Abstract
The brain and the cardiovascular system influence each other during the processing of emotion. The study of the interactions of these systems during emotion regulation has been limited in human functional neuroimaging, despite its potential importance for physical health. We have previously reported that mental expertise in cultivation of compassion alters the activation of circuits linked with empathy and theory of mind in response to emotional stimuli. Guided by the finding that heart rate increases more during blocks of compassion meditation than neutral states, especially for experts, we examined the interaction between State (compassion vs. neutral) and Group (novice, expert) on the relation between heart rate and BOLD signal during presentation of emotional sounds presented during each state. Our findings revealed that BOLD signal in the right middle insula showed a significantly stronger association with heart rate (HR) across state and group. This association was stronger in the left middle/ posterior insula when experts were compared to novices. The positive coupling of HR and BOLD was higher within the compassion state than within the neutral state in the dorsal anterior cingulate cortex for both groups, underlining the role of this region in the modulation of bodily arousal states. This state effect was stronger for experts than novices in somato-sensory cortices and the right inferior parietal lobule (group by state interaction). These data confirm that compassion enhances the emotional and somatosensory brain representations of others' emotions, and that this effect is modulated by expertise. Future studies are needed to further investigate the impact of compassion training on these circuits.
Empathy can broadly be referred to as the capacity to understand and share another person's emotional experience. Recent functional magnetic resonance imaging (fMRI) studies have demonstrated that observing another person's emotional state activates parts of the neuronal network involved in processing that same state in oneself, whether it is disgust, touch, or pain (for reviews see (de Vignemont and Singer 2006; Sommerville and Decety 2006)). For instance, the anterior insula (AI) and anterior cingulate cortex (ACC), which participate in the sensory and affective processing of pain (Wiech, Ploner, Tracey 2008), was activated when participants experienced pain themselves as well as when they saw an arrow cue indicating that their partner had experienced pain ((Singer et al., 2004) for review (de Vignemont and Singer 2006)). These data are consistent with perception-action models of empathy (Preston and de Waal 2002), in which observing and imagining another person in a particular state is thought to activate a similar state in the observer (simulation theory (Rizzolatti, Fogassi, Gallese 2001)). They also fit with a similar framework that proposes that the observed and executed actions are ‘coded in a common cognitive and neural framework,’ enabling individuals to construct ‘shared representations of self and others’ ((Sommerville and Decety 2006)).
Recent studies have started to assess both state and trait factors modulating empathic brain responses(de Vignemont and Singer 2006; Silani et al., 2008; Sterzer et al., 2007). As state factors, top-down processes such as cognitive appraisal or attention were found to influence empathy. For instance, empathic brain responses were diminished when participants believed that the other participant received pain as therapeutic treatment (Lamm et al., 2007). As a trait factor, characteristics of the empathizer were found to modulate the magnitude of empathic brain responses. For instance, Cheng et al. (2007) compared physicians who practice acupuncture to naive participants while observing animated visual stimuli depicting needles being inserted into different body parts (Cheng et al., 2007). The results indicated less empathy-related pain activity in AI and ACC for the expert group as compared with the control group, suggesting a reduction of empathic brain responses if the participants are familiar with such stimuli. Similarly, inter-individual differences in neural empathy responses were found to correlate with self-reported measures of empathy: the higher participants scored on these empathy questionnaires, the higher their activation was in insula and ACC (e.g. (Jabbi, Swart, Keysers 2007; Singer et al., 2004)).
Along these lines, we recently studied the impact of a state of compassion (short for “compassion and loving-kindness meditation state”) on this empathy-related network (insula and ACC) in both expert and novice meditation practitioners (Lutz et al., 2008a). We studied compassion as both state and trait (experts vs. novices) factors influencing empathy. Loving-kindness, the wish of happiness for others, and compassion, the wish to relieve others' suffering, are empathic responses which are motivated by an altruistic concern for others. In many traditions, these qualities are cultivated through specific meditation practices designed to prime behaviors compatible with these wishes in response to actual interpersonal encounters (Gethin 1998; Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa 1995). Despite the potential social and clinical importance of these affective processes, the possibility that they can be trained in a manner comparable to attentional (Slagter et al., 2007) or sensory-motor skills (Maguire, Woollett, Spiers 2006) has received very limited scientific attention even though our recent study supports this hypothesis. To cultivate these affective qualities, practitioners in a number of traditions have developed meditative practices, which are thought to be essential to counteract self-centered tendencies (Gethin 1998; Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa 1995). To investigate these questions, we previously assessed brain activity using fMRI while novice and expert meditation practitioners generated a loving-kindness–compassion meditation state. To probe affective reactivity, we presented emotional (positive and negative) and neutral sounds during the meditation and comparison periods. This meditation is said to enhance loving-kindness when the joy of others is perceived or compassion when the suffering of others is perceived. Therefore, we predicted that during this meditation state, both negative sounds (sounds of a distressed woman) and positive sounds (a baby laughing) would induce greater physiological changes (BOLD response and heart rate changes) than neutral sounds (background noise in a restaurant). The presentation of the emotional sounds was associated with increased pupil diameter and activation of limbic regions (insula and cingulate cortices) during meditation (versus rest). During meditation, activation in the insula was greater during the presentation of negative sounds than positive or neutral sounds in expert versus novice meditators (Group by State by Valence interaction). The strength of activation in the insula was also associated with self-reported intensity of the meditation for both groups. Together these data indicate that compassion and loving-kindness, along with the mental expertise to cultivate these qualities, alter the activation of circuits previously linked to empathy in response to emotional stimuli.
Because practitioners reported that a task would disrupt their ongoing meditation, our previous study did not include a behavioral measure of the compassion state. However, classical descriptions of this practice indicate that the generation of intense compassionate feelings during meditation can produce specific bodily changes including increased heart rate, goose bumps, or even tears (Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa 1995). Guided by these descriptions, in this report we analyze cardiac changes during this meditation practice and examine the relation between heart rate (HR) changes and simultaneously acquired (with fMRI) measures of brain function in experts and novices. In particular, we use variations in HR during the task as a peripheral marker of the visceral alterations that occur during the generation of compassion. Voxelwise correlations of this marker to the blood-oxygen-level dependent (BOLD) signal allow us to identify the neural processes that accompany the autonomic changes during the voluntary generation of compassion and to examine how the neurovisceral coupling during meditation might differ between experts and novices.
Previous neuroimaging studies have addressed the question of central control of heart rate in emotions by collecting parallel measurement of heart rate changes and changes in activation as indexed by fMRI ((Critchley et al., 2003; Critchley et al., 2005; Yang et al., 2007) and PET (Lane et al., 2009)). In one study where brain activity and heart rate were simultaneously measured, the authors reported that the level of activity in emotion-related regions (amygdala, insula, anterior cingulate) predicted subjects' heart rate responses to the presentation of emotional facial expressions (Yang et al., 2007). Of importance for this study, Critchley et al. measured simultaneous electrocardiography and brain activity during performance of cognitive and motor tasks. Activity in the dorsal anterior cingulate cortex (ACC) was found to mediate the modulation of bodily arousal states (sympathetic activity) during these tasks (Critchley et al., 2003). In a study from our laboratory where functional MRI of the heart and brain were acquired nearly simultaneously, we found that greater activation in the amygdala, anterior cingulate and the right middle frontal gyrus predicted greater cardiac contractility during a stimulus paired with threat versus safety (Dalton et al., 2005).
Based on the findings reviewed above, our main hypothesis was that changes in heart rate during compassion would be positively correlated with BOLD signal, as a function of compassion meditation versus neutral state (State) and as a function of expertise (Group), in regions important for feelings and emotions (e.g., insular cortex) and autonomic control (e.g, dorsal ACC). In a first analysis, we investigated whether changes in HR across both states were associated with changes in the brain. In this first analysis, the HR BOLD coupling parameters across states were influenced both by the main effect of state and by changes in the degree of HR/BOLD couplings within each state. We then specifically investigated the changes in the HR/BOLD couplings within each state. We used a voxel-wise analysis performed on the HR/BOLD coupling parameter within each state using a 2 × 2 factorial design with the first factor representing “Group” (10 experts vs. 12 novices) and the second factor being the “State” (compassion meditation vs. neutral state). This analysis provided a way to identify the brain regions which specifically correlated with the fluctuations in emotional arousal during compassion meditation. In addition, we tested whether these associations differed as a function of expertise (“Group”, expert vs. novice practitioners).
Materials and methods
Participants
We selected the participants from our previous studies(Brefczynski-Lewis et al., 2007; Lutz et al., 2008a) for whom the heart beats were visible in the signal recorded by the pulse oximeter during the whole session. Participants from the current analysis included 10 long-term Buddhist practitioners whom we classified as experts (mean age = 40.0 years, std = 9.6 years,) and 13 healthy volunteers (mean age = 38.3 years, std = 9.1 years). The two groups did not differ in age (t-test, t(1,21) = -0.3, p = 0.78) or gender (t-test, t(1,21) = -0.5, p = 0.65). All participants were right-handed, except for one ambidextrous expert, as assessed by Edinburgh Handedness Inventory (Oldfield 1971) and all but 4 were male (2 experts and 2 age-matched novices). Buddhist practitioners, recognized as experts, were 5 of Asian origin, 5 of Caucasian origin. To navigate the cultural differences among scientists and long-term practitioners and for recruitment purposes, communication was facilitated by Dr. Matthieu Ricard, an interpreter for the Dalai Lama who is a Western Buddhist monk with scientific training and 35 years of meditative training in Nepal. Experts had previously completed mental training in the similar Tibetan Nyingmapa and Kagyupa traditions for 10,000 to 50,000 hours, which included a variety of meditation practices including this compassion meditation. The length of their training was estimated based on their daily practice and the time they spent in meditative retreats. Ten hours of meditation per day of retreat was estimated as an average. The control participants were recruited via advertisements in local newspapers and consisted of members of the UW Madison community. The advertisement specifically recruited participants who had an interest in meditation, but who had no prior meditative training. One week before the actual fMRI scan session, novices were given written instructions on how to perform the meditation practices, written by Dr. Ricard, and practiced this compassion meditation and two other meditations for one hour a day for a week (20 minutes per meditation). Written informed consent was obtained prior to scanning, in accordance with procedures and protocols approved by the UW-Madison Institutional Review Board. A proficient Tibetan speaking translator gave detailed procedural instructions and read the consent form to three non-English speaking participants.
Meditative instruction
The state of loving-kindness and compassion is described as an “unconditional readiness and availability to help living beings”. This practice does not require concentration on particular objects, memories or images, although in other meditations that are also part of their long-term training, practitioners focus on particular persons or groups of beings. Because “benevolence and compassion pervades the mind as a way of being”, this state is called “pure compassion” or “non-referential compassion” (dmigs med snying rje in Tibetan, pronounced “mig mey nying jey” – literally: compassion (nying jey) without (mey) object (mig)). As described in Dr. Ricard's instructions for the novices: “During the training session, the subject will think about someone he cares about, such as his parents, sibling or beloved, and will let his mind be invaded by a feeling of altruistic love (wishing well-being) or of compassion (wishing freedom from suffering) toward these persons. After some training the subject will generate such feeling toward all beings and without thinking specifically about someone. While in the scanner, the subject will try to generate this state of loving kindness and compassion.” The Resting state (Tib. “sem lung ma bstan”, pronounced “sem lung ma ten” – literally: neutral (lung ma ten) mind (sem)) was a non-meditative state without specific cognitive content and with a lack of awareness or clarity of the mind. Novice's instructions were the following: “Neutral here means that your emotional state is neither pleasant nor unpleasant and that you remain relaxed. Try to be in the most ordinary state without being engaged in an active mental state.” The controls' ability to follow the instruction was assessed orally prior to the data collection.
Protocol
Before the MRI scanning session, participants had a simulation session during which they viewed an abbreviated version of the experimental paradigm while lying in a mock MRI scanner (including head coil and digitized scanner sounds). This simulation session served to acclimate all participants to the fMRI environment. We used a block design, alternating ∼3min of the state of meditation (4 cycles) with ∼1.6 min of a resting, neutral state (5 cycles), twice on separate days. Per session, there was an average of 643 seconds of meditation and 550 seconds of neutral state (264 seconds and 190 seconds respectively for expert participant 2). A total of 25 2-second auditory sounds from the International Affective Digitized Sounds (IADS) (Bradley and Lang 2000) for each valence (positive, neutral and negative) were randomly presented across these two sessions. These sounds were presented every 6-10 seconds after the first 40 seconds of the meditative blocks and after 15 seconds of the resting blocks. Participants were instructed to maintain their practice during the presentation of the sounds. During the meditation and neutral states, eyes remained open and directed toward a fixation point on a black screen. In this study we did not include a behavioral task because practitioners reported that a task would disrupt their ongoing meditation. Two scans were run on two separate days (1 day apart for experts, in general less than 1 week apart for novices) due to the length of the scan run.
Data collection
MR images were collected with a GE Signa 3.0 Tesla scanner equipped with a high-speed, whole-body gradient and a whole-head transmit-receive quadrature birdcage headcoil. Whole-brain anatomical images were acquired at the end of each session using an axial 3D T1-weighted inversion-recovery fast gradient echo (or IR-prepped fast gradient echo) sequence. The field of view (FOV) was 240×240 mm with a 256×256 matrix. The slice thickness was 1.0-1.2 mm, with 0.9×0.9mm in-plane resolution. Functional data were collected using whole-brain EPI (TR = 2000, TE = 30ms). For functional images, sagittal acquisition was used to obtain 30 interleaved 4 mm slices with a gap of 1 mm between slices. The resulting voxel size was 3.75×3.75× 5 mm (FOV = 240mm, matrix = 64×64).
To ensure a high signal-to-noise ratio in areas prone to susceptibility artifacts, the field inhomogeneities were lessened during data collection using high-order shim coils that applied small correction gradients. In addition, acquisition of a 3D field map of the magnetic field provided a complementary strategy to further reduce distortion (these data were not acquired for the first three Buddhist experts). Based on these field maps, echo planar imaging (EPI) data were unwarped so that accurate alignment to anatomical images could be made. During the fMRI session, head movement was restricted using a vacuum pillow (Vac Fix System, S&S Par Scientific). A Silent Vision system (Avotec, Inc., Jensen Beach, FL) displayed the fixation point for the concentrative task. Eye movements, fixations and pupil diameter were continuously recorded during the fMRI scan using an iView system (sampling rate, 60 Hz) with a remote eye-tracking device (SensoMotoric Instruments, 2001). We collected pupil data from 13 controls and 10 expert participants. Cardiac data were extracted from the photoplethysmograph (PPG) data collected at 1000 Hz using GE's standard internal infrared pulse oximeter.
Data analysis
Analysis techniques were similar to those described previously in our lab (Lutz et al., 2008a). Briefly, data processing was implemented via AFNI (Analysis of Functional Neural Images) (Cox 1996). Data processing steps included image reconstruction in conjunction with smoothing in Fourier space via a Fermi filter, correction for differences in slice timing, 6-parameter rigid-body motion correction, and removal of skull and ghost artifacts. The motion estimates over the course of the scan for translation (inferior-superior, right-left, and anterior-posterior) and rotation (yaw, pitch, roll) were charted. Time points with more than 0.5 mm of motion were removed from the analysis. Two of the experts could not complete the second session for scheduling reasons. Seven of the novices completed only one session as they were part of an additional control group (see (Brefczynski-Lewis et al., 2007)). One of the two sessions was omitted for two of the experts and two of the novices, due to excessive head motion. One of the sessions of one novice was omitted due to sleepiness. The final N for the neuroimaging part of the study was 10 expert and 12 novice meditators. Between the two subject groups, participants with only one session of data were not significantly different (t-test, t(1,20) = -1.3, p = 0.2).
BOLD correlates of HR across states
The BOLD time series was modeled with a least-squares general linear model (GLM) fit that included the heart rate, event-related sound responses across all states (compassion and neutral), motion parameters in six directions and fourth-order polynomial baseline function. For the event-related sound responses, a standard gamma function was used to model the shape of the hemodynamic response. For each subject, the HR effect was modeled as 16 one-minute blocks (8 during each state) occurring during the presentation of the sounds. The HR time series was positive during the time course of the HR blocks and set to zero otherwise. The amplitude of each block was weighted by the average HR value in this time window and z-transformed compared to all HR values (HR mean across states was subtracted from each HR value and divided by the global HR standard deviation across states). The beta coefficient of the HR regressor was converted to percentage signal change using the mean overall baseline and spatially smoothed using a 6mm RMS Gaussian filter: we referred to the resulting measure as the “HR/BOLD coupling parameter”. The resultant parametric maps were transformed into the standardized Talairach space via identification of anatomical landmarks on the high-resolution anatomical image.
The analysis of the HR/BOLD coupling parameter was performed using voxelwise t-test comparing the HR/BOLD coupling parameter maps across both groups to 0. A group comparison was performed using a voxelwise t-test comparing the HR/BOLD coupling parameter maps between the two groups. Monte Carlo simulations were run to correct for multiple testing to achieve an overall corrected mapwise alpha of 0.05. We found that the minimum cluster size was 1030 contiguous voxels with the data thresholded at an uncorrected voxelwise p-value of p = 0.01. The data were then overlaid onto a high-resolution anatomical image.
BOLD correlates of HR within each state
As some of the effects found in the above analysis on the HR/BOLD coupling parameter might merely reflect the meditation state effect in the BOLD (see Lutz et al. 2008) and in the HR (see Table 1) (i.e. creating a bi-modal distribution of these values between states), we further estimated a HR/BOLD coupling parameter within each state (neutral and compassion). The analytical strategy was identical to the above, except that we used two separate HR regressors in the GLM, one for each state. The z-transformation of HR values was run separately within each state.
Table 1. Heart Rate (HR) Results (Repeated-Measured ANOVA).
| Factors | F(1,147) | p-Value |
|---|---|---|
| State | 9.9 | 0.005 |
| State × Group | 4.7 | 0.043 |
| Block | 1.7 | 0.12 |
| Group × Block | 0.62 | 0.74 |
| State × Block | 1.67 | 0.12 |
| State × Group × Block | 1.48 | 0.18 |
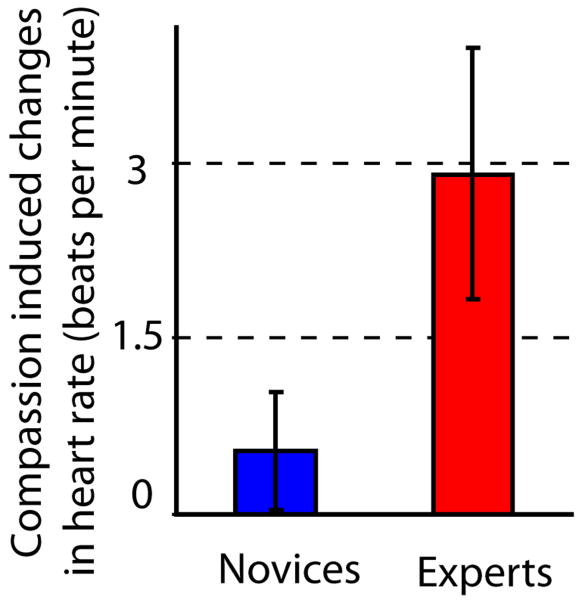
The HR analysis was performed with HR Compassion as the dependent variable, State (Neutral vs. Compassion) and Block (8 blocks across the session) as within-subject factors and Group (Experts vs. Novices) as the between-subject factor. Note that HR increases for both groups during compassion, and that this increase was larger for experts than novices (paired t-test, t(1,21) = -2.1, p < 0.05, 10 experts and 13 novices).
The main analysis of the HR/BOLD coupling parameter was performed using a 2×2 factorial design (voxelwise 2-way ANOVA) with State (resting and meditation states) as a within-subjects factor varying within subject and with Group as a between-subjects factor (Matlab package for AFNI, C. Chen). Monte Carlo simulations were run to correct for multiple testing to achieve an overall corrected mapwise alpha = 0.05. For the State effect and Group by State effect, we found that the minimum cluster sizes were, respectively, of 1030 and 3580 contiguous voxels with the data thresholded at an uncorrected voxelwise p-value of p = 0.01 and p = 0.05 respectively.
Heart Rate Data
Timing of heart beats was extracted as the peaks in the photoplethysmograph (PPG) signal recorded by the pulse oximeter. Individual peaks in the PPG were picked using in-house developed Matlab software that allows for modification of peak (beat) detections while simultaneously viewing the resulting interbeat interval (IBI) series superimposed on a spline interpolated (at 10 samples per second) version. Missed and artifactual beats can be identified by visual inspection of the IBI series, and corrected by manual addition or removal of individual beats. Since it has been shown (Wendelken et al.,) that PPG data can be used for an estimation of heart rate variability (HRV), it was decided to include these parameters in the analysis along with simple heart rate.
For each subject and for each state (compassion or neutral), we extracted from the IBI series 8 1-minute intervals of cardiac data during the presentation of emotional sounds. Heart rate (HR), HRV and respiratory sinus arrhythmia (RSA) were computed using the CMetX freeware program (Allen, Chambers, Towers 2007) for each of these 16 1-minute intervals. We investigated changes in HR, HRV and RSA as a function of State (compassion versus neutral), Block (8 1-minute intervals) and Group (experts versus novices) using an 8 × 2 × 2 factorial design (repeated measures analysis of variance (ANOVAs)).
Heart rate correlation with qualitative report on meditation intensity
After each scan run, participants were asked to verbally report the meditative intensity of each block on a scale from 1 to 9. Some participants, not comfortable using the number scale to quantify or qualify their meditative states, simply identified the two blocks, from among the four recorded, that were the best and the worst of the day. The collection of metrics of intensity of meditation was incomplete; we had meditation reports for 8 practitioners and 2 controls. For each subject, we computed the correlation HR / intensity of meditation in each session, averaged the correlation coefficients across sessions, and normalized the mean coefficient using the Fisher transformation (z=0.5*log((1+c)/(1-c))). We tested whether there was a positive correlation between HR and the intensity of meditation by comparing the mean normalized coefficient to 0 with a t-test.
Respiration data
Among the participants with respiration data (3 experts and 8 controls), we were able to visually detect respiratory rates on 2 experts and 8 controls. We visually counted the numbers of breaths per blocks of neutral or meditation. A paired t-test was used to assess for possible changes in respiratory rates from rest to meditation.
Results
Effects of compassion state on HR
We investigated changes in HR as a function of Block, State and Group using an 8 × 2 × 2 factorial design (repeated measures analysis of variance (ANOVA)). Table 1 and Figure 1 display the results. We found that HR was higher during compassion versus neutral states (main effect of State), and that this increase was stronger for the experts than the novices (the interaction between State and Group is displayed in Table 1 and in Figure 1). For experts, the mean HR was 64.2 (8.2 S.D.) and 67.1 (9.8 S.D.) beats per minute in the neutral and compassion states respectively. For novices, the mean HR was 64.3 (7.0 S.D.) and 64.8 (6.7 S.D.) beats per minute in the neutral and compassion states respectively. We investigated whether the HR increase was reflecting the state of compassion alone or the interaction between the state of compassion and the emotional sounds. The mean HR during the 40 seconds interval without sounds in each block of compassion was not significantly different from the mean HR during the interval with sounds during compassion (paired t-test, t(1,19)= -0.9, p=0.38). The mean HR was 66.3 (8.3 S.D.) and 66.0 (8.6 S.D.) beats per minute for compassion without sounds and compassion with sounds. Consistent with figure 1, we found an increase in HR during from neutral state with sounds to compassion state with sounds (paired t-test, t(1,19)=3.1, p<0.01). Finally, we did not find a difference in HR between the two groups during the 10-15 second intervals of resting state periods without sounds (t-test, t(1,18)=0.57, p=0.57), suggesting that meditation-related effects on HR were state specific.
Figure 1. Compassion increases emotional cardiovascular arousal.
Average HR significantly increased from neutral to compassion states for 10 experts (red) and 13 novices (blue) (main effect of State, see Table 1). During both states, emotional vocalizations were presented to the participants. Note that the practitioners showed significantly larger HR increase than the novices (State by Group interaction, see Table 1).
For a subset of participants (8 experts and 2 novices), we were able to correlate HR in each block with the subjective intensity of the meditation, as verbally reported. We found that the mean correlation coefficient was positive (on average 0.31 with 0.45 S.D.) and the normalized coefficient was significantly larger than 0 (one-tailed, t-test, t(1,9)=2.0, p<0.05), supporting the relevance of HR as a peripheral marker of this state. We also ran identical exploratory ANOVAs on heart rate variability (HRV) and respiratory sinus arrhythmia (RSA). HRV reflects the dynamic relationship between sympathetic and parasympathetic nervous system influences, and RSA is thought to measure only the parasympathetic influence (Allen, Chambers, Towers 2007). There were no significant results in the analysis of these metrics and consequently, they were not used in the subsequent analysis with BOLD data. In brief, the generation of compassion during the task increases the cardiovascular arousal compared to the neutral state, and this increase was modulated by meditation expertise.
Effect of meditation on respiratory rate
The respiratory rate was not significantly different between the blocks of neutral state and compassion meditation (paired t-test on the 10 participants, t(1,9)=1.06, p=0.34). The mean breaths/minute were 12.5 (4.0 S.D.) and 12.1 (4.0 S.D.) during neutral and compassion respectively. This finding is unlikely to reflect a type-II error, as the heart significantly increases from neutral to meditation for these participants (one tailed paired t-test, t(1,9)=-2.1, p<0.05).
BOLD correlates of HR across states
In this first analysis, the HR/BOLD coupling parameters across states can be influenced both by the main effect of state and by changes in the degree of HR/BOLD couplings within each state. For both groups, there was a positive coupling between HR and average BOLD signal across neutral and compassion states in right middle insula cortex, somatosensory cortex, right inferior parietal cortex and premotor cortex (BA 6) (voxel-wise t-test, corrected, see Table 2). To test our main hypothesis on the role of the insula in compassion, we contrasted the maps containing the intra-subject HR/BOLD coupling parameters from the experts with the ones from the novices. As predicted, the amplitude of HR/BOLD coupling parameter across states was higher for experts than novices in the left middle/posterior insula (voxel-wise t-test, corrected, Figure 2.A, Table 3). There was a trend for this cluster to be more strongly coupled on the left than the right side for experts more than novices (interaction side by group, ANOVA, F(1,20)=3.55, p=0.074). Positive couplings were also found in the left somato-sensory cortex, mid-anterior cingulate cortex and in several regions associated with mirror-neuron-like systems (premotor cortex, BA 6 and left inferior parietal lobule, see Table 3). Considering our hypothesis, we next evaluated whether this cluster in the insula showed compassion-specific neural correlates of HR. We contrasted the HR/BOLD coupling parameter in this region of interest (ROI) between the two conditions (compassion and neutral) and found that this association was presented only for experts during the compassion condition, and the coupling during compassion was higher for experts than novices (Figure 2.B). Together these results support our main hypothesis that the brain regions underlying emotions and feelings were positively coupled with heart rate as a function of state and expertise.
Table 2. BOLD correlates of HR across states.
Clusters extracted from the ROIs showing a positive HR/BOLD coupling parameter across the blocks of neutral and compassion states (average t score in each cluster) (10 experts and 12 novices). No cluster with negative HR/BOLD coupling parameter was found.
| Location | Side R | Volume (mm3) |
Peak Coordinates | t score |
|---|---|---|---|---|
| Middle insula / pre- and postcentral gyri | R | 12827 | 40 3 11/ 45 -16 39 | 3.1 |
| Cerebellum | R/ L | 5287 | -34 -70 -34 | 3.1 |
| Inferior occipital gyrus | R/ L | 4906 | -33 -86 -3 | 3.0 |
| Paracentral lobule, medial frontal gyrus | R/L | 2236 | -1 -18 53 | 3.1 |
| Parahippocampal gyrus/ hippocampus | L | 1594 | -32 -27 -12 | 3.0 |
| Angular gyrus/ middle temporal gyri (TPJ) | R | 1571 | 41 -61 29 | 3.1 |
| Medial frontal gyrus | L | 1360 | -10 48 24 | 3.1 |
| Medial frontal gyrus | L | 1192 | -9 36 53 | 3.0 |
| Inferior parietal lobule | R | 1100 | 36 -44 48 | 3.1 |
Figure 2. Expertise effect on HR/BOLD coupling parameter across states.
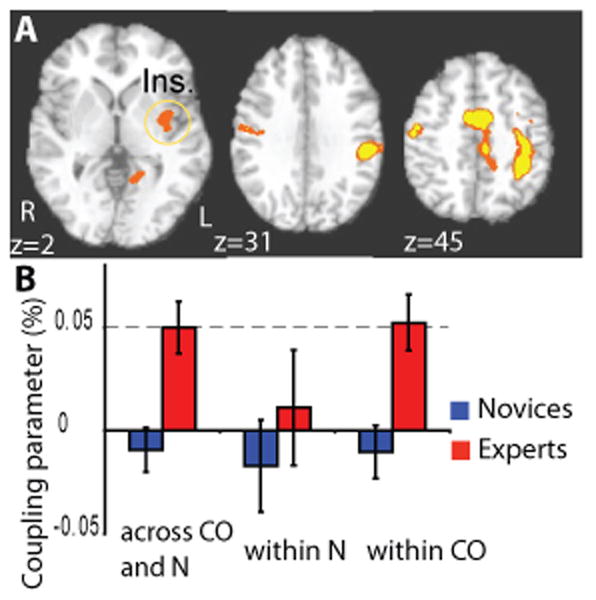
A. Voxel-wise analysis comparing the coupling between HR and BOLD across states (compassion and neutral) between 10 experts (red) and 12 novices (blue) (z = 2, corrected, color code: orange, p < 0.05, yellow, p < 0.02) in insula (ins.). B. The cluster in the left insula shows an expertise-related effect consistent with our previous study (Lutz, 2008). The average HR/BOLD coupling in the left insula (Ins.) was larger for experts than novices (t-test, t(1,20) = 3.6, p < 0.005) and significantly positive for experts (t-test, t(1,9) = 3.9, p < 0.005). The coupling in this cluster shows additional emotion-specific effects: for both groups, there was no significant coupling within the neutral state (t-test, at least p>0.45 with each group), but, within the compassion state, the coupling was higher for experts than novices (t-test, t(1,20) = 3.8, p < 0.005) and was significantly positive only for the experts (t-test, t(1,9) = 4.0, p < 0.005). The HR/BOLD coupling represents the percent BOLD signal change per ratio of change in heart rate.
Table 3. Expertise-related effect in BOLD correlates of HR across states.
Clusters extracted from the ROIs showing a stronger HR/BOLD coupling parameter for experts than novices across the blocks of neutral and compassion states (average t score in each cluster) (10 experts and 12 novices).
| Location | Side | Volume (mm3) |
Peak Coordinates | t score |
|---|---|---|---|---|
| Post- and pre-central gyri | L | 8553 | -28 -26 56 | 3.4 |
| Post-central gyrus | L | 1203 | -59 -13 23 | 3.0 |
| Inf. frontal gyrus/ Pre-central gyrus | R | 2269 | 45 -5 26 | 3.1 |
| Medial frontal gyrus/ paracentral lobule | L | 7131 | -6 -18 55 | 3.1 |
| Cerebellum | R/L | 5460 | 24 -41 27 | 3.1 |
| Inferior parietal lobule | L | 4073 | -42 -36 43 | 3.3 |
| Anterior mid-cingulate cortex | L | 3334 | -8 -10 40 | 3.2 |
| Middle frontal gyrus | R | 2729 | 22 -17 62 | 3.1 |
| Insula | L | 1771 | -35 -3 0 | 3.1 |
| Parahippocampal gyrus | L | 1281 | -31 -30 -12 | 3.0 |
| Parahippocampal gyrus | R | 1301 | 31 -29 -12 | 3.3 |
Compassion-specific correlates of HR
In addition, we explored emotion-specific correlates of HR as a function of state (compassion versus neutral) and group (experts versus novices) using a 2×2 factorial design. This second analysis provided a way to identify the brain regions which specifically correlated with the fluctuations in emotional arousal during compassion meditation. The main effect for State showed stronger HR/BOLD coupling parameter during meditation than rest in dorsal ACC/premotor cortex and right somatosensory cortex (voxel-wise repeated ANOVA, Figure 3, Table 4), supporting our hypothesis regarding the role of dorsal ACC in autonomic control. No cluster in the insula passed our correction criteria. A cluster in the orbito-frontal cortex, a region important in emotion and reward, showed the only decrease of coupling from neutral to compassion (Table 4). This cluster will not be further discussed as this region is particularly susceptible to drop-out and artifacts and was not an a priori region of interest. Voxelwise analysis of Group by State interactions showed experts to have had stronger HR/BOLD coupling parameter in a cluster encompassing the right somatosensory cortex and right inferior parietal lobule (IPL) (Figure 4) and a large cluster including parahippocampal / fusiform gyrus / occipital gyrus (Table 5). In the cluster that included IPL, the magnitude of the Group-by-State interaction was driven by a larger difference in the HR/BOLD coupling parameter between compassion and rest for the experts than the novices (see Figure 4.B and its legend). The coupling parameter for experts during compassion was positive and larger than the one during the neutral state (Figure 4). These results highlighted that brain regions associated with mirror-neuron-like systems (Iacoboni 2005; Rizzolatti, Fogassi, Gallese 2001)), such as IPL, were positively coupled with heart rate as a function of the interaction between state and expertise, and that this effect was driven by experts during the generation of compassion.
Figure 3. Compassion-specific neural correlates of HR.
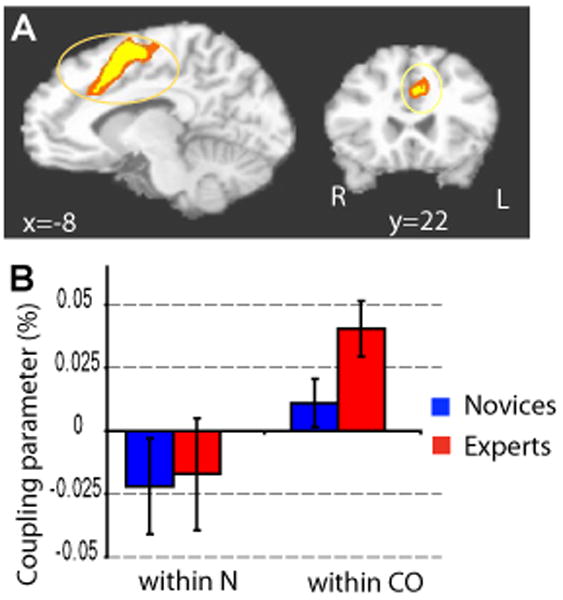
A. Voxel-wise analysis of the main effect of State (compassion vs. neutral) on the HR/BOLD coupling parameter in the dorsal ACC/premotor cortex (y = 22, corrected, color code: orange, p < 0.05, yellow, p < 0.02) 10 experts (red) and 12 novices (blue)). B. In this cluster the HR/BOLD coupling parameter was larger during the compassion state than the neutral state for 10 experts (red) and 12 novices (blue) (repeated ANOVA, main effect of state, F(1,20) = 8.1, p = 0.01). This finding suggests stronger cardiovascular autonomic control from the dorsal ACC during the compassion state than the neutral state.
Table 4. Compassion-specific effect on HR/BOLD coupling parameters: main effect of State.
These clusters were driven by a stronger HR/BOLD coupling parameter within compassion than neutral state across Group. In these clusters, HR/BOLD coupling parameters were higher during compassion than neutral state, except in one cluster in the orbito-frontal cortex (10 experts and 12 novices).
| Location | Side | Volume (mm3) |
Peak Coordinates | F score |
|---|---|---|---|---|
| Middle occipital gyrus/ cuneus | 7137 | R | 25 -86 10 | 6.7 |
| Middle occipital gyrus/ cuneus | 6845 | L | -21 -91 8 | 6.7 |
| Orbito-frontal cortex | 5802 | L | -36 45 -7 | 6.5 |
| Dorsal anterior cingulate cortex | 5943 | L | -9 9 47 | 6.0 |
| Post- and precentral gyri | 3608 | R | 34 -15 46 | 5.8 |
Figure 4. Expertise effect on compassion-specific neural correlates of HR.
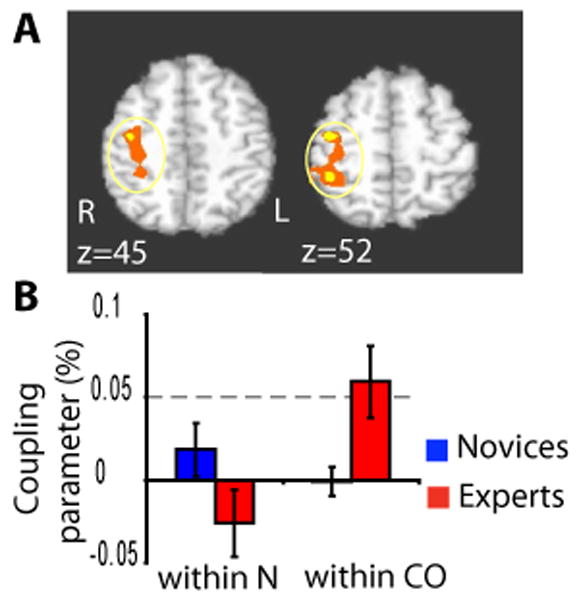
A. Voxel-wise analysis of Group (experts vs. Novices) by State (compassion vs. neutral) interaction on the HR/BOLD coupling parameter in somatosensory and inferior parietal lobule (z = 52, corrected, color code: orange, p < 0.05, yellow, p < 0.02, 10 experts (red) and 12 novices (blue)). B. Compassion expertise affects the HR/BOLD coupling parameter, in particular in the inferior parietal lobule (IPL). In the cluster from A., the experts showed a larger HR/BOLD coupling parameter in compassion than neutral states and than the novices (repeated ANOVA, F(1,20) = 7.2, p < 0.05).
Table 5. Compassion-specific effect on HR/BOLD coupling parameter: State by Group interaction.
These clusters showed a stronger HR/BOLD coupling parameter within compassion than neutral state more strongly for experts than novices (10 experts and 12 novices).
| Location | Side | Volume (mm3) |
Peak Coordinates | F score |
|---|---|---|---|---|
| Pre- and post central gyri/ IPL | R | 4141 | 38 -29 50 | 5.3 |
| Parahippocampal/ occip./ fusiform gyri | R | 5173 | 23 -76 11 | 6.5 |
Discussion
1) From empathy to compassion
In the current study, we found that changes in HR across states were positively associated with the right middle insula and somatosensory regions, right IPL and premotor regions (BA6), as well as right TPJ (Table 2). This pattern of neurovisceral coupling was more pronounced for experts than novices in the left hemisphere (left insula, left somatosensory cortex and left IPL) and in the mid-ACC (Table 3, Figure 2). A growing number of neuroimaging studies have reported a similar pattern of activation in the insular cortex, somatosensory cortex, parietal cortex, and mid-ACC/premotor cortex when participants are asked to imagine anothers' pain from a somatosensory perspective (e.g. while looking at body parts in painful situations) (e.g. (Danziger, Faillenot, Peyron 2009; Lamm et al., 2007)). Thus, the coupling between HR and BOLD signal in these regions may be interpreted within an ‘embodied’ or ‘simulation’ framework, as proposed by several authors(Preston and de Waal 2002; Rizzolatti, Fogassi, Gallese 2001; Sommerville and Decety 2006) which emphasizes the importance of motor predictions and motor simulations in processing the emotional significance of affective events. This finding was confirmed in our second analysis, during which we specifically compared the neurovisceral coupling within each state. Again we found that, during the compassion state only, HR and BOLD activity was positively coupled in somatosensory regions, and, more strongly for the experts, in the IPL, a region important in the imitation or understanding of others' actions(Iacoboni 2005; Rizzolatti, Fogassi, Gallese 2001).
While the present study confirms our previous finding on the role of the insular cortex in the state of compassion, these results also differ from our previous report (Lutz et al., 2008a) in several ways. In particular, our previous study showed a stronger activation during compassion (State effect) and more for the experts than the novices (Group by State interaction) in regions commonly recruited during high-level social-cognitive tasks (e.g. mentalizing see for review (Decety and Lamm 2007)) and during the redirection of attention to salient events (TPJ, posterior superior temporal gyrus, medial prefrontal cortex and posterior cingulate cortex/precuneus). In the current study, this circuit was not coupled with the HR changes either across states or within states, except for a positive HR/BOLD coupling parameter in the right TPJ across State and for both groups (Table 2). These differences may be attributed to the different analytical strategies between these two studies. In our previous analysis, we primarily modeled the perceptual response to the emotional sounds and its modulation by the states (compassion vs neutral) and expertise (experts vs. novices). By contrast, here we modeled the brain activity in 1-minute intervals in which the sounds were presented (block effect) while removing from the BOLD signal the variance attributed to the evoked response to sounds per se. This choice was guided by the necessity to compute our cardiac measures (HR, RSA, HRV) within time windows of at least 60 seconds (Allen, Chambers, Towers 2007). Because the current analysis reflected the block effect more than the evoked brain responses to sounds, the brain regions showing a state effect or a state by expertise effect in the current study might reflect some of the contextual processes that participated in the modulation of the perceptual responses to the sounds identified in our previous study. In this view, the generation of a state of compassion recruited empathy-related regions and regions which are part of a motor-neuron-like system that differentially modulates the perception of the emotional sounds.
In the present study, our neuroimaging and physiological observations provide evidence for an association between dorsal ACC activity and modulation of heart rate: the more heart rate increases across blocks of compassion meditation, the more ACC was activated for both groups (Figure 3). This link was not detected during the neutral state. Accumulating evidence supports the view (Critchley et al., 2003) that this region of dorsal ACC is mediating the regulation of bodily states of arousal so the organism can meet behavioral demands; for this reason some authors (e.g. (Craig 2007), (Heimer and Van Hoesen 2006)) have coined the term “limbic motor cortex” for the cingulate cortex. This neurovisceral coupling in ACC was previously observed during cognitive and emotional tasks(Critchley et al., 2003; Critchley et al., 2005). Our finding adds to this literature by showing that a purely mental state (without an explicit behavioral task) is sufficient to produce autonomic changes via this circuitry. In this view, observations of abulia and motivational impairments in patients with large cingulate lesions, in the absence of attentional or frontal executive impairments, broadly illustrate the role of this region in generating self-initiated preparatory bodily states(Devinsky, Morrell, Vogt 1995). According to that framework, the activity in dorsal ACC during the increase in HR during compassion may mediate some of the motivational processes underlying this prosocial state.
2) Exploring the potential impact of compassion meditation on emotional balance
Meditation can be conceptualized as a family of complex emotional and attentional regulatory training regimes developed for various ends, including the cultivation of well-being and emotional balance (for a recent review see (Lutz et al., 2008b)). The impact of such mental training on peripheral biological processes that are important for physical health and illness is not yet well understood. Increasing evidence suggests that meditation may impact physiological pathways, including the cardio-vascular(Benson 1997; Phongsuphap et al., 2008; Wu and Lo 2008), immune and neuroendocrine systems (Carlson et al., 2004; Carlson et al., 2007; Davidson et al., 2003; Pace et al., 2009), which are critical for disease development and progression. To date, the majority of studies examining the impact of meditation on peripheral biology have investigated practices that aim to calm the mind, improve focused attention, or develop mindfulness (for a review of these practices see (Lutz et al., 2008b)). These studies have commonly reported that meditation causes parasympathetic changes in cardiac measures (Benson 1997; Phongsuphap et al., 2008; Wu and Lo 2008), which are known to predict various health benefits (for review (Thayer and Lane 2007)). In contrast, we observed that compassion meditation produced elevated heart rate which also correlated with the intensity of the meditation as verbally reported. Increase in heart rate can occur as a result of decreased parasympathetic activity, increased sympathetic activity or a combination of both. Since we found that compassion meditation had no significant effect on a standard cardiac measure of parasympathetic activity (i.e. RSA), it may be that the heart rate increases observed during compassion meditation in the experts are more driven by increased sympathetic activity. Such increase in heart rate has already been reported during specific Kundalini Yoga breathing techniques (Peng et al., 2004). Increase in HR was also previously associated with helping in ambiguous emergencies, even if these studies did not differentiate between sympathetic and personal distress reactions (Gaertner and Dovidio 1977; Sterling and Gaertner 1984). Our current finding that compassion amplified the emotional saliency of emotional stimuli is consistent with the traditional function of this meditation (Dalai Lama X 1995) and with a recent study showing a positive correlation between emotional awareness and dorsal ACC activity in the context of viewing highly arousing pictures (McRae et al., 2008). In combination with previous findings, our finding suggests that one outcome of compassion training is to sensitize the practitioner to the emotional experience of others. Traditional accounts of the compassion practice suggest that, even if strong personal distress can occur during compassion meditation, this distress is alleviated by two factors: 1) cultivating an equanimity stance toward suffering, in particular one's own suffering, such that one is able to bring suffering to mind without being swept away by it; and 2) a conviction that, from the longest term perspective, complete relief of others' suffering is definitely achievable (Dalai Lama X 1995; Pace et al., 2009). For that reason, compassion meditation is often preceded by conceptual training that helps practitioners develop cognitive schemas which cultivate a sense of equanimity and hopefulness and meditation training that develops a non-judgmental, non-reactive stance toward one's habitual patterns of emotional reactivity. These two factors are said to protect the meditators from suffering from compassion burnout. Recent studies provide preliminary evidence for this protective effect of compassion on affective reactivity: self-compassion was associated with less anxiety in response to a mock job interview and less distress after getting neutral feedback in response to a videotaped speech performance (Leary et al., 2007), and the amount of practice correlated with improvements in immune and behavioral responses to a psychosocial stressor within the group of participants randomized to the type of compassion-based conceptual training mentioned above (Pace et al., 2009). Although the present study did not detect any parasympathetic effects, the impact of compassion meditation on emotion regulation might result from a complex pattern of interaction between sympathetic and parasympathetic influences. The trend toward a stronger neurovisceral coupling in the left insula versus the right insula for experts than novices might reflect a stronger parasympathetic control during the increase in HR induced by compassion meditation, as suggested by some models (Craig 2007) and supported by the localization of the observed ACC modulation with state and expertise on the left side (see Tables 3 and 4, and (Matthews et al., 2004; and Wittling et al., 1998)). This possibility needs to be further explored in future studies.
3) Limitations of the study and direction for future studies
Because novices and experts differ in many respects other than simply the extent of mental training, such as culture of origin and first language, longitudinal research that follows individuals over time in response to compassion training will be needed to further substantiate our findings. It will also be essential to assess the impact of such emotional and empathic training on behavioral tasks involving altruism and more generally involving emotional reactivity and regulation. The long-term question is to evaluate whether repeated practice in such techniques could result in enduring changes in emotional reactivity and affective style(Davidson 2004). The fact that systematic changes in cardiac function and neurovisceral coupling were observed during the mental practice of compassion and in response to auditory emotional stimuli presented during the practice, and the fact that robust differences were observed between experts and novices, suggests that the next steps to evaluate the behavioral impact of this training and to assess its effects longitudinally are warranted.
Acknowledgments
We would like to acknowledge Dr. Matthieu Ricard for assistance with task design, participant recruitment and written meditation instructions and Dr. John Dunne for Tibetan translation and clarifications on Buddhist meditative techniques. We also thank the Mind and Life Institute for helping to facilitate this work and Helen Weng, for helpful comments on a preliminary version of the manuscript. This work was supported by grants from the National Institute of Mental Health (NIMH P50-MH069315 to R.J.D.), the National Center for Complementary and Alternative Medicine (P01-AT004952 to R.J.D. and to A.L, and U01AT002114-01A1 to A.L.), and gifts from Bryant Wangard and Ralph Robinson, Keith and Arlene Bronstein and the John W. Kluge Foundation (to R.J.D.).
Footnotes
Author contributions AL, and RJD designed the study. AL was involved in data collection. AL, LLG and D.P. analyzed the data. AL wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Benson H. The relaxation response: Therapeutic effect. Science (New York, NY) 1997;278(5344):1694–1695. doi: 10.1126/science.278.5344.1693b. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–215. [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity. 2007;21(8):1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J. Expertise modulates the perception of pain in others. Current Biology : CB. 2007;17(19):1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: A neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3rd. Guilford Press; New-York, NY: 2007. [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24(3):751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain : A Journal of Neurology. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Dalai Lama X. The world of tibetan buddhism: An overview of its philosophy and practice. Wisdom Publisher; Boston: 1995. [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17(6):969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61(2):203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: Neural substrates and biobehavioural correlates. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2004;359(1449):1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: How, when and why? Trends in Cognitive Sciences. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : A Journal of Neurology. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Gaertner SL, Dovidio JF. The sublety of white racism, arousal, and helping behavior. Journal of Personality and Social Psychology. 1977;35:691–707. [Google Scholar]
- Gethin R. The foundations of buddhism. Oxford University Press; Oxford; 1998. [Google Scholar]
- Gyatso Tenzin, the XIV Dalai Lama, Jinpa GP. The world of tibetan buddhism: An overview of its philosophy and practice. Wisdom Publications; 1995. [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews. 2006;30(2):126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Current Opinion in Neurobiology. 2005;15(6):632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007;2(12):e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Leary MR, Tate EB, Adams CE, Allen AB, Hancock J. Self-compassion and reactions to unpleasant self-relevant events: The implications of treating oneself kindly. Journal of Personality and Social Psychology. 2007;92(5):887–904. doi: 10.1037/0022-3514.92.5.887. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS ONE. 2008a;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008b;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22(3):1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41(2):648–655. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CK, Henry IC, Mietus JE, Hausdorff JM, Khalsa G, Benson H, Goldberger AL. Heart rate dynamics during three forms of meditation. International Journal of Cardiology. 2004;95(1):19–27. doi: 10.1016/j.ijcard.2003.02.006. [DOI] [PubMed] [Google Scholar]
- Phongsuphap S, Pongsupap Y, Chandanamattha P, Lursinsap C. Changes in heart rate variability during concentration meditation. International Journal of Cardiology. 2008;130(3):481–484. doi: 10.1016/j.ijcard.2007.06.103. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. The Behavioral and Brain Sciences. 2002;25(1):1–20. 20–71. doi: 10.1017/s0140525x02000018. discussion. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews. Neuroscience. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: An fMRI study. Social Neuroscience. 2008;3(2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science (New York, NY) 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Decety J. Weaving the fabric of social interaction: Articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychonomic Bulletin & Review. 2006;13(2):179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Sterling B, Gaertner SL. The attribution of arousal and emergency helping: A bidirectional process. Journal of Experimental Social Psychology. 1984;20:286–296. [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. NeuroImage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wendelken SM, McGrath SP, Akay M, Blike GT. Using a forehead reflectance pulse oximeter to detect changes in sympathetic tone. 1:325–8. doi: 10.1109/IEMBS.2004.1403158. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Wittling W, Block A, Genzel S, Schweiger E. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia. 1998;36(5):461–468. doi: 10.1016/s0028-3932(97)00129-2. [DOI] [PubMed] [Google Scholar]
- Wu SD, Lo PC. Inward-attention meditation increases parasympathetic activity: A study based on heart rate variability. Biomedical Research (Tokyo, Japan) 2008;29(5):245–250. doi: 10.2220/biomedres.29.245. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Bischoff-Grethe A, Frank GK, Arce E, Paulus MP. Increased amygdale activation is related to heart rate during emotion processing in adolescent subjects. Neuroscience Letters. 2007;428(2-3):109–114. doi: 10.1016/j.neulet.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]