Abstract
Graves disease represents a systemic autoimmune process targeting the thyroid, orbit, and pretibial skin. The thyroid dysfunction is treatable, but no consistently effective medical therapy has yet been described for the orbital manifestations of Graves disease, also known as thyroid-associated ophthalmopathy or thyroid eye disease. Several autoantigens are potentially relevant to the pathogenesis of thyroid eye disease. Activating antibodies generated against the thyrotropin receptor can be detected in a majority of patients, and these drive hyperthyroidism. However, stimulating antibodies against the insulin-like growth factor-1 receptor (IGF-1R) may also play a role in the extra-thyroid manifestations of GD. IGF-1R is over-expressed by orbital fibroblasts derived from patients with TED, while IGF-1R+ T and IGF-1R+ B cells are considerably more frequent in GD. Actions of several cytokines and the molecular interplay peculiar to the orbit appear to provoke the inflammation, fat expansion, and deposition of excessive extracellular matrix molecules in thyroid eye disease. Based upon these new insights, several therapeutic strategies can now be proposed that, for the first time, might specifically interrupt its pathogenesis.
Keywords: Graves ophthalmopathy, immunology, thyroid
I. Introduction
The unique features of Graves disease (GD) have both fascinated and frustrated the medical community for the 200 years since its first description. GD is an autoimmune disease where circulating antibodies cause hyperthyroidism and lead to thyrotoxicosis. These antibodies, originally referred to as long-acting thyroid stimulators, are directed against the thyrotropin receptor (TSHR). They mimic the agonist activity of TSH but are not subject to the normal feedback in the anterior pituitary. GD is approximately 7 to 10 fold more frequent in women, and typically occurs between 20 and 50 years of age.72
Clinical manifestations of GD include thyroid enlargement and thyrotoxicosis, inflammation and remodeling of the orbit, and rarely dermopathy. The orbital disease is collectively known as thyroid-associated ophthalmopathy or thyroid eye disease (TED). It is unclear why anatomically unrelated tissues undergo coordinate and selective immune infiltration and remodeling. Furthermore, the mechanistic basis for the self-limited course of the orbital disease is unclear, but identifying these underlying factors could provide insights necessary for the development of effective therapies.
This article summarizes our current understanding of TED, focusing on the fundamental aspects of its molecular pathogenesis. In it we identify attractive potential targets for interrupting the disease.
II. Immunology of GD
Adults normally exhibit tolerance to antigens that are present during fetal life and thus are recognized as “self.” Under certain circumstances, however, tolerance may be lost, leading to immune reactions against self that manifest clinically as autoimmune disease. Proposed mechanisms for autoimmunity include molecular mimicry, abnormal protein modification, release of ordinarily sequestered antigens, and epitope spreading.
Development of disease requires participation of self-reactive helper (CD4+) T cells. These Th1, Th2, and Th17cells can support both cell and antibody-mediated autoimmune responses. It remains likely that common mechanisms for auto antigen generation and both T and B cell activation link several, if not all, autoimmune diseases. Thus, rheumatoid arthritis, type 1 diabetes mellitus, and systemic lupus erythematosis may share pathogenic features with GD. This is the rationale for exploring whether therapeutic agents exhibiting activities in one disease might benefit those with another. While GD is a systemic disease, its manifestations exhibit an anatomic-site selective predilection. Thyroid dysfunction is the principal hallmark of GD and occurs in greater than 90% of patients sometime during the course of their disease.
Hyperthyroidism results from activating antibodies that bind to TSHR on thyroid epithelial cells and mimic the actions of TSH. In addition to stimulating antibodies directed against TSHR (TSAb), those blocking that receptor can also be detected in patients with hyperthyroidism, and a shift in the balance between these two types of antibodies can result in hypothyroidism in as many as 15% of patients.61 In addition to these pathogenic antibodies, those generated against thyroid peroxidase (TPO) and thyroglobulin (TG) can often be detected. TSAb uniformly belong to IgG1 subclass, whereas antibodies against other thyroid antigens are not IgG1 restricted.98,100 Overall, the clinical manifestations of glandular GD are predictable and can be treated with relative ease in the vast majority of patients.86 A detailed description of the endocrine derangements associated with GD is beyond the scope of this article, and can be found elsewhere.47,77,105
III. Clinical Course of TED
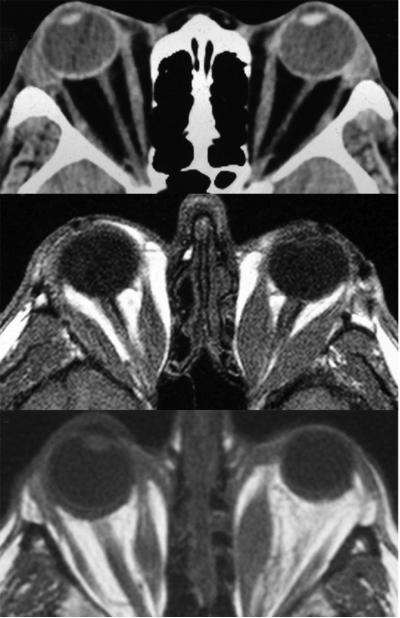
Approximately 25-50% of patients with GD develop TED, while sight-threatening disease occurs in 5% of patients.7 Conversely, 10% of those manifesting with TED fail to become hyperthyroid. Regardless of whether thyroid dysfunction or TED develops first, the other becomes apparent within 18 months in 85% of patients with GD. Rundle was the first to divide the course of TED into active (dynamic) and inactive (static) disease phases. Signs and symptoms of active TED include proptosis, conjunctival injection, chemosis, diplopia, corneal ulceration, and rarely loss of sight from optic nerve compression. The tissue expansion occurs within the relatively fixed volume imposed by the bony orbit and results from inflammation, accumulation of glycosaminoglycans (GAGs), and increased fat content. Compression within the tight orbit can compromise venous drainage, potentially increasing the retrobulbar pressure and leading to chemosis and periorbital edema. Idiosyncratic variations in orbital shape and vessel location may render a subset of patients with GD more susceptible to severe TED.45 The disease is often asymmetrical. Computed tomography can demonstrate predominant expansion of muscle, fat, or both as illustrated in Fig. 1.
Figure 1.
The three predominant forms of soft tissue involvement in TED. Predominantly fat expansion (top), predominantly muscle enlargement (middle), or a combination of both (bottom).
Inactive disease is characterized by stable proptosis, eyelid retraction, and may be accompanied by persistent restrictive strabismus, with resolution of inflammation, usually within 18-24 months of its first appearance. The self-limited nature of TED is peculiar among human autoimmune diseases. Anti-inflammatory therapy, such as corticosteroids are effective only during the active phase, whereas surgical intervention is usually performed once this phase has subsided.
Genetic factors appear to contribute to disease susceptibility, as is suggested by increased incidence of concordance among monozygotic twins.19,98 Specific genetic alterations peculiar to GD, including polymorphisms, have been difficult to identify across multiple ethnicities; however, candidates include major histocompatibility complex (MHC) class II, protein tyrosine phosphatase-22, CD40, and cytotoxic T lymphocyte antigen 4 (CTLA4).47,51,98
Environmental factors, particularly, cigarette smoking, appear to increase the incidence and severity of TED. Moreover, tobacco use reduces the response to therapies.26,103,112 Cawood et al in an in vitro study demonstrated that cigarette smoke extract induced adipogenesis and increased hyaluronic acid production by orbital fibroblasts, and was synergistic with IL-1 in inducing adipogenesis.26 Tissue hypoxia leading to the formation of superoxide radicals may induce orbital fibroblasts (OFs) from patients with TED to proliferate, synthesize GAGs and undergo adipogenesis.21,26,87
Thyroid dysfunction appears to run a clinical course that is independent of TED, but treatment of hyperthyroidism might carry important consequences to the orbital process. Radioiodine therapy can be associated with mild, transient worsening of TED.71 Routine use of steroids immediately before and following radioiodine therapy remains controversial.9,114 It may not be necessary in all patients, but is widely recommended for those with severe orbitopathy. Persistence of either hypo- or hyperthyroidism correlates with increased severity of TED.9 Hence, maintenance of the euthyroid state may be of importance and is therefore strongly advocated.
IV. Immunology of TED
The active phase of TED is characterized by orbital and periorbital inflammation targeting connective tissue and fat.101,102 Electron and light microscopy suggest that the muscle cells remain intact early in the disease;59,128 however, intense infiltration between extraocular muscle fibres and in orbital fat of T lymphocytes, mast cells and occasional B cells suggests that connective tissue represents the primary autoimmune target.134,135
Immunohistochemical evidence of cytokines, including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1α (IL-1α) has been reported in the connective tissues, and their presence is associated with T cell infiltration.59 These cytokines may be produced by infiltrating mononuclear cells and resident fibroblasts since they are also detected in areas devoid of mononuclear infiltration.55 Specifically, IL-1α is a proinflammatory cytokine produced by monocytes, macrophages, and fibroblasts that may play a critical role in promoting inflammation and extracellular matrix proteins.48 Extensive deposition of hyaluronan in the interstitium dominates the histological picture of TED, and is associated with orbital tissue expansion.62,63,84,93,113,128
The stable phase of TED is defined by resolution of inflammation associated with clinical improvement.10,11,12 The pattern of Th1 cytokine predominance found in active disease may skew toward Th2 cytokines such as IL-4, IL-5, and IL-13 during the stable phase.4,49 This shift could alter immune cell trafficking, promote tissue fibrosis, or promote disease resolution.57,90 Th2 cytokine involvement in the pathogenesis of GD was discovered by chance. Patients diagnosed with the Th1 predominant disease, multiple sclerosis, were treated with a monoclonal antibody against CD52, which depletes >95% of circulating T lymphocytes.28 Amelioration of that disease appeared secondary to decreasing Th1 T cells. However, 18 months after treatment, patients underwent B cell expansion, presumably due to unopposed Th2 cytokines. One-third of these patients developed GD with detectable TSAb.28
Factors underlying the spontaneous resolution of inflammation in TED remain unidentified. The possibilities include declining auto-antigen abundance or reduced antigen presentation.3,33 The targets of other autoimmune diseases, such as synovial tissue in rheumatoid arthritis, exhibit recognizable lymphoid structures.25,80,83,127 In contrast, the orbit lacks these structures. Thus, TED is not associated with the lymphoid neogenesis that might be crucial to sustained immune activation.
A. ROLE OF ORBITAL FIBROBLASTS (OF) IN THE PATHOGENESIS OF TED
Several studies have demonstrated that OF, especially those from patients with GD, are unique with respect to how they respond to several proinflammatory cytokines.27,122,136 The divergent phenotype of these cells may underlie the anatomic site-selective manifestations of GD.118,119,123 Cao and Smith reported some time ago that OF, unlike dermal fibroblasts, fail to generate adequate levels of soluble IL-1 receptor antagonist. This would allow poorly opposed IL-1β signaling.23 In comparison to control OF, those from patients with GD over-produce prostaglandin E2 (PGE2) in response to IL-1β, CD154, and leukoregulin as a result of the coordinate induction of prostaglandin endoperoxide H synthase-2 (PGHS-2) and the microsomal PGE2 synthase genes.23,50 They also exhibit enhanced production of extracellular matrix components such as hyaluronan in response to these cytokines (Fig. 2). Thus, GD OF produce proinflammatory molecules and components of connective tissue that lend themselves to the site-specific tissue remodeling occurring in TED.62,121
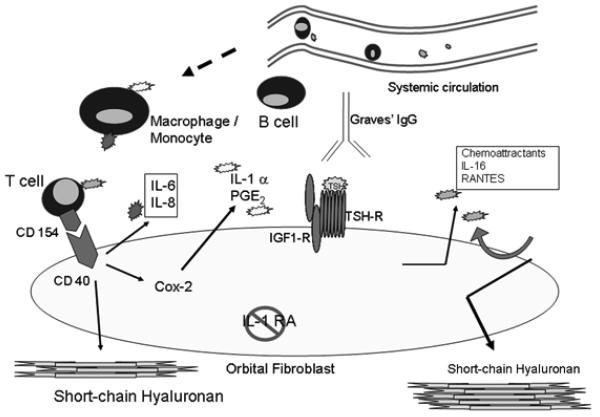
Figure 2.
Cartoon of our current model for the interaction between orbital fibroblasts and members of the professional immune system and the small molecules they produce. Chemoattractant molecules such as IL-16 and regulated on activation, normal T cell expressed (RANTES) are generated in response to Graves disease-IgG (GD-IgG) acting on the fibroblast. This in turn leads to the recruitment of T cells and other mononuclear cell members of the immune system. When activated, these cells produce a number of proinflammatory cytokines such as IL-1a, IL-1b, CD154 (CD40 ligand), and IL-6. Cytokines in turn activate proinflammatory genes such as those encoding prostaglandin endoperoxide H synthase-2 (PGHS-2), IL-6, IL-8, hyaluronan synthase (HAS), and UDP glucose dehydrogenase (UGDH). The major factor thus far identified as explaining the exaggerated responses to cytokines concerns the low levels of IL-1 receptor antagonist (IL-1RA) expressed by orbital fibroblasts. In addition, IL-4 and IL-13 induce 15-lipoxygenase exclusively in orbital fibroblasts from patients with GD, perhaps accounting for the different patterns of inflammation found in TAO.
T cells may also play an important role in OF activation through increased expression of CD40 on the latter.109 CD40 binds CD40 ligand (aka CD154) displayed on the surface of T lymphocytes and provides T cell co-stimulation that results in clonal expansion of naïve T lymphocytes and enhances pro-inflammatory cytokine production, including that of IL-1, IL-6 and IL-8.104 Actions of these in turn activate the expression of PGHS-2, hyaluronan synthase (HAS), and UDP glucose dehydrogenase (UGDH) genes, leading to inflammation and hyaluronan production.24,125 (Tsui, Chen, and Smith, unpublished observations). Thus, disruption of fibroblast – T cell interactions mediated by CD40-CD40 ligand could represent an important therapeutic target in TED. Administration of therapeutic blocking antibodies against the CD40 ligand already has proven effective in pre-clinical mouse models of diabetes and inflammatory bowel disease.16,34,89
Orbital connective tissue comprises a heterogeneous population of OF and this cellular diversity may provide the basis for variations in the clinical presentation of TED. Expression of the surface glycoprotein, Thy-1 has been used to delineate phenotype and function of OF subsets. Those expressing Thy-1, such as perimysial fibroblasts, can differentiate into myofibroblasts, and their capacity for undergoing adipogenesis may be limited.68,120,124 Orbital fat and connective tissue contains both Thy-1+ and Thy-1− fibroblasts.131 Thy-1+ OF differentiate into myofibroblasts when treated with TGF-β.68 They may promote inflammation and orbital fibrosis through their production of IL-6, IL-8 and extracellular matrix components. Thy-1− OF can differentiate into adipocytes.120 An important molecular trigger of adipocyte differentiation is the peroxisome proliferator-activated receptor γ (PPARγ). When activated by agonists, OF and subcutaneous preadipocytes undergo adipogenesis.1, 132 When these agents are administered therapeutically to patients with diabetes, they can exacerbate tissue expansion in TED.30,73,75 A predominance of Thy-1− fibroblasts could contribute to fat expansion in proptotic disease. Thus, the proximate determinants of fibroblast differentiation might represent targets for disease-modifying therapies.120
B. ROLE OF T LYMPHOCYTES IN TED AND ITS THERAPEUTIC IMPLICATIONS
The inflammatory phase of TED is characterized by T cell infiltration, often accompanied by mast cells, B lymphocytes, and macrophages.65 Endogenous ligation of the T cell receptor (TCR) in the absence of co-stimulation is insufficient to activate T cells, but can lead to T cell anergy, tolerance, or depletion.6,14 Activated CD4+CD45RO+ T cells appear numerous in the early orbital infiltrate. They produce cytokines and chemoattractants, which in turn further amplify immune responses.
Given the diverse roles attributed to T cells, their depletion should attenuate these responses.54,65 Down-regulating pathogenic CD4+ T cell activity during autoimmune disease has provided an important rationale for work involving anti-CD3 antibodies that bind to the TCR complex. A number of deleterious side-effects associated with this strategy have been overcome by “humanizing” monoclonal antibodies, reducing Fc receptor binding.115,116,117 These studies have provided encouraging results in type 1 diabetes mellitus.115,116,117 Preclinical studies by Smith et al demonstrate that humanized anti-CD3 [hOKT3γ1 (Ala-Ala)] can either deplete or induce anergy in IL-2 or interferon-γ producing T cells (Th1 cells). Conversely, T cells that produce IL-10 or IL-4 (Th2 cells) may be stimulated by anti-CD3.54,64 These effects occur in activated T cells but are absent in their naïve counterparts. hOKT3γ1 (Ala-Ala) was found to improve glycemic control and preserve residual beta cell function during the first year of type 1 diabetes mellitus.54,64 Side-effects of therapy occur in 50-75% of patients but have not proven to be life-threatening.76 A further refinement of this therapeutic strategy, including the generation of a non-mitogenic form of anti-CD3 (IgG2a Ala–Ala), appears to reduce cytokine release but remains equally efficacious.13,92
Expression of CD25 and the transcription factor Foxp3 is characteristic of regulatory T cells (Tregs).20,85 Mutations of Foxp3 are associated with severe immunopathology.36,58 Reduced frequency of Tregs can result in particularly severe autoimmune disease while increases may be associated with disease remission.111 Although details concerning the mechanisms by which Tregs exert immune suppression remain incomplete, CD4+ and CD8+ T cell function appears to be mediated through IL-4, IL-10 and TGF-β.67
T cell depletion has yet to be examined as a potential therapy in TED, despite evidence that these cells are critical to cell-mediated responses and antibody production. The prominent role for both in the pathogenesis of GD and its orbital manifestations suggests that this avenue of therapeutic intervention might prove rewarding (Table 1). Interruption of T cell activation mediated through CTLA4 can be achieved with antibodies directed against the protein (CTLA4 Ig). This agent blocks CTLA4 association with CD80 and CD86 on antigen-presenting cells, leading to T cell anergy.133 Results with CTLA4 Ig have been promising in an open label phase I trial in rheumatoid arthritis and multiple sclerosis.18,97
Table 1.
Immunotherapy for Thyroid Eye Disease
| Therapy | Target Tissues | Agents |
|---|---|---|
| B-cell targeted therapy |
Membrane proteins, Survival factors, or ligands |
Eprantuzumab, Belimumab, Abatacept, LJP394 |
|
T-cell targeted therapy |
CTLA4 | CTLA4 Immunoglobulin |
| Cytokine mediated therapy |
Etanercept |
C. B LYMPHOCYTES IN GD AND THEIR IMPLICATIONS IN THERAPY DESIGN
In addition to their function as precursors for antibody-secreting plasma cells, B cells efficiently present antigen and produce important cytokines. B cell-deficient mice cannot generate T cell responses following immunization with TSHR, and thus these cells are probably essential to the initiation of autoimmune thyroid disease.5,130 Early plasma cell survival can be mediated by B cell-activating factor (BAFF) receptors that appear critical to the production of autoantibodies.43,81 Autoantibody generation is also dependent on the complex interplay between B and T cells.90
Thus, B cell-depleting therapies and those that interrupt interactions between cognate molecules on B cell surfaces offer great promise in the context of autoimmune disease (Table 2). An important example is rituximab (RTX), a monoclonal antibody that binds the B cell surface antigen CD20. RTX blocks cell proliferation and attenuates CD20-dependent B cell maturation. Plasma cells do not express CD20 and are thus spared from the cell-depleting actions of RTX. Despite this lack of plasma cell depletion, the agent reduces antibody-mediated responses by blocking antigen presentation and cytokine production.17,82 RTX was developed for the treatment of B cell non-Hodgkin's lymphomas and has been used in rheumatoid arthritis and lupus only relatively recently.38 In a multi-center, randomized, double-blind study, a short course of RTX provided patients with rheumatoid arthritis symptomatic improvement for 48weeks. The effect was observed when RTX was used as a single agent or in combination with anti-metabolites such as cyclophosphamide.78 A subsequent dose-escalation study using RTX as monotherapy in 17 patients with lupus found a strong association between reduced disease activity and B cell depletion.110,129 In these studies, peripheral B cell depletion was associated with reduced levels of rheumatoid factor and B cell activation associated antigens.110 In addition, T cell expression of CD40 ligand, CD69, and HLA-DR declined following RTX therapy in lupus.39 Reduction of CD40 ligand levels may be critical since CD40-CD40 ligand interactions are critical to both B and T cell function.41,108
Table 2.
B Cell–Targeted Agents in Autoimmune Disease.
| Agent | Target | Target characteristics |
Mode of action |
|---|---|---|---|
| Rituximab | CD-20 | Membrane protein |
Cytolysis through antibody dependent cellmediated cytotoxicity, complement, and=or apoptosis |
| Eprantuzumab DT2219 |
CD-22 CD-19 and CD- 22 |
Membrane protein Membrane proteins |
Cytolysis with or without agonist is inhibitory Cytolysis through immunotoxin bispecific binding to CD19 and CD22 |
| Belimumab TACI- immunoglobulin |
BAFF BAFF and APRIL |
B-cell survival factor B-cell survival factors |
Sequestration and=or neutralization Sequestration and=or neutralization |
| Abatacept | CTLA4- immunoglobulin |
Negative cell- surface costimulatory ligand |
Modulation of costimulatory pathways |
| LJP394 | BCR | Cell-surface ligand |
Antigen decoy to induce tolerance |
Experience with B cell depletion in TED has been limited to uncontrolled studies but remains encouraging. Two case reports describe reduction in the clinical activity in patients with TED unresponsive to steroids.15,108 A prospective, controlled study demonstrated sustained remission of hyperthyroidism in GD patients treated with RTX, even though the drug failed to influence autoantibody levels. In another open, non-randomized study of patients with TED, RTX was compared to intravenous glucocorticoid therapy. Patients receiving RTX demonstrated greater improvement of the clinical activity score with fewer side effects (33% vs. 45% of patients) than those treated with glucocorticoids. Thyroid function and TRAb levels were unaltered following RTX treatment. Adverse effects related to RTX include transient hypotension, cough, itching, mild temperature elevation, multifocal leucoencephalopathy and potentially, infection.22,29 However, most studies have failed to demonstrate significantly increased infection rates.8,38,123 Thus, RTX appears to represent a promising therapeutic agent in a subset of patients with TED.91 Well-controlled, prospective, and adequately-powered studies remain essential to fully evaluate its role.
D. AUTOANTIGENS IN TED
The search for relevant antigenic triggers in GD and TED has broadened considerably in the wake of findings that other autoimmune processes involve multiple autoantigens. Both genetic and environmental factors have been implicated. The role of TSHR is firmly established in the pathogenesis of hyperthyroidism in GD. But the other facets of this disease, including those occurring in the connective tissue are not easily reconciled with TSHR as the single pathogenic antigen. IGF-1R has been implicated in the pathogenesis of TED by our research group.88
Other potential autoantigens expressed by extraocular muscles include tropomodulin, G2s, which is the terminal 141 amino acids of the winged-helix transcription factor FOXP1, and the calcium binding protein calsequestrin. Antibodies to each of these proteins have been detected in patients with GD and their levels may correlate with myopathy.37 TG and TPO have also been proposed; however, levels of anti-TG and anti-TPO antibodies do not correlate with the presence, clinical activity, or severity of TED.79 TG shares physical attributes with acetylcholinesterase (ACHE), prompting the question of whether a shared epitope might provide the link between thymus nd orbit.60 Anti-ACHE antibodies were detected in 8% of sera from patients with TED, but their levels failed to correlate with disease activity.37,46 Multiple non-pathogenic autoantibodies are frequently detected in autoimmune disease, generated as a consequence of tissue damage. Thus, a role for any of these proteins and the antibodies directed against them in TED remains to be demonstrated.
E. ROLE OF TSHR
The role of TSHR and its antibodies in the pathogenesis of TED remains uncertain. Several interesting correlations between antibody levels and disease activity have been reported. TSHR expression in human fat tissue was first suggested when TSH was found to mediate lipolysis in fetal and newborn adipocytes but not in adult adipocytes.42,56,126 TSHR mRNA has been detected in orbital tissues and OF, albeit at extremely low levels.129,131 Undifferentiated fibroblasts fail to respond to rhTSH;2,131,132 however, functional TSHR appears following differentiation into adipocytes where rhTSH modestly enhanced cAMP.44 The role of TSHR in T cell activation is unclear. Two of eighteen T cell lines derived from orbital tissue of patients with GD exhibited increased migration following treatment with TSH, suggesting the absence of an important role for TSHR in orbital T cell activation.40,52,74 Clearly additional studies will be required if we are to establish an important role for TSHR in the pathogenesis of TAO.
F. ROLE OF IGF-1R
The IGF-1/IGF-1R pathway has been implicated in the pathogenesis of many malignant and autoimmune diseases. Crohn disease, pulmonary fibrosis, and multiple sclerosis are examples of presumed autoimmune diseases where IGF-1R might be over-expressed. More than 20 yrs ago, IGF-1 immunoreactivity was demonstrated on the surface of extra-ocular muscle and orbital fat cells from 2 patients with TED.101 Subsequently, the fraction of IGF-1R+ fibroblasts cultured from the orbit, skin, and thyroid of patients with GD was found to be increased.99,100,101 Treatment of these fibroblasts with either IGF-1 or GD-IgG results in the synthesis of two powerful T cell chemoattractants, IL-16 and RANTES, as well as the generation of hyaluronan.101,119,123 Importantly, neither IGF-1 nor GD-IgG elicited these responses in fibroblasts from individuals without autoimmune disease. These findings suggest that the increased levels of IGF-1R may play a role in the pathogenesis of GD; however, serum IGF levels are normal in euthyroid patients with GD. Elevated IGF-1 levels in orbital tissues appear to be independent of serum IGF-1.70 Anti-IGF-1R antibodies were detected in most patients with GD, but in few individuals without the disease.31
Like fibroblasts, T and B cells from patients with GD exhibit a striking phenotypic skew toward the IGF-1R+ phenotype.31,32 Notably, CD45RO+ T cells, representing memory T cells, exhibit remarkable IGF-1R skew, especially those with the CD8+ phenotype.129 Display of IGF-1R imparts a growth advantage and protects from Fas mediated apoptosis among T cells and is associated with the production of anti-TSHR antibodies in B cells.32 These findings suggest that IGF-1R may participate in the development of GD. Evidence that TSHR and IGF-1R might be functionally linked was strengthened recently when these proteins were found to co-localize.129 These receptors may form both physical and functional complexes since TSHR signaling to ERK activation could be attenuated by an IGF-1R blocking antibody.53,95,107
Several strategies for disrupting IGF-1R signaling have been developed recently and are currently being evaluated as therapy for cancer. These include several small molecules and antibodies. CP-751,871 (Pfizer) is an anti IGF-1R antibody currently undergoing phase III clinical trials in patients with non-small cell lung cancers. Phase I trials with IMC A-12 (Imclone), a human monoclonal antibody against IGF-1R, has also shown promise.69
G. ROLE OF CYTOKINES AND IMMUNE MEDIATORS
TNF-α levels may be elevated during the inflammatory phase of TED.96,106 Disruption of this pathway has become a major and highly successful approach to the therapy of rheumatoid arthritis and Crohn's disease.35,66 Three biological anti-TNF-α agents are currently in wide clinical use including the monoclonal antibodies infliximab and adalimumab. Etanercept, a recombinant human soluble TNF-α receptor fusion protein, binds and inhibits TNF-α activity. Two separate reports of infliximab use in patients with TED suggest that it might reduce inflammation and improve visual function without side effects.35 In the first study, nearly complete resolution of inflammation was observed within 72 hours following drug administration and improvement in visual acuity and color vision occurred over the subsequent week.94 In another, Paridaens et al found that the clinical activity score was reduced by 60% among 10 patients with TED, although 3 exhibited a disease flare following therapy withdrawal. No serious adverse event was noted during a mean follow-up of 18 months.94
V. Conclusion
Despite intensive study, identity of the proximate antigenic target initiating TED and the relationship between the orbital disease and the other components of GD remain uncertain. Lack of a preclinical disease models continues to plague our efforts to better understand this disease. Important insights concerning the pathogenesis of allied autoimmune diseases and increasing knowledge about their successful treatment should shed new light on the fundamental factors underlying TED and facilitate development of therapies for this particularly vexing process.
VI. Method of Literature Search
The primary search was performed on the accessible literature as of September, 2008. The MeSH database was used to target the search of Medline/PubMed and included available reports of Graves disease and thyroid eye disease. Various synonyms and eponyms of TED were searched. Additional MeSH terms included “autoimmune disease” and “thyroid” which were combined with “TED” to identify relevant citations. Full-text manuscripts of relevant English-language abstracts were reviewed. Additional references were identified by examining the bibliographies of retrieved articles and relevant textbooks of ophthalmology and immunology. English language abstracts for non-English language articles were included and referenced where relevant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VKK, O'Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100(12):3149–53. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agretti P, De Marco G, De Servi M, Marcocci C, Vitti P, Pinchera A, Tonacchera M. Evidence for protein and mRNA TSHr expression in fibroblasts from patients with thyroid-associated ophthalmopathy (TAO) after adipocytic differentiation. Eur J Endocrinol. 2005;152(5):777–84. doi: 10.1530/eje.1.01900. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 4.Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves' ophthalmopathy. J Clin Endocrinol Metab. 2000;85(2):776–80. doi: 10.1210/jcem.85.2.6333. [DOI] [PubMed] [Google Scholar]
- 5.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112(2):286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacchetta R, Gregori S, Roncarolo MG. CD4+ regulatory T cells: Mechanisms of induction and effector function. Autoimmunity Reviews. 2005;4(8):491–6. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Bahn RS, Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993;329(20):1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 8.Bahn RS. TSH receptor expression in orbital tissue and its role in the pathogenesis of Graves' ophthalmopathy. J Endocrinol Invest. 2004;27(3):216–20. doi: 10.1007/BF03345269. [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Marcocci C, Pinchera A. Graves' ophthalmopathy: a preventable disease? Eur J Endocrinol. 2002;146(4):457–61. doi: 10.1530/eje.0.1460457. [DOI] [PubMed] [Google Scholar]
- 10.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(4):284–90. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 11.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. Chronology of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(4):426–34. doi: 10.1016/s0002-9394(14)70439-8. [DOI] [PubMed] [Google Scholar]
- 12.Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 13.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17(6):638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5(4):343–9. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 15.Bonara P, Vannucchi G, Campi I, Rossi S, Cantoni F, Frugoni C, Sbrozzi F, Guastella C, Avignone S, Beck-Peccoz P, Salvi M. Rituximab induces distinct intraorbital and intrathyroidal effects in one patient satisfactorily treated for Graves' ophthalmopathy. Clin Rev Allergy Immunol. 2008;34(1):118–23. doi: 10.1007/s12016-007-8024-3. [DOI] [PubMed] [Google Scholar]
- 16.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114(7):979–87. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody Rituximab. Ann Oncol. 2003;14(4):520–35. doi: 10.1093/annonc/mdg175. [DOI] [PubMed] [Google Scholar]
- 18.Braley-Mullen H, Yu S. Early requirement for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2000;165(12):7262–9. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- 19.Brix TH, Petersen HC, Iachine I, Hegedus L. Preliminary evidence of genetic anticipation in Graves' disease. Thyroid. 2003;13:447–51. doi: 10.1089/105072503322021106. [DOI] [PubMed] [Google Scholar]
- 20.Brunkow ME, J E, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 21.Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves' ophthalmopathy. Exp Eye Res. 1997;65(2):311–6. doi: 10.1006/exer.1997.0353. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese LH, Molloy ES. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann Rheum Dis. 2008;67:64–5. doi: 10.1136/ard.2008.097972. [DOI] [PubMed] [Google Scholar]
- 23.Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999;277:1075–85. doi: 10.1152/ajpcell.1999.277.6.C1075. [DOI] [PubMed] [Google Scholar]
- 24.Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615–25. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 25.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104(10):3021–7. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 26.Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. Smoking and Thyroid-associated ophthalmopathy: A novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92(1):59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Tsui S, Boeglin WE, Douglas RS, Brash AR, Smith TJ. Interleukin-4 induces 15-Lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves disease: Evidence for anatomic site-selective actions of Th2 cytokines. J Biol Chem. 2006;281(27):18296–306. doi: 10.1074/jbc.M603484200. [DOI] [PubMed] [Google Scholar]
- 28.Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, Weetman A, Hale G, Chatterjee VK, Waldmann H, Compston A. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. The Lancet. 1999;354(9191):1691–5. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 29.Cooper N, Stasi R, Cunningham-Rundles S, Feuerstein MA, Leonard JP, Amadori S, Bussel JB. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–9. doi: 10.1111/j.1365-2141.2004.04889.x. [DOI] [PubMed] [Google Scholar]
- 30.Dorkhan M, Lantz M, Frid A, Groop L, Hallengren B. Treatment with a thiazolidinedione increases eye protrusion in a subgroup of patients with type 2 diabetes. Clin Endocrinol (Oxf) 2006;65(1):35–39. doi: 10.1111/j.1365-2265.2006.02542.x. [DOI] [PubMed] [Google Scholar]
- 31.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the Insulin-like growth factor-1 receptor by T Cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178(5):3281–7. doi: 10.4049/jimmunol.178.5.3281. [DOI] [PubMed] [Google Scholar]
- 32.Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181(8):5768–74. doi: 10.4049/jimmunol.181.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 34.Durie FH, Foy TM, Noelle RJ. The role of CD40 and its ligand (gp39) in peripheral and central tolerance and its contribution to autoimmune disease. Res Immunol. 145(3):200–205. doi: 10.1016/s0923-2494(94)80184-3. [DOI] [PubMed] [Google Scholar]
- 35.Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005;24(2):117–9. doi: 10.1080/01676830590912562. [DOI] [PubMed] [Google Scholar]
- 36.Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, Bluestone JA. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115(1):3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein AK, Plicht M, Lax H, Hirche H, Quadbeck B, Mann K, Steuhl KP, Esser J, Morgenthaler NG. Clinical results of anti-inflammatory therapy in Graves' ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol (Oxf) 2004;61(5):612–8. doi: 10.1111/j.1365-2265.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- 38.Edwards JCW, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with Rituximab in patients with Rheumatoid Arthritis. N Engl J Med. 2004;350(25):2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 39.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. The rationale for B lymphocyte depletion in Graves' disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol. 2006;154(5):623–32. doi: 10.1530/eje.1.02140. [DOI] [PubMed] [Google Scholar]
- 40.El Yafi F, Winkler R, Delvenne P, Boussif N, Belaiche J, Louis E. Altered expression of type I insulin-like growth factor receptor in Crohn's disease. Clin Exp Immunol. 2005;139(3):526–33. doi: 10.1111/j.1365-2249.2004.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fassi DE, Nielsen CH, Hasselbalch HC, Hegedus L. Treatment-resistant severe, active Graves' ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16(7):709–10. doi: 10.1089/thy.2006.16.709. [DOI] [PubMed] [Google Scholar]
- 42.Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves' disease retro-orbital tissue. Lancet. 1993;342(8867):337–8. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 43.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 44.Forster G, Otto E, Hansen C, Ochs K, Kahaly G. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol. 1998;112(3):427–34. doi: 10.1046/j.1365-2249.1998.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142(1):147–53. doi: 10.1016/j.ajo.2006.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2000;52(3):267–71. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 47.Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, Graves' disease, and Thyroid-associated ophthalmopathy. Thyroid. 2008;18:953–8. doi: 10.1089/thy.2007.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han R, Smith TJ. Induction by IL-1 beta of tissue inhibitor of metalloproteinase-1 in human orbital fibroblasts: modulation of gene promoter activity by IL-4 and IFN-gamma. J Immunol. 2005;174(5):3072–9. doi: 10.4049/jimmunol.174.5.3072. [DOI] [PubMed] [Google Scholar]
- 49.Han R, Smith TJ. T Helper type 1 and type 2 cytokines exert divergent Influence on the induction of prostaglandin E2 and hyaluronan synthesis by Interleukin-1{beta} in orbital fibroblasts: Implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology. 2006;147(1):13–9. doi: 10.1210/en.2005-1018. [DOI] [PubMed] [Google Scholar]
- 50.Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355–64. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 51.Han S, Zhang S, Zhang W, Li R, Li Y, Wang Z, Xie Y, Mao Y. CTLA4 polymorphisms and ophthalmopathy in Graves' disease patients: Association study and meta-analysis. Hum Immunol. 2006;67(8):618–26. doi: 10.1016/j.humimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Harrison NKCA, Myers AR, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci (Lond) 1994;86(2):141–8. doi: 10.1042/cs0860141. [DOI] [PubMed] [Google Scholar]
- 53.Hartog H, Wesseling J, Boezen HM, Van der Graaf WT. The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer. 2007;43(13):1895–904. doi: 10.1016/j.ejca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 55.Heufelder AE, Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993;23(1):10–7. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 56.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves' ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3(4):297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 57.Hiromatsu Y, Kaku H, Miyake I, Murayama S, Soejima E. Role of cytokines in the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):217–21. doi: 10.1089/105072502753600160. [DOI] [PubMed] [Google Scholar]
- 58.Huang X, Zhu J, Yang Y. Protection against autoimmunity in nonlymphopenic hosts by CD4+ CD25+ regulatory T cells is antigen-specific and requires IL-10 and TGF-beta. J Immunol. 2005;175(7):4283–91. doi: 10.4049/jimmunol.175.7.4283. [DOI] [PubMed] [Google Scholar]
- 59.Hufnagel TJ, Hickey WF, Cobbs WH, Jakobiec FA, Iwamoto T, Eagle RC. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves' disease. Ophthalmology. 1984;91:1411–9. doi: 10.1016/s0161-6420(84)34152-5. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson DM. Acetylcholine receptor antibodies in patients with Graves' ophthalmopathy. J Neuroophthalmol. 1995;15(3):166–70. [PubMed] [Google Scholar]
- 61.Jameson JL, Weetman AP. Disorders of the Thyroid Gland” (Chapter) In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 17th Edition [Google Scholar]
- 62.Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by Interleukin-1{beta} in human orbital fibroblasts: potential insight into the molecular pathogenesis of Thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84(11):4079–84. doi: 10.1210/jcem.84.11.6111. [DOI] [PubMed] [Google Scholar]
- 63.Kahaly G, Forster G, Hansen C. Glycosaminoglycans in thyroid eye disease. Thyroid. 1998;8(5):429–32. doi: 10.1089/thy.1998.8.429. [DOI] [PubMed] [Google Scholar]
- 64.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin J-M, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach J-F, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 Diabetes. N Engl J Med. 2005;352(25):2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 65.Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174(8):4525–34. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 66.Komorowski J, Jankiewicz-Wika J, Siejka A, Lawnicka H, Klysik A, Gos R, Majos A, Stefanczyk L, Stepien H. Monoclonal anti-TNFalpha antibody (infliximab) in the treatment of patient with thyroid associated ophthalmopathy. Klin Oczna. 2007;109(10-12):457–60. [PubMed] [Google Scholar]
- 67.Koulova L, Clark EA, Shu G, Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173(3):759–62. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 Expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291–300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krassas GE, Heufelder AE. Immunosuppressive therapy in patients with thyroid eye disease: an overview of current concepts. Eur J Endocrinol. 2001;144(4):311–8. doi: 10.1530/eje.0.1440311. [DOI] [PubMed] [Google Scholar]
- 70.Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A. Free and total insulin-like growth factor (IGF)-I, -II, and IGF binding protein-1, -2, and -3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab. 2003;88(1):132–5. doi: 10.1210/jc.2002-021349. [DOI] [PubMed] [Google Scholar]
- 71.Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves' disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79(2):542–6. doi: 10.1210/jcem.79.2.7913934. [DOI] [PubMed] [Google Scholar]
- 72.Larsen PR, Davies TF, Schlumberger MJ. Thyrotoxicosis. In: Larsen PR, Kronenberg HM, Melmed S, editors. Williams Textbook of Endocrinology. W.B. Saunders Co.; Philadelphia: 2003. pp. 374–421. [Google Scholar]
- 73.Lee S, Tsirbas A, Goldberg RA, McCann JD. Thiazolidinedione induced thyroid associated orbitopathy. BMC Ophthalmol. 2007;7:8. doi: 10.1186/1471-2415-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee TC, Gold LI, Reibman J, Aston C, Bégin R, Rom WN, Jagirdar J. Immunohistochemical localization of transforming growth factor-beta and insulin-like growth factor-I in asbestosis in the sheep model. Int Arch Occup Environ Health. 1997;69(3):157–64. doi: 10.1007/s004200050132. [DOI] [PubMed] [Google Scholar]
- 75.Levin F, Kazim M, Smith TJ, Marcovici E. Rosiglitazone-induced proptosis. Arch Ophthalmol. 2005;123(1):119–21. doi: 10.1001/archopht.123.1.119. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Davis J, Bracht M, Carton J, Armstrong J, Gao W, Scallon B, Fung R, Emmell E, Zimmerman M, Griswold DE, Li L. Modulation of antigen-specific T cell response by a non-mitogenic anti-CD3 antibody. Int Immunopharmacol. 2006;6(6):880–91. doi: 10.1016/j.intimp.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Liu C, Papewalis C, Domberg J, Scherbaum WA, Schott M. Chemokines and autoimmune thyroid diseases. Horm Metab Res. 2008;40(6):361–8. doi: 10.1055/s-2008-1073153. [DOI] [PubMed] [Google Scholar]
- 78.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50(8):2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 79.Ludgate M, Swillens S, Mercken L, Vassart G. Homology between thyroglobulin and acetylcholinesterase: An explanation for pathogenesis of Graves' ophthalmopathy? Lancet. 1986;2(8500):219–20. doi: 10.1016/s0140-6736(86)92515-8. [DOI] [PubMed] [Google Scholar]
- 80.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 81.Macht LM, Corrall RJ, Banga JP, Elson CJ. Control of human thyroid autoantibody production in SCID mice. Clin Exp Immunol. 1993;91(3):390–6. doi: 10.1111/j.1365-2249.1993.tb05914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–66. [PubMed] [Google Scholar]
- 83.Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, Fitzgerald O, Bresnihan B, Caporali R, Montecucco C, Uguccioni M, Pitzalis C. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- 84.Martins JR, Furlanetto RP, Oliveira LM, Mendes A, Passerotti CC, Chiamolera MI, Rocha AJ, Manso PG, Nader HB, Dietrich CP, Maciel RM. Comparison of practical methods for urinary glycosaminoglycans and serum hyaluronan with clinical activity scores in patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2004;60(6):726–33. doi: 10.1111/j.1365-2265.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- 85.McGinness JL, Bivens M-MC, Greer KE, Patterson JW, Saulsbury FT. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) associated with pemphigoid nodularis: A case report and review of the literature. J Am Acad Dermatol. 2006;55(1):143–8. doi: 10.1016/j.jaad.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 86.McKenzie JM, Zakarija M, Sato A. Humoral immunity in Graves' disease. Clin Endocrinol Metab. 1978;7(1):31–45. doi: 10.1016/s0300-595x(78)80034-6. [DOI] [PubMed] [Google Scholar]
- 87.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf) 1994;40(1):67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 88.Mizokami T, Salvi M, Wall JR. Eye muscle antibodies in Graves' ophthalmopathy: pathogenic or secondary epiphenomenon? J Endocrinol Invest. 2004;27(3):221–9. doi: 10.1007/BF03345270. [DOI] [PubMed] [Google Scholar]
- 89.Mohan C, Shi Y, Laman JD, Datta SK. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154(3):1470–80. [PubMed] [Google Scholar]
- 90.Naik V, Khadavi N, Naik MN, Hwang C, Goldberg RA, Tsirbas A, Smith TJ, Douglas RS. Biologic therapeutics in thyroid-associated ophthalmopathy: translating disease mechanism into therapy. Thyroid. 2008;18(9):967–71. doi: 10.1089/thy.2007.0403. [DOI] [PubMed] [Google Scholar]
- 91.Nielsen CH, Fassi DE, Hasselbalch HC, Bendtzen K, Hegedus L. B-cell depletion with rituximab in the treatment of autoimmune diseasesGraves' ophthalmopathy the latest addition to an expanding family. Expert Opin Biol Ther. 2007;7(7):1061–78. doi: 10.1517/14712598.7.7.1061. [DOI] [PubMed] [Google Scholar]
- 92.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10(8):801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 93.Pappa A, Jackson P, Stone J, Munro P, Fells P, Pennock C, Lightman S. An ultrastructural and systemic analysis of glycosaminoglycans in thyroid-associated ophthalmopathy. Eye. 1998;12(Pt 2):237–44. doi: 10.1038/eye.1998.57. [DOI] [PubMed] [Google Scholar]
- 94.Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves' ophthalmopathy: a pilot study. Eye. 2005;19(12):1286–9. doi: 10.1038/sj.eye.6701768. [DOI] [PubMed] [Google Scholar]
- 95.Paz K, Hadari YR. Targeted therapy of the insulin-like growth factor-1 receptor in cancer. Comb Chem High Throughput Screen. 2008;11(1):62–9. doi: 10.2174/138620708783398313. [DOI] [PubMed] [Google Scholar]
- 96.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6(6):644–53. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 97.Pichurin P, Aliesky H, Chen CR, Nagayama Y, Rapoport B, McLachlan SM. Thyrotrophin receptor-specific memory T cell responses require normal B cells in a murine model of Graves' disease. Clin Exp Immunol. 2003;134(3):396–402. doi: 10.1111/j.1365-2249.2003.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–35. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 99.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942–50. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 100.Pritchard J, Tsui S, Horst N, Cruikshank WW, Smith TJ. Synovial fibroblasts from patients with Rheumatoid Arthritis, like fibroblasts from Graves' disease, express high levels of IL-16 when treated with Igs against Insulin-like growth factor-1 receptor. J Immunol. 2004;173(5):3564–9. doi: 10.4049/jimmunol.173.5.3564. [DOI] [PubMed] [Google Scholar]
- 101.Pritchard Jane, HR, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348–54. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 102.Prummel M. Pathogenetic and clinical aspects of endocrine ophthalmopathy. Exp Clin Endocrinol Diabetes. 1999;107(Suppl 3):S75–8. doi: 10.1055/s-0029-1212155. [DOI] [PubMed] [Google Scholar]
- 103.Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993;269(4):479–82. [PubMed] [Google Scholar]
- 104.Ramsdell F, Seaman MS, Clifford KN, Fanslow WC. CD40 ligand acts as a costimulatory signal for neonatal thymic gamma delta T cells. J Immunol. 1994;152(5):2190–7. [PubMed] [Google Scholar]
- 105.Rapoport B, McLachlan SM. The Thyrotropin receptor in Graves' disease. Thyroid. 2007;17(10):911–22. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 106.Rothe A, Power BE, Hudson PJ. Therapeutic advances in rheumatology with the use of recombinant proteins. Nat Clin Pract Rheumatol. 2008;4(11):605–14. doi: 10.1038/ncprheum0909. [DOI] [PubMed] [Google Scholar]
- 107.Sachdev D, Yee D. Inhibitors of Insulin-like growth factor signaling: A therapeutic approach for breast cancer. J Mammary Gland Biol Neoplasia. 2006;11(1):27–39. doi: 10.1007/s10911-006-9010-8. [DOI] [PubMed] [Google Scholar]
- 108.Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, Guastella C, Avignone S, Pirola G, Ratiglia R, Beck-Peccoz P. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154(4):511–7. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 109.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274(3 Pt 1):C707–14. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 110.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–13. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 111.Shevach EM. CD4+CD25+ Suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 112.Shine B, Fells P, Edwards OM, Weetman AP. Association between Graves' ophthalmopathy and smoking. Lancet. 1990;335(8700):1261–3. doi: 10.1016/0140-6736(90)91315-2. [DOI] [PubMed] [Google Scholar]
- 113.Shishido M, Kuroda K, Tsukifuji R, Fujita M, Shinkai H. A case of pretibial myxedema associated with Graves' disease: an immunohistochemical study of serum-derived hyaluronan-associated protein. J Dermatol. 1995;22(12):948–52. doi: 10.1111/j.1346-8138.1995.tb03952.x. [DOI] [PubMed] [Google Scholar]
- 114.Sisson JC, Schipper MJ, Nelson CC, Freitas JE, Frueh BR. Radioiodine therapy and Graves' ophthalmopathy. J Nucl Med. 2008;49(6):923–930. doi: 10.2967/jnumed.107.049437. [DOI] [PubMed] [Google Scholar]
- 115.Smith JA, Bluestone JA. T cell inactivation and cytokine deviation promoted by anti-CD3 mAbs. Curr Opin Immunol. 1997;9(5):648–54. doi: 10.1016/s0952-7915(97)80044-1. [DOI] [PubMed] [Google Scholar]
- 116.Smith JA, Tang Q, Bluestone JA. Partial TCR signals delivered by FcR-nonbinding anti-CD3 monoclonal antibodies differentially regulate individual Th subsets. J Immunol. 1998;160(10):4841–9. [PubMed] [Google Scholar]
- 117.Smith JA, Tso JY, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med. 1997;185(8):1413–22. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151(2):317–22. [PMC free article] [PubMed] [Google Scholar]
- 119.Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, Insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076–80. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 120.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87(1):385–92. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 121.Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268(2 Pt 1):C382–8. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 122.Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 123.Smith TJ. The putative role of fibroblasts in the pathogenesis of Graves disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity. 2003;36(6-7):409–15. doi: 10.1080/08916930310001603000. [DOI] [PubMed] [Google Scholar]
- 124.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81(9):3428–31. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 125.Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273(39):25117–24. doi: 10.1074/jbc.273.39.25117. [DOI] [PubMed] [Google Scholar]
- 126.Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30(3):369–80. doi: 10.1677/jme.0.0300369. [DOI] [PubMed] [Google Scholar]
- 127.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in Rheumatoid synovitis. J Immunol. 2001;167(2):1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 128.Tallstedt L, Norberg R. Immunohistochemical staining of normal and Graves' extraocular muscle. Invest Ophthalmol Vis Sci. 1988;29(2):175–84. [PubMed] [Google Scholar]
- 129.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181(6):4397–405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tuscano JM, Harris GS, Tedder TF. B lymphocytes contribute to autoimmune disease pathogenesis: current trends and clinical implications. Autoimmun Rev. 2003;2(2):101–8. doi: 10.1016/s1568-9972(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 131.Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84(7):2557–62. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 132.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87(5):2352–8. doi: 10.1210/jcem.87.5.8472. [DOI] [PubMed] [Google Scholar]
- 133.Viglietta V, Bourcier K, Buckle GJ, Healy B, Weiner HL, Hafler DA, Egorova S, Guttmann CR, Rusche JR, Khoury SJ. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71(12):917–24. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 134.Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989;75(2):222–7. [PMC free article] [PubMed] [Google Scholar]
- 135.Weetman AP, Yateman ME, Ealey PA, Black CM, Reimer CB, Williams RC, Jr, Shine B, Marshall NJ. Thyroid-stimulating antibody activity between different immunoglobulin G subclasses. J Clin Invest. 1990;86(3):723–7. doi: 10.1172/JCI114768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine-target cell interactions. Proc Natl Acad Sci U S A. 1998;95(15):8904–9. doi: 10.1073/pnas.95.15.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]