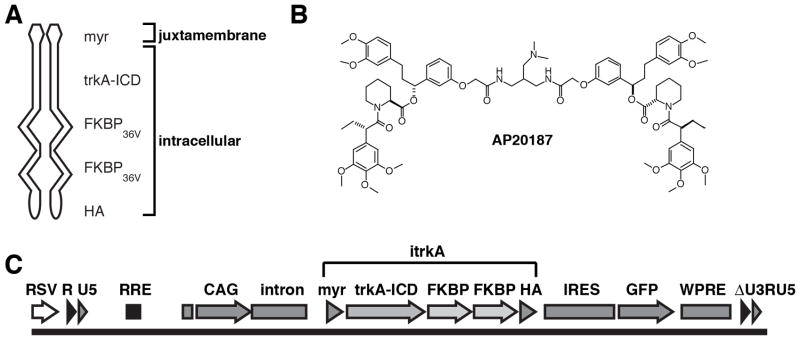
To regulate trkA signal transduction and to allow for stable gene expression in neurons, we generated a lentiviral vector expressing the intracellular domain of trkA including the juxtamembrane and activation loop domains (trkA-ICD), fused to two modified FK506 binding protein domains (FKBP36V) and an amino-terminal myristoylation signal (myr) for membrane localization. A carboxy-terminal hemagglutinin tag (HA) was included for immunocytochemical detection (Fig. 1), and an internal ribosome entry site followed by a GFP expression cassette monitored gene expression.
Figure 1. Schematic vector map and components of the inducible trkA receptor (ItrkA) system.
(A) A chimeric protein was constructed by fusing the entire intracellular domain of the trkA receptor (trkA-ICD), including the juxtamembrane and activation loop domains, to two modified FK-506 binding protein domains (FKBP36V), an amino-terminal myristoylation signal (myr) for membrane localization (juxtamembrane) and a carboxy-terminal hemagglutinin tag (HA). The entire protein is located intracellularly. (B) Chemical structure of the small molecule ligand AP20187, a derivative of FK506, used for the dimerization of modified FK-506 binding protein domains. (C) Lentiviral construct for the expression ItrkA also expressed GFP from an internal ribosome entry site (IRES), allowing vector-transduced cells to be identified by GFP labeling. Control vectors expressed GFP alone (Lenti-GFP). RSV: Rous Sarcoma Virus promoter/enhancer, RRE: rev response element, CAG Promoter: CMV enhancer/chicken β-actin promoter, WPRE: Woodchuck posttranscriptional response element.
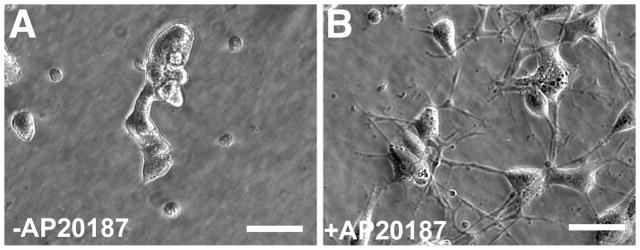
To examine the ability of this inducible trkA (ItrkA) construct to activate trkA signaling cascades in response to a small molecule ligand for dimerization (AP20187), an ItrkA expressing cell line was generated by transducing PC12 cells with ItrkA lentivirus followed by fluorescence activated cell sorting for GFP expression. Treatment of transduced PC12 cells with AP20817 in the cell culture medium for 3 days resulted in robust extension of neurites whereas vehicle treated PC12 cells showed very little neurite extension. These findings indicate that morphological responses normally observed after NGF treatment can be obtained by AP20187-induced dimerization of ItrkA (Fig. 2).
Figure 2. PC12 cell neurite outgrowth in response to ItrkA dimerization by AP20187.
Phase contrast images of PC12 cells transduced by lentiviral gene transfer to express ItrkA. PC12 cells were treated with (A) vehicle (0.1% ethanol) or (B) 1.25 μM AP20187 in 0.1% ethanol. Neurite extension in (B) AP20187 treated cells was robust, with 90 percent of cells responding by day 4. In contrast, very little neurite extension was observed in (A) vehicle treated cells. Scale bar=22 μm in (A, B).
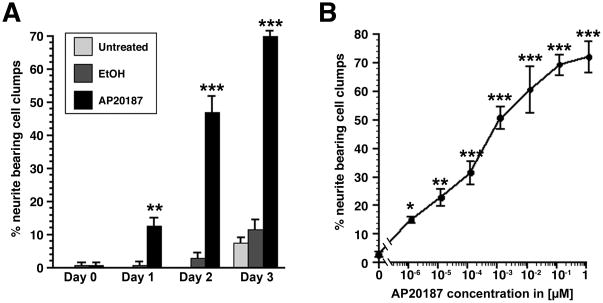
A time course analysis of neurite outgrowth in the presence or absence of AP20187 indicated that as early as 1 day after addition of AP20187 to the cell culture medium, neurite growth responses could be observed, with increasing numbers of cells extending neurites after 2 and 3 days of AP20187 treatment (Fig. 3A, repeated measures ANOVA p<0.0001 for time, and treatment versus time, posthoc analysis p<0.001 compared to untreated and vehicle treated cells at each time point). In contrast, untreated cells or cells treated with vehicle (0.1% ethanol) showed very few neurites and were not significantly different from each other at any time point.
Figure 3. Dose dependence and kinetics of neurite extension in ItrkA transduced PC12.
(A) Time course analysis demonstrates that that neurite outgrowth can be induced as early as 1 day after AP20187 addition and subsequent increases in the percentage of responding cells after 2 and 3 days. In contrast only very few neurite bearing cell clumps are found in untreated cells or cells treated with vehicle (0.1% ethanol). (repeated measures ANOVA p<0.0001 for time, and treatment versus time, posthoc analysis **p<0.001, *** p<0.0001 compared to untreated and vehicle treated cells) (B) With increasing doses of AP20187 in the cell culture medium the percentage of neurite-bearing clumps increases. Significant neurite extension compared to vehicle treated control conditions can be observed at picomolar concentrations with an ID50 of about 1 nM after 3 days of treatment (ANOVA p<0.0001; Posthoc analysis *p<0.05, **p<0.001, *** p<0.0001 compared to control)
In initial experiments, cells were treated with 1.25 μM AP20187. However, substantially lower levels of AP20187 might be capable of inducing ItrkA signaling, and ideally various levels of morphological responses should be obtainable depending on the amount of AP20187 present. Analysis of the dose response curve of ItrkA transfected PC12 cells exposed to various doses of AP20187 demonstrated that concentrations of AP20187 as low as 1 picomolar induced significant neurite outgrowth compared to vehicle treated cells (Fig. 3B, ANOVA p<0.0001; posthoc analysis p<0.05 for concentrations of 1.25 pM). No significant further increases in neurite outgrowth were observed at concentrations above 125nM (p=0.62 comparing 125nM to 1.25 μM) and half-maximal responses within the AP20187 concentration range tested were obtained around1 nM. Thus, morphological responses are dependent on the concentration of AP20187 in the medium and can be accordingly modulated, similar to dose-response curves following NGF modulated neurite outgrowth.
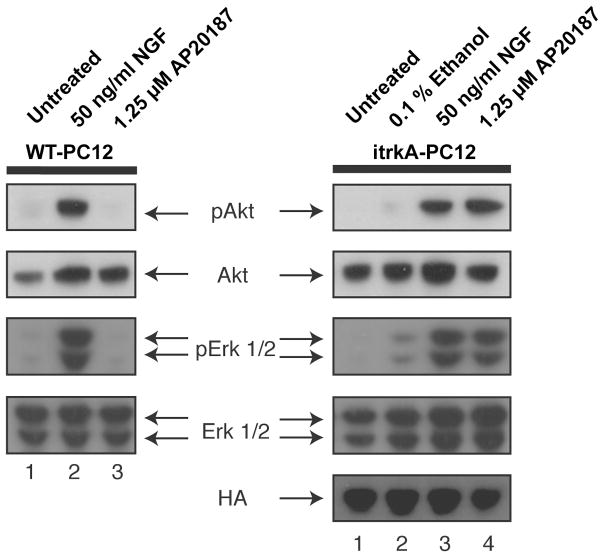
NGF-induced cell signaling via trkA is mediated through two separate signaling cascades. Signals for differentiation are thought to depend on the activation of Erk1/2 and signals for cell survival utilize the PI3K/AKT cascade (Ashcroft et al. 1999; Cowley et al. 1994; Patapoutian and Reichardt 2001). To determine whether ItrkA dimerization induces the same downstream signaling cascade as NGF binding to trkA, phosphorylation of AKT and ERK1/2 were investigated in naïve and ItrkA transduced PC12 cells in response to NGF and AP20187.
Stimulation of naïve and ItrkA-transduced PC12 cells with 50 ng/ml NGF resulted in similar increases in AKT and ERK1/2 phosphorylation in comparison to untreated control cells indicating that ItrkA overexpression did not interfere with normal NGF downstream signaling (Fig. 4). Treatment of ItrkA-transduced cells with AP20817 resulted in similar downstream signaling events as NGF binding to the full-length receptor. AP20817 increased phosphorylation of AKT and ERK1/2 compared to untreated cells or cells treated with vehicle (0.1% ethanol). In contrast, AP20187 treatment of naïve control cells did not stimulate AKT and ERK1/2 phosphorylation.
Figure 4. Western blot analysis of trkA downstream signaling in naïve and ItrkA-transduced PC12 cells.
Naïve untransduced PC-12 cells (WT-PC12, left) and ItrkA-transduced cells (ItrkA-PC12, right) were untreated or treated for 40 min with 0.1% ethanol (diluent for AP20187), 50 ng/ml NGF or 1.25 μM AP20187 in 0.1% ethanol and processed for Western blotting. AP20187 increases phosphorylation of AKT and ERK1/2 (pAKT, pERK1/2) to a similar extent as NGF in ItrkA transduced cells (right) but not in naïve PC12 cells (left). Total amounts of AKT and ERK1/2 are similar between groups. Labeling for the hemagglutinin tag (HA) in ItrkA indicates similar levels of ItrkA in transduced cells, no HA immunoreactivity was observed in naïve PC12 cells (not shown).
Taken together, ItrkA dimerization by AP20817 appears to result in the same downstream signaling events as NGF binding to full-length trkA. Baseline levels of phosphorylated ERK1/2 and AKT are similar in ItrkA-transduced and naïve cells indicating a tight control of ItrkA phosphorylation. Furthermore, ItrkA overexpression does not interfere with normal NGF signaling mechanisms in PC12 cells.
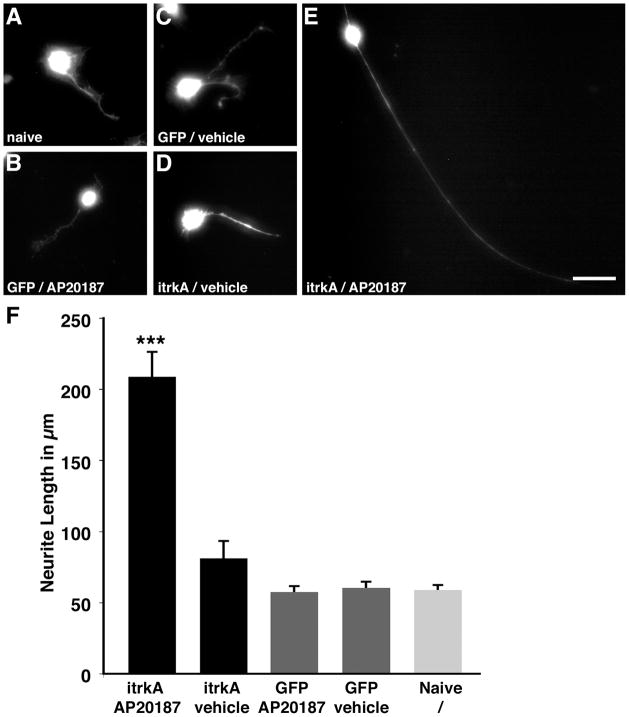
To determine whether ItrkA signaling can also induce classical responses to neurotrophin receptor activation in primary neurons, embryonic DRG neurons were isolated and transduced with ItrkA lentivirus or GFP virus as control. Examination of GFP fluorescence indicated that virtually all cells were transduced by lentivirus. Incubation of ItrkA transduced DRG neurons with AP20187 potently stimulated neurite growth compared to ItrkA transduced neurons incubated with vehicle. In contrast, neurite length of cells transduced with GFP control virus did not differ between AP20187 or vehicle treated cells and was not significantly different from non-transduced naïve neurons (Fig. 5A–E). Quantification of mean neurite lengths indicated a highly significant 3–4 fold increase in neurite length in AP20187-stimulated ItrkA-transduced neurons compared to all other groups (ANOVA; p < 0.0001). Neurite length of vehicle-treated ItrkA transduced neurons was not significantly different from GFP-transduced neurons, and AP20187 treatment did not affect neurite length in GFP-transduced neurons (Fig. 5F).
Figure 5. Inducible neurite outgrowth in E15 DRG neurons.
E15 dorsal root ganglion neurons were plated under low neurotrophin (0.2 ng/ml NGF) conditions and remained (A) untransduced, or were transduced with (B, C) GFP expressing control lentivirus or (D, E) ItrkA virus. Treatment with AP20187 (125 nM) results in increased neurite growth only in (E) ItrkA-transduced DRG cultures but not in (B) GFP expressing DRG neurons. (F) Quantification of mean neurite length after Calceine live cell staining demonstrates that AP20187 treatment of ItrkA significantly increases neurite length (ANOVA p<0.0001, Fischer’s posthoc test p<0.0001 comparing AP20187-ItrkA to all other groups). Scale bar=25 μm.
DISCUSSION
The present study investigated whether trkA signal transduction and biological responses can be regulated by a chimeric fusion protein containing only the membrane anchored intracellular domain of trkA fused to two domains for small ligand-induced dimerization. Our data indicate that expression of a regulated, inducible trkA fusion protein (ItrkA) by lentiviral gene transfer can mimic NGF activity in PC12 cells and primary neurons but only in the presence of the dimerizing agent, AP20187. Undetectable baseline activity, tight regulation of signaling activity and graded biological responses that can be detected at picomolar concentrations of dimerizer are additional characteristics of the system described.
Notably, the ItrkA system activates signaling pathways important for both survival (Akt) and differentiation (MAPK) suggesting that this system is able to mimic endogenous NGF-trkA signaling (Patapoutian and Reichardt 2001). ItrkA overexpression does not interfere with normal NGF signal transduction in PC12 cells either by quenching or dimerizing with naive trkA, because ItrkA transduced PC12 cells respond with similar levels of Erk and Akt phosphorylation and neurite outgrowth as naïve PC12 cells following NGF stimulation. Thus, the system described in this study satisfies several important criteria for regulated signal activation: low basal levels of signal transduction, activation at very low concentration of dimerizing agent, activation of the same intracellular signaling cascades as naive ligand-receptor interactions, and lack of interference with normal ligand-receptor interaction.
We did not subclone ItrkA transduced PC12 cells to examine whether intracellular signaling and neurite outgrowth differ between high ItrkA and low ItrkA expressing cells. It is possible that very high expression levels of ItrkA result in autoactivation of ItrkA in the absence of the small molecule ligand AP20187 and thereby increase the leakiness of this system. Alternatively, very high expression levels might be necessary for cells to respond to AP20187 signaling and therefore only a subpopulation of ItrkA-transduced PC12 cells responded to AP20187 treatment. However, our results do not support these conclusions: 1) PC12 cells were transduced with a low multiplicity of infection and only a small subset of PC12 cells were found to be GFP positive before FACS. Thus, the majority of cells likely contained only a single viral copy and this was also reflected in the relatively uniform GFP expression. 2) Western blot analysis indicated similar increases in Akt and Erk phosphorylation after NGF and AP20187 treatment of ItrkA transduced PC12 cells. Since the vast majority of PC12 cells are known to respond to NGF with Akt and Erk phosphorylation, similar amounts in pAkt and pErk after NGF and AP20187 treatment indicate that the percentage of cells responding to AP20187 and NGF is similarly high. 3) Quantification of neurite length in primary DRG neurons did not indicate large variations in neurite growth in ItrkA-transduced DRG neurons after AP20187 treatment indicating responses from the majority of ItrkA-transduced neurons. Furthermore, no significant differences in neurite length were found between ItrkA-transduced neurons and control GFP-transduced neurons in the absence of ItrkA. Thus, signaling and biological responses were tightly regulated under the conditions investigated.
Recent improvements in viral gene therapy vectors have provided for the first time the opportunity to test whether neurotrophic factor delivery can augment neuronal survival and function in neurodegenerative disorders such as AD and Parkinson’s disease (Tuszynski 2007; Tuszynski et al. 2005). Neurotrophic factor gene therapy could benefit from the use of reliable systems that control either the amount of neurotrophic factor expression or from the use of cell-specific means to regulate signal transduction. We and others have previously explored the possibility of regulating neurotrophin gene expression using tetracycline-regulated expression systems to control neuronal survival and axonal growth (Blesch et al. 2005; Blesch et al. 2001; Blesch and Tuszynski 2007; Georgievska et al. 2004; Regulier et al. 2002). These studies have provided proof-of-concept; however, immune responses to prokaryotic components of tetracycline-regulated systems remain a concern (Favre et al. 2002; Latta-Mahieu et al. 2002). While detailed studies investigating the potential immunogenicity of modified FKBP-containing signal transduction have not been conducted to date, the fact that this system is entirely derived from human proteins may minimize potential adverse immune effects. As an additional advantage, downstream effects can be limited to selected cells in conjunction with an appropriate delivery vector and a cell specific promoter, thereby further limiting the risk for potential adverse effects of neurotrophic factors on non-targeted structures. Application of this system in animal models of neurodegenerative disorders will require sufficient concentrations of AP20187 to cross the blood-brain barrier. Although no information has been reported to date about the distribution and pharmacokinetics of AP20187 in vivo, it is likely that sufficient concentrations of AP20187, a derivative of FK506, which can readily cross the blood-brain barrier (Klettner and Herdegen 2003), can be achieved within the CNS.
ItrkA activation might also provide a novel means of enhancing axonal regeneration after injury. While our experiments were not designed to distinguish between localized axonal and somal ItrkA signaling and retrograde transport of activated ItrkA signaling complexes, our results suggest that both survival and differentiation signals can be activated by membrane proximal ItrkA dimerization in the absence of NGF. Future experiments using compartmentalized neuronal cultures in vitro or localized application of the small molecule ligand AP20187 to axons distant from the cell body in vivo will allow us to investigate potential retrograde ItrkA signaling and its effects on neuronal survival and/or axon growth.
In summary, we have generated an inducible trkA signaling system that, when paired with lentiviral delivery, permits dose-dependent spatiotemporal regulation of trkA signaling. We have demonstrated the utility of this system in vitro in PC12 cells and primary neurons. Future experiments will examine whether ItrkA delivery and signaling can prevent neuronal degeneration and induce axon outgrowth in animal models of neurodegenerative disease and spinal cord injury.
Acknowledgments
Supported by grants from NIH (NS46466, NS54833, NS047101, NS09881), the International Spinal Research Trust, Veterans Administration, Adelson Medical Research Foundation, Craig H. Neilsen Foundation and Wings for Life. R.W.A. was supported by the Stein Institute for Research on Aging Student Investigator Program, UC Leads undergraduate research program and an Amylin Pharmaceuticals Undergraduate Research award. We thank Ariad Pharmaceuticals for providing the plasmid pC4M-FV2E and dimerizer AP20187.
References
- Allocca M, Di Vicino U, Petrillo M, Carlomagno F, Domenici L, Auricchio A. Constitutive and AP20187-induced Ret activation in photoreceptors does not protect from light-induced damage. Invest Ophthalmol Vis Sci. 2007;48(11):5199–5206. doi: 10.1167/iovs.07-0140. [DOI] [PubMed] [Google Scholar]
- Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR. The selective and inducible activation of endogenous PI 3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite outgrowth. Oncogene. 1999;18(32):4586–4597. doi: 10.1038/sj.onc.1202814. [DOI] [PubMed] [Google Scholar]
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33(2):164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Blesch A, Conner J, Pfeiffer A, Gasmi M, Britton W, Alfa R, Verma I, Tuszynski MH. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol Ther. 2005;11(6):916–925. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Blesch A, Conner JM, Tuszynski MH. Modulation of neuronal survival and axonal growth in vivo by tetracycline-regulated neurotrophin expression. Gene Therapy. 2001;8:954–960. doi: 10.1038/sj.gt.3301480. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J Neurosci. 2007;27(39):10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, Lu X, Cerasoli F, Jr, Gilman M, Holt DA. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci U S A. 1998;95(18):10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Delort JP, Capecchi MR. TAXI/UAS: A molecular switch to control expression of genes in vivo. Human Gene Therapy. 1996;7(7):809–820. doi: 10.1089/hum.1996.7.7-809. [DOI] [PubMed] [Google Scholar]
- Fan L, Freeman KW, Khan T, Pham E, Spencer DM. Improved artificial death switches based on caspases and FADD. Hum Gene Ther. 1999;10(14):2273–2285. doi: 10.1089/10430349950016924. [DOI] [PubMed] [Google Scholar]
- Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, Marme F, Cherel Y, Salvetti A, Hurtrel B, Heard JM, Riviere Y, Moullier P. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76(22):11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nature Genetics. 2000;25(2):217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Freche B, Guillaumot P, Charmetant J, Pelletier L, Luquain C, Christiansen D, Billaud M, Manie SN. Inducible dimerization of RET reveals a specific AKT deregulation in oncogenic signaling. J Biol Chem. 2005;280(44):36584–36591. doi: 10.1074/jbc.M505707200. [DOI] [PubMed] [Google Scholar]
- Fussenegger M, Morris RP, Fux C, Rimann M, von Stockar B, Thompson CJ, Bailey JE. Streptogramin-based gene regulation systems for mammalian cells. Nat Biotechnol. 2000;18(11):1203–1208. doi: 10.1038/81208. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Jakobsson J, Persson E, Ericson C, Kirik D, Lundberg C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum Gene Ther. 2004;15(10):934–944. doi: 10.1089/hum.2004.15.934. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klettner A, Herdegen T. FK506 and its analogs - therapeutic potential for neurological disorders. Curr Drug Targets CNS Neurol Disord. 2003;2(3):153–162. doi: 10.2174/1568007033482878. [DOI] [PubMed] [Google Scholar]
- Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I, Loquet I, Dedieu JF, Mahfoudi A, Trannoy E, Thuillier V. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther. 2002;13(13):1611–1620. doi: 10.1089/10430340260201707. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Welm BE, Freeman KW, Spencer DM, Rosen JM, Isaacs HV. An inducible system for the study of FGF signalling in early amphibian development. Dev Biol. 2003;256(1):89–99. doi: 10.1016/s0012-1606(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Regulier E, Pereira de Almeida L, Sommer B, Aebischer P, Deglon N. Dose-dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington’s disease. Hum Gene Ther. 2002;13(16):1981–1990. doi: 10.1089/10430340260355383. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262(5136):1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):179–189. doi: 10.1097/WAD.0b013e318068d6d2. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, UHS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(6):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Weber W, Fux C, Daoud-el Baba M, Keller B, Weber CC, Kramer BP, Heinzen C, Aubel D, Bailey JE, Fussenegger M. Macrolide-based transgene control in mammalian cells and mice. Nat Biotechnol. 2002;20(9):901–907. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- Welm BE, Freeman KW, Chen M, Contreras A, Spencer DM, Rosen JM. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J Cell Biol. 2002;157(4):703–714. doi: 10.1083/jcb.200107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72(12):9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]