Abstract
Asthma in inner city children is prevalent, has increased severity, and for many patients poses difficulty in achieving control.. Although some of the limitations to asthma control in inner city children include socioeconomic burdens, other factors, like environmental allergens, pollutants, infections, and stress, contribute significantly to the disease burden found in these children. As a consequence of these unmet needs for asthma control in inner city children, the National Institutes of Allergy and Infectious Diseases (NIAID) established research networks with an ultimate goal of improving care for this at risk population. The Inner City Asthma Network Program began in 1991 and has, over the ensuing years, funded three distinct networks which have a common goal, to improve care for the child with asthma. Each of these networks has expanded our knowledge about asthma in the inner cities, and asthma in general. The NIAID efforts continue with the current inner city network, Inner City Asthma Consortium (ICAC2). The following article will discuss the application of these inner city networks to the study of asthma, the contributions of each of the individual networks, and how newly proposed efforts under ICAC2 propose to improve our understanding and eventual control of asthma in inner city children.
Keywords: Asthma, inner city, cockroach sensitivity, asthma treatment
Introduction
In 2007, the Journal of Allergy and Clinical Immunology1 published a series of articles that reviewed the importance of asthma in inner city children and the commitment of the National Institute of Allergy and Infectious Diseases (NIAID) to establish research networks to study asthma in this population. The funded networks were charged to identify risk factors, gain greater insight into the mechanisms of how asthma in this population may differ from that found in children not living in the inner city, and evaluate potentially effective immune-based treatments,. Many of the previously identified issues with asthma, or for that matter other illnesses, in the inner city continue to exist today despite significant advances at many fronts. For example, the burden of asthma on affected children, and their families, remains significant with ongoing compromises to their day-to-day lifestyle.2;3 These limits to a normal lifestyle in the inner city extend beyond the pathophysiology of asthma to include socioeconomic hurdles such as availability of care, access to healthcare givers, costs, both direct and indirect, and an absence of follow-up for improved management.4 What has not been established is whether asthma in the inner city is distinct from asthma in other locales in terms of pathogenesis and pathophysiology, and, if so, what are the differences and how may they affect treatment. These questions remain important but not fully answered. Nonetheless, for children living in our inner cities and having asthma, this disease continues to be highly prevalent and many of these children continue to have significant symptoms, despite treatment.
Overview of asthma and its severity in inner city children
It is helpful to first define who are the children in these networks. Children participating with the NIAID Networks live in the inner cities in neighborhoods that have been identified on the basis of census tracts. Each of these census tracts has been designed to be homogeneous with respect to population characteristics, economic status, and living conditions. In general, the study populations are residents of urban census tracts in which at least 20% of the households have incomes below the federal poverty level.
Based on this selection process, there has been speculation that children living in these inner city neighborhoods have asthma of greater severity. Severity, as discussed by Szefler et al. in this issue,5 can, however, be assessed and expressed in a number of ways. First, severity can be judged by the amount of medication needed to achieve asthma control. Whether the child with asthma in the inner city requires a greater amount of medication to achieve the same level of control as their counterpart in the suburban areas is not fully defined, but this information will be important to eventually achieve improved management and understanding of the pathophysiology in this population. Asthma severity can also be reflected in the need for care, or health care utilization. Care for acute asthma is a frequent occurrence for inner city children; this need, however, may relate to many factors, other than disease severity, including a lack of available or convenient follow up care or the use of the emergency departments for non-acute care. Severity can also be determined by the response to medication or ease by which control is achieved. Evidence that the child with asthma in the inner city may be resistant, or have impaired responsiveness, to corticosteroids is not established, but is of interest. Finally, whether the degree and, possibly, type of airway inflammation in asthma found in inner city patients is greater, or distinct, from a non-inner city population and whether this differential in airway injury contributes to disease severity, has also yet to be defined.
Although asthma severity remains of considerable interest and importance, attention has expanded, or perhaps shifted, to identify factors that limit control of asthma. Although asthma severity is integrally linked with the response to treatment and control, other factors need to be considered in determining methods to achieve control and to use this approach to elucidate what limits this goal. These factors can include environmental allergens, the load of these allergens, pollutants, nutritional aspects, smoke exposure, stress, obesity, and microbes. The NIAID-sponsored networks represent a coordinated approach to learn more about asthma in inner city children, their interactions with multiple environmental factors, and the complexities of these factors to the disease process, and with this information, formulate approaches to improve the treatment and control of asthma in this population.
To appreciate and understand the development and achievements of the NIAID programs in inner city asthma, and the direction that they have given to research, the following discussion will focus, briefly, on a number of aspects of these programs: a description of the inner city programs and their findings as well as an overview and description of the recently renewed Inner City Asthma Consortium (ICAC) 2 with its proposals and rationale for future research.
What are the NIAID Sponsored Inner City Asthma Programs and what has been learned from their individual and collective efforts?
For nearly the past 20 years, the NIAID has funded networks to study asthma in the inner cities; each of the three funded networks has had distinct goals and research approaches to address asthma in the inner city (Table 1). Importantly, the findings of these networks have constructed a foundation of expanding knowledge about asthma, in general, and particularly about this disease in inner city children. To more fully appreciate where we are today in terms of understanding the issues, their inherent complexities, and approaches tied to control asthma in children living in inner cities of the United States, it is helpful to briefly review the goals and findings of each of the three individual inner city networks.
Table 1.
Objectives and key findings of NIAID inner-city asthma programs
NCICAS: 1991–1997
|
ICAS: 1994–2001
|
ICAC: 2002 to the present
|
National Cooperative Inner City Asthma Study (NCICAS, 1991–1997)
The goals of this initial network were directed into two phases: (1) to identify environmental factors that may be responsible for the increase in asthma prevalence in inner city children and (2) to develop an intervention strategy based on these initial findings. NCICAS was a very productive effort in terms of new knowledge and publications. The brief summary below reflects only a small component of their efforts, but is intended to focus on outcomes that have influenced the new ICAC. In one of their initial efforts, a large population of 1,528 children with asthma and living in major U.S. cities was recruited from inner city neighborhoods; they were young (mean age of 6.2 years), primarily black or Hispanic, and highly atopic to a battery of environmental allergens evaluated.6 These children and their home environments were comprehensively evaluated in terms of allergen sensitization, allergen presence and distribution, and exposure as well as the associated, or resulting, clinical picture of asthma in these patients.
A number of key findings emerged from this first network to indicate that there is not a single factor, or cause, associated with the observed high morbidity, but, rather, many components contribute to the pattern of asthma in children living in the inner city. These components include access to care, associated psychological factors of either the child or family or both, and difficulties with adherence to treatment. However, and important to an understanding the contributors to asthma in this population, the affected children were found to have a high incidence of allergen sensitization, with the presence of sensitization having a strong influence on disease morbidity. Moreover, the allergens dominating the sensitization profile of these children, cockroach (36.8%), house dust mite (34.9%) and mouse (22.7%), were identified, along with their environmental exposure. Sensitization and exposure to cockroach, in particular but not exclusively, were associated with critical and important clinical outcomes: increased hospitalizations, wheezing, and unscheduled medical visits. The increased need for health care utilization is a clear outcome indicator that reflects a greater severity of underlying asthma in these children. These observations were important, and directive, as they further emphasized the relevance of the cockroach allergen, and other antigens such as house dust mite, Alternaria, and mouse, as an indication of asthma severity and as an environmental factor that limited the ability of these patients to achieve good asthma control. These findings have also directed future research activity towards one population of patients, the child with cockroach allergen sensitization and exposure, as being potentially an example of a key factor that needs to be addressed to improve asthma control in the inner city children. The approach and findings of NCICAS have set the stage for the subsequent networks.
Inner City Asthma Study (ICAS, 1994–2001)
The goal of ICAS, the second NIAID-sponsored network, was to design and conduct multicenter intervention trials that would reduce asthma morbidity among inner city children. The findings from NCICAS were a strong indication that one environmental component of inner cities that contributed to disease severity was cockroach exposure and sensitization, which was associated with greater markers of asthma severity, i.e., increased asthma symptom days and more unscheduled medical visits. These findings suggested that a key intervention, or target, to achieve better asthma control was a reduction in environmental exposure to cockroach. From the population of children previously recruited in NCICAS, 937 asthma patients were identified and selected for an intervention study. Approximately half of these recruited children were assigned to an intervention that consisted of remediation of exposure to house dust mite, passive smoking, cockroaches, pets, rodents, and molds; the intervention was carefully tailored to the allergic sensitivity of the individual patient. The other half of the recruited population served as a comparative control group.
As reported by Morgan et al.,7 this environmental allergen avoidance intervention led to a significant reduction in the average maximal number of days with asthma symptoms and unscheduled asthma-related healthcare use. This latter effect is a key beneficial outcome as it reflects a lessening of the asthma burden to the patient and family and a reduced need for acute care due to uncontrolled asthma. Despite the significant benefit noted on these outcomes, allergen avoidance had no effect on lung function. These findings were important as they pointed to the differential effects that treatments may have on the various domains of asthma control. They also indicated that different interventions may have different effects, i.e. reduction in symptoms versus improvement in lung function, These observations need to be considered in the design of treatment trials.
The findings from ICAS were also important as they suggested the feasibility for achieving greater patient benefit through a reduction of the environmental allergen burden. Moreover, these observations suggested which of the components of asthma control will likely be responsive to an avoidance approach, i.e., symptoms and exacerbations, but not necessarily lung function. Moreover, the ICAS findings underscored the importance of allergen sensitivity of a patient to their asthma morbidity, and identified cockroach as a key inner city allergen which could limit the achievement of optimal disease control. Finally, the ICAS findings served as an excellent transition to the third network in inner city asthma, the Inner City Asthma Consortium (ICAC), where cockroach antigen sensitization and exposure continue to emerge as key factors in determining asthma severity and limitation to achieving disease control, as well as a target for treatment in the inner city population.
Inner City Asthma Consortium (ICAC1, 2002–2009, and ICAC2 2009–2014)
The third NIAID-sponsored inner city asthma network, ICAC, began in 2002 and entered its renewal phase, ICAC2, following in October 2009. During the first funding period, 2002–2009, ICAC made significant progress in a number of areas: the establishment of a birth cohort (Urban Environment and Childhood Asthma [URECA]) and the completion and publication of the results from the Asthma Control Evaluation (ACE) trial. The results of this trial are reviewed in detail by Szefler, et al. in this issue of JACI.5 In addition, ICAC has completed the Inner City Anti-IgE Therapy in Asthma (ICATA) trial; these data are undergoing analysis. In addition, ICAC has begun to assess the potential effectiveness of immunotherapy (IT) treatment with cockroach antigen in asthma, since this allergen continues to be a, if not the, dominant allergic factor in asthma for inner city children.
What questions will ICAC2 address in its efforts to further characterize asthma in inner city children and the underlying mechanisms?
The current ICAC program, ICAC2, has identified a number of projects and has begun to develop protocols to meet their research goals over the next 5 years.
ICAC is composed of three components that are designed to direct efforts of the program: ICAC administrative component and its site principal investigators; the NIAID, represented by Drs. Peter Gergen and Alkis Togias, and the Statistical and Clinical Coordinating Center (Dr. Herman Mitchell). The ICAC Steering Committee is composed of representatives from NIAID, the Statistical and Clinical Coordinating Center, and representatives from the ICAC Administrative Center (Drs. William Busse, James Gern and Christine Sorkness) along with principal investigators from the eight investigative sites (Baltimore, Boston, Chicago, Dallas, Denver, Detroit, New York, and Washington, D.C.) as well as the two investigators of the Basic Science Sites (Drs. Homer Boushey and David Schwartz). The Steering Committee is responsible for the overall direction of the consortium including the planning of its clinical trials, pilot studies, and preparation of manuscripts.
Urban Environment and Childhood Asthma (URECA)
URECA’s primary goal, as detailed by James Gern in this issue of the Journal of Allergy and Clinic Immunology,8 is to establish a birth cohort of newborns who are high-risk for asthma. These inner city children were recruited at birth and have been closely followed and studied to determine the immunologic causes of asthma in this group. To accomplish this goal, the recruited children have had extensive evaluations of their immune system and its development since birth. Furthermore, extensive environmental information on this cohort has been and continues to be gathered, including key environmental factors, such as respiratory viral infections and allergen exposure and sensitization, along with the influence of stress, diet (i.e., vitamin D), pollutants, and obesity. From these observations, it will be possible to dissect and discover the “gene-by-environment” interactions that occur in these high risk inner city children, how these immune aspects may either contribute to or cause asthma, and perhaps provide clues to the eventual prevention of this disease. Not only do these observations help determine the developmental aspects of immune function in inner city children in relationship to environmental influences and the eventual expression of asthma, but these data will also provide information that can be compared to other non-inner city birth cohorts. These observations can then be a key step in our efforts to determine if differences do indeed exist between inner city and non-inner city asthma, what immune-based mechanisms may be involved to lead to these distinctions, and how might modifications of immune responses or environmental factors benefit asthma control in this population.
The Development of Immune-based Treatment Protocols
Immunotherapy for Inner City Children with Asthma
A major component of ICAC 2 will be the design, development and implementation of clinical interventional protocols to evaluate immune-based therapies to control asthma in inner city children. To conduct the newly designed clinical trials, eight research sites have been identified and are responsible for the recruitment and conduct of these studies. They include Drs. Rebecca Gruchalla (Dallas, TX), Meyer Kattan (New York, NY), Andrew Liu (Denver, CO), George O’Connor (Boston, MA), Jacqueline Pongracic (Chicago, IL), Stephen Teach (Washington, DC), Robert Wood (Baltimore, MD), and Edward Zoratti (Detroit, MI). All of these sites have demonstrated access to inner city children and have extensive experience in the conduct of studies involving an urban population. As noted above, sensitization to cockroach is highly prevalent in the inner city pediatric populations, which comprise these networks and is an apparent major factor in determining the severity of asthma in this population. Morgan et al.7 have already shown the clinical benefit of reducing cockroach allergen exposure in sensitized children on relevant asthma control outcomes. Another approach to diminish the host’s allergic reaction to environmental allergens is to modify the immune-based responses to environmental allergens. Our evaluation of omalizumab in the ICATA trial has provided insight into how the use of a “broad-based” anti-IgE regulation can affect asthma and what clinical outcomes appear most responsive to this form of treatment. To extend these findings, it is known that IT, either subcutaneous or sublingual, has clinical benefit with pollens and house dust mite antigen in allergic rhinitis and asthma.9–14 However, there are no, or at best limited, data on the effects of IT with cockroach in inner city children with asthma.
Since IT has been shown to have sustained benefit associated with its use, this immune-based approach has considerable appeal for this highly exposed, allergen-sensitive population of inner city children, and raises the possibility that IT might be disease modifying, an outcome not noted with current pharmacological interventions.14 Studies in young children have shown that IT may attenuate the eventual expression of asthma in allergic sensitized children.15;16 Consequently, a major initiative over the next 5 years will be to establish the most effective IT program with cockroach allergen in inner city asthma, i.e., sublingual or parenteral, and begin to evaluate its effect, and effectiveness, in asthma. A key component to structuring these IT trials will be the identification of a patient biomarker, e.g., change in antigen-specific IgE or increase in IgG4 levels, which may reflect an immune response to treatment, potentially correlate to clinical outcomes, and provide insight into the immune basis of this therapy in an inner city population.
Reducing future risks – preventing exacerbations
The recent revision of the U.S. Guidelines for Asthma Diagnosis and Management17 has proposed two major domains to judge the effectiveness of asthma control: (1) impairment and (2) future risks. Measures of control that are based upon impairments include symptoms, need for short-acting beta agonists for rescue treatment, and lung function measurements. Instruments for these measurements are established, and current asthma treatments have shown efficacy based on impairment outcomes. Future risks in asthma control include exacerbations, progressive loss of lung function, and side effects from medication. Asthma exacerbations may have a profound effect on patients, and their families, due to the associated morbidity, costs, inconveniences, and general disruption of lifestyle, as well as the rare, but still real risk for a fatal outcome.
What is known about asthma exacerbations in children?
Our understanding and appreciation of asthma exacerbations has increased considerably in the last 15 years. For children, and adults to a lesser degree, viral respiratory infections, particularly the common cold virus, rhinovirus, are the major cause of these attacks.18 Secondly, there is a seasonal pattern to asthma exacerbations which shows a marked increase in September and October. These “September” epidemics occur predictably every year and coincide with children’s return to school and contracting a cold.19;20 For many children, these exacerbations occur despite optimal medical management and well-controlled asthma prior to development of a respiratory infection. Although respiratory viral infections “trigger” these exacerbations, underlying allergic sensitization also appears as another, and important, risk factor in this interaction. Treatment of asthma has been primarily directed towards reducing existing airway inflammation and not necessarily treating, or modifying, the underlying allergic processes, which may be a major factor in the susceptibility for viral respiratory infections. For most children with asthma, whether living in the inner city or elsewhere, treatment that targets airway inflammation has been of considerable benefit and has reduced both impairments and exacerbations.21 Despite the achievement of presumably good asthma control with anti-inflammatory medications, many children still have “breakthroughs” in their treatment coverage and experience exacerbations of asthma when they “catch a cold.” Improved control of exacerbations remains a major unmet need in the treatment of asthma.
Given this unmet need in treatment, protocols for ICAC2 are being developed that will focus on interventions which might be of greater effectiveness in reducing asthma exacerbations. Although our treatment will target a traditional outcome, i.e. exacerbations, the use of an anti-IgE intervention will also attempt to modify underlying allergic sensitization, as this patient characteristic appears as a major risk factor for wheezing with a respiratory infection.22–24 Thus, it is our hypothesis that underlying allergic sensitization and exposure to putative allergens make the host more susceptible to an exacerbation with a viral respiratory infection. Concomitant with this goal will be the conduct of mechanistic studies to determine how the interplay between allergic sensitization and respiratory viruses leads to a loss of asthma control and an exacerbation, and what are the biological mechanisms that drive this process and may be future targets for treatment.
The incorporation of basic science or translational projects in ICAC2
A new component to ICAC2 will be the inclusion of two basic science projects. The goal behind the addition of this research effort to ICAC2 is to apply novel, basic science investigation to asthma in the inner city, and, through these interactions, determine basic, underlying mechanisms of asthma, and mechanisms that are potentially unique to this high risk group of patients. Moreover, it is anticipated that the application of innovative investigation, through the inclusion of basic mechanisms, to the inner city population of patients will provide insights to novel treatments and management that are potentially more effective for asthma patients in these environments.
Epigenetics
Multiple genes have been identified in asthma and with features of asthma.25;26 Expanded exploration into the genetic regulation of asthma will need to take advantage of genomic-wide investigations to more fully determine the “pattern” of gene products which may be involved and related to clinical features of asthma. To date, however, studies have not given us definitive information on how asthma in the inner city is regulated, is possibly unique, or how asthma in the inner city may be differentiated from asthma in non-inner city environments.
Epigenetics is an approach that should allow us to explore the possibility that environmental exposures can bring about functional changes by methylating, for example, selective genes, and, through this process, lead to altered function that is relevant to asthma.27 As methylation, or other changes to genes, can arise from environmental influences and can occur relatively quickly, an epigenetic investigative approach of well defined and characterized populations promises to provide novel and unexplored insights into how environmental influences of the inner city may have an influence on asthma, particularly, for inner city patients. David Schwartz from National Jewish Health, Denver, CO will lead this effort.
Microbiota
Microbes also have a tremendous influence on host immune responses. However, the microbes in our intestine or our environment are not one organism but a family of different organisms, i.e. bacteria, fungi, etc. The collective influence of this microbiota on the host’s immune system can arise from a variety of environmental sources.28;29 To begin to determine whether differences exist in the microbiota of inner city home environments, collections of household dust will be analyzed to define the mixture of bacteria, fungi, chitin, and allergens, and, eventually, assess the overall influence of this microbiota on asthma. It is hypothesized that inner city home environments have a unique microbiota profile given high concentrations of cockroach, mouse droppings, and house dust mite found in these dwellings. Moreover, as there are interactions between bacteria, allergen proteases, fungus, and chitin, for example, the pattern of microbes that emerges in this mixture may influence the environment’s allergenicity and the resulting immune response in the host. Dr. Homer Boushey from the University of California in San Francisco will lead these studies.
What is known about asthma phenotypes in the inner city and what has been learned from ICAC?
Studies from ICAC1 have begun to shed some light on this question. The ACE study has, indirectly, addressed differences in asthma severity, as a potential feature, or phenotype, and can be assessed by the amount of treatment necessary to achieve asthma control.21 The conduct and results of the ACE trial are reviewed in greater detail in the article by Stanley Szefler et al. in this issue.5 Briefly and relevant to a discussion of disease severity, the recruitment of patients for ACE identified subjects between 12 and 20 years of age, who had an established diagnosis of asthma and symptoms of persistent asthma or evidence of uncontrolled disease. At the initial study visit for ACE, each subject was assessed for ongoing symptoms, measures of pulmonary function, and the overall level of asthma control. Based upon these entry assessments, the recruited subjects were treated according to six-step escalating algorithm designed to achieve asthma control; treatment began with fluticasone, 100 mcg/day, and ranged up to fluticasone 500 mcg/Salmeterol 50 mcg, twice a day, along with either montelukast or low dose theophylline.
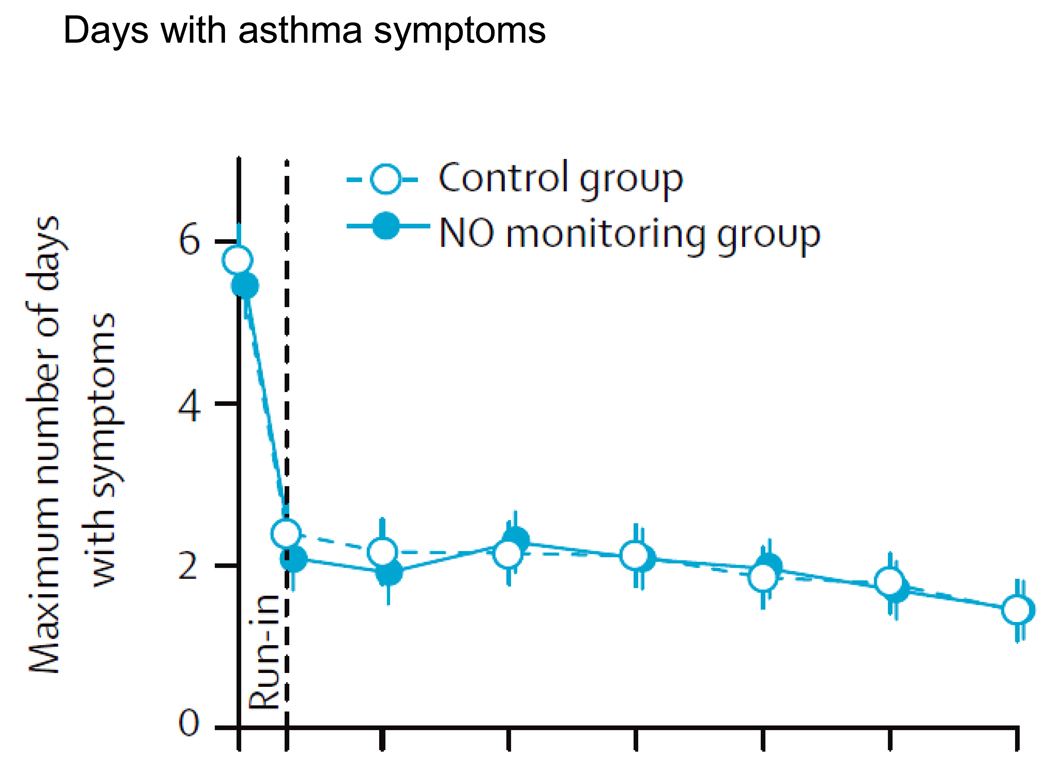
Despite the use of asthma control medication at the screening visit, the recruited subjects recorded an average (SD) of 5.6 (4.6) maximum days/2 weeks of asthma symptoms (Fig. 1). These values promptly fell to 2.1 (2.7) and 2.4 (3.0) days in the FeNO-treated and control treatment groups, respectively, and were sustained at this level of control for the entire study. Although some subjects in the FeNO group reached a higher level of step care, based upon elevated FeNO levels, the level of symptom control was similar in the two groups.
Figure 1.
A comparison of the addition of NO monitoring with Guideline directed care to Guideline directed care alone on maximum number of days with symptoms. During the “run-in,” patients asthma treatment was adjusted prior to entering the control vs. NO monitoring group. Reprinted with permission from Szefler SJ, et al. Lancet 2008; 372: 1065–72.
As noted by Szefler et al.,21 the majority of enrolled subjects, in both the guideline and FeNO plus guideline directed groups, achieved a level 1 control status, which reflects 0–3 days of symptoms and 0–1 nights of symptoms over the 2 week interval, and FEV1 values were ≥ 80% predicted. Therefore, under the “right” management strategy and proper medication doses, asthma control can be achieved and maintained in an inner city group of asthma patients.
A number of factors were felt to lead to this level of asthma control. First, the enrolled patients were closely monitored throughout the trial by skilled personnel. Second, medications were provided as part of the study, thus relieving the patient of the need to secure their own drugs. Finally, considerable effort was extended to maintain regular use of the medications and a high level of treatment adherence, an approach that resulted in a mean adherence of 86.6% during the study. These data suggest that with guideline-directed treatment and medication adherence, asthma control can be achieved and maintained in an inner city group of patients.
A number of conclusions may be derived from the ACE study as to the treatment of asthma, in general, and, specifically, to an inner city population and whether asthma severity is greater in these populations.21 Of considerable interest was the observation that an “algorithm-directed treatment” with step-care adjustments, based on guideline recommendations, led to good asthma control in a large portion of inner city patients. At face value, these observations raise the possibility that asthma severity in the inner city may not be as different from other populations, and facilitated efforts to use the correct medications and at the correct dosages, along with a dedication to adherence on the part of the patient, will lead to good asthma control.
Does this really mean that asthma severity may not be distinct in inner city children? For a number of reasons, such a conclusion is premature and speculative at the present. First, recruitment for ACE did not include the patients with more severe disease. Second, the relative responsiveness to the step-care approach did not include a non-inner city comparative group. Finally, recruitment was not random, but rather included patients known to the study centers and interested in being part of this trial. The question as to whether greater asthma severity exists in inner city children with asthma remains, largely, unanswered, but may be an important facet of disease in this population and factor in determining the most appropriate treatment for individual patients.
Are there any distinct asthma phenotypes in inner city populations?
In an editorial accompanying their issue devoted to asthma in 2006, Lancet issued a challenge and “a plea to abandon asthma as a disease concept.” They went on to say, “The general consensus now emerging is that, even in adults, asthma is unlikely to be a single disease entity. Sally Wenzel30 describes an approach to distinguish different phenotypes and subphenotypes, aspirin-sensitive, late onset, and obesity-related, for example. Whether these phenotypes simply represent different aspects of disease in a single underlying pathological process – airway inflammation – in people with different predispositions who are susceptible to different triggers driven by specific cellular and molecular responses, is not known. Or perhaps, asthma, as a symptom is really only the clinical manifestations of several “distinct diseases.”31 The latter is most likely.
To gain insight into the existence of various phenotypes, or subgroups, various investigative groups have begun to explore the features of different asthma populations. As part of the NIH-supported Severe Asthma Research Program (SARP), Moore et al.32 recruited patients and from this cohort has begun to define the clinical features of patients with severe disease. In the study by Moore et al.,32 recruited patients, who were classified with severe asthma, had clinical characteristics defined by an American Thoracic Society (ATS) Workshop, which proposed two major and seven minor criteria to define this group.33 The suggested major criteria centered on the need for large doses of inhaled corticosteroids, with, or without, systemic corticosteroids, to maintain asthma control.
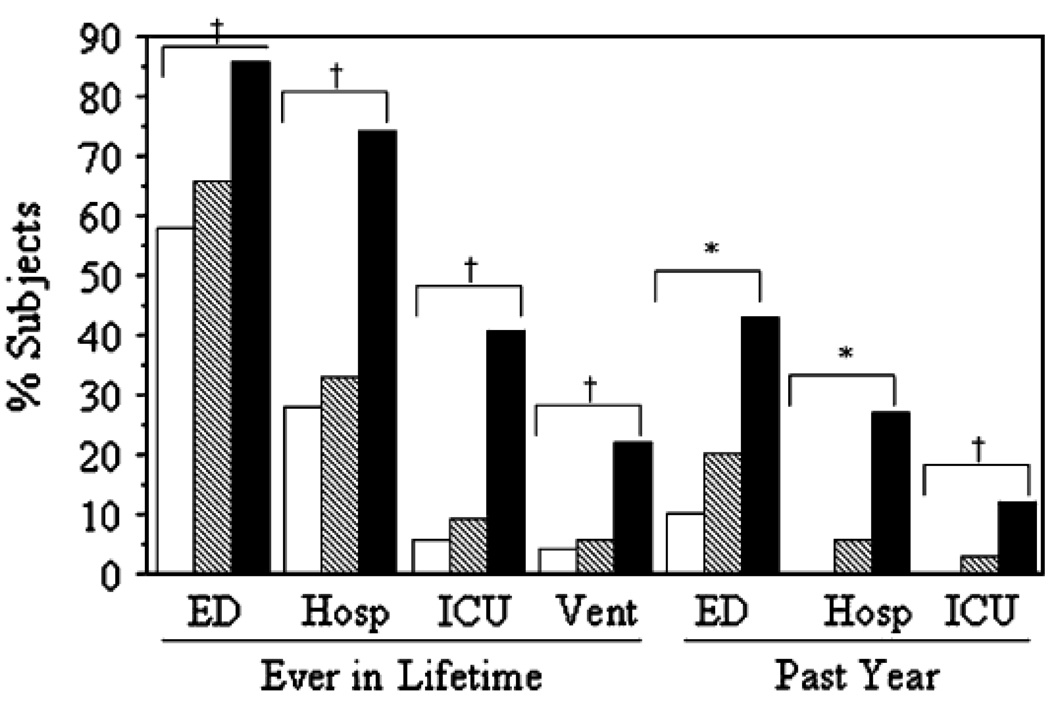
When clinical features of asthma patients with mild and moderate asthma were compared to those meeting the ATS criteria for severe disease, a number of differentiating features emerged between the severe and non-severe asthma clusters. First, by recruiting based on the definition of the ATS Workshop, patients with severe asthma required more medications, particularly corticosteroids, to achieve disease control. However, when compared to subjects with moderate asthma, indices of airflow obstruction were similar to severe asthma. In contrast, when patients with mild, moderate and severe asthma were assessed for frequency of health care utilization, distinctions between these groups became strikingly apparent. Patients with severe asthma had a greater use of emergency care and hospitalizations as well as need for treatment in intensive care units or requiring assisted ventilation and intubation (Fig. 2).
Figure 2.
Urgent HCU increased with disease severity. Data are shown as lifetime (ever) events and visits in the past year. Hosp, Inpatient hospitalizations; Vent, need for mechanical ventilation. White bars, mild (n 5 164); hatched bars, moderate; black bars, severe asthma. *P < .0001, all groups are different from each other; HP < .0001, mild and moderate groups are similar, but different from severe group. Reprinted with permission from Moore, et al. J Allergy Clin Immunol 2007; 119: 405–13.
These differences were consistently noted at two timeframes, ‘ever in a lifetime’ or ‘in the past year.’ Therefore, a feature of severe asthma is a greater frequency and need for health care utilization, likely arising as the result of asthma exacerbations, which occurred despite aggressive treatment with corticosteroids. An obvious question arising from these assessments is whether patients with more severe disease are unresponsive or partially responsive to corticosteroids.
Characterization of clinical features associated with severity in children with asthma have not been extensively defined. However, when Fitzpatrick et al.34 compared features of severe and non-severe asthma in school-age children in Atlanta, GA, a number of clinical aspects emerged to distinguish these levels of disease severity. Children with more severe asthma had increased symptoms, greater airflow obstruction, and higher prevalence of allergen sensitization, a feature that contrasted with the adult population, where skin test positivity tended to be less in patients with more severe disease. In patients with severe asthma, there was also a greater variability in airflow obstruction and FeNO, a marker felt to reflect underlying inflammation that was not responsive to treatment, including large doses of inhaled corticosteroids, again raising the possibility of resistance to the effects of corticosteroids. Finally, the frequency of exacerbations was greater in those with severe disease, a characteristic shared with the adult population.
Therefore, a pattern is emerging that distinguishes severe asthma from other forms of this disease. First, clinical features that reflect ongoing impairments are greater – symptoms, airflow obstruction, and interference with day-to-day activity. Furthermore, those with more severe disease have a greater frequency for future risks, i.e., asthma exacerbations, as reflected in the need for more healthcare utilizations. These markers of disease exist despite the use of more medications, particularly inhaled and systemic corticosteroids. The underlying mechanisms, particularly those associated with airway inflammation, which likely contribute to these clinical characteristics, are not well established. Nor is known whether certain phenotypes for asthma constitute clusters of greater disease severity and whether one aspect of this differential is resistance to treatment, including corticosteroids.
What have we learned about subpopulations in inner city asthma?
Observations from ACE may have begun to shed insight on clinical characteristics in inner city children that may reflect more severe disease and possibly distinct phenotypes. Higher doses of ICS were required to achieve asthma control in subjects who were obese, a greater frequency of allergen sensitization and higher IgE levels.21 There has been considerable interest in the role of obesity in asthma as a distinct phenotype and as a potential risk factor for more severe disease.35;36 The mechanisms by which obesity affects asthma severity in our inner city population are not established, but because of the greater frequency of obesity in the inner city, this relationship will continue to be of considerable interest and importance. We have already discussed the importance of allergen sensitivity for asthma exacerbations with viral respiratory infections. Whether sensitization to inner city allergens, like cockroach, results in a distinct phenotype has not been explored in detail, but will be of interest as we define differences between asthma in inner city and non-inner city populations where unique characteristics may arise in the cockroach vs. house dust sensitized asthmatic patient. Important in these comparisons will be an evaluation of responses to treatment.
What have we learned from our ICAC studies?
Analyzing the ACE population, which had a number of historical characteristics and biomarkers determined prior to initiating the intervention protocol, Gruchalla et al.37 evaluated a panel of patient factors which might predict future asthma control. Included in this evaluation were determinations of FeNO, total IgE, allergen-specific IgE, allergen skin test reactivity, asthma symptoms prior to enrollment, peripheral blood eosinophils, airway hyperresponsiveness, and sputum eosinophils. Using this approach, maximum symptom days during the ACE trial were modestly predicted by entry levels of symptoms, use and need for rescue beta-agonists, and a history of exacerbations. To a similarly modest degree, symptoms, albuterol use, previous exacerbations, and lung functions predicted future exacerbations. Overall, these findings in ACE suggest that the control domains of impairment and future risk are influenced by similar factors, at least in this particular population. Unfortunately, these assessments did not appear sufficient to serve as highly sensitive surrogates to link to future asthma outcomes.
What should be the next steps in establishing the various phenotypes of asthma in the inner city?
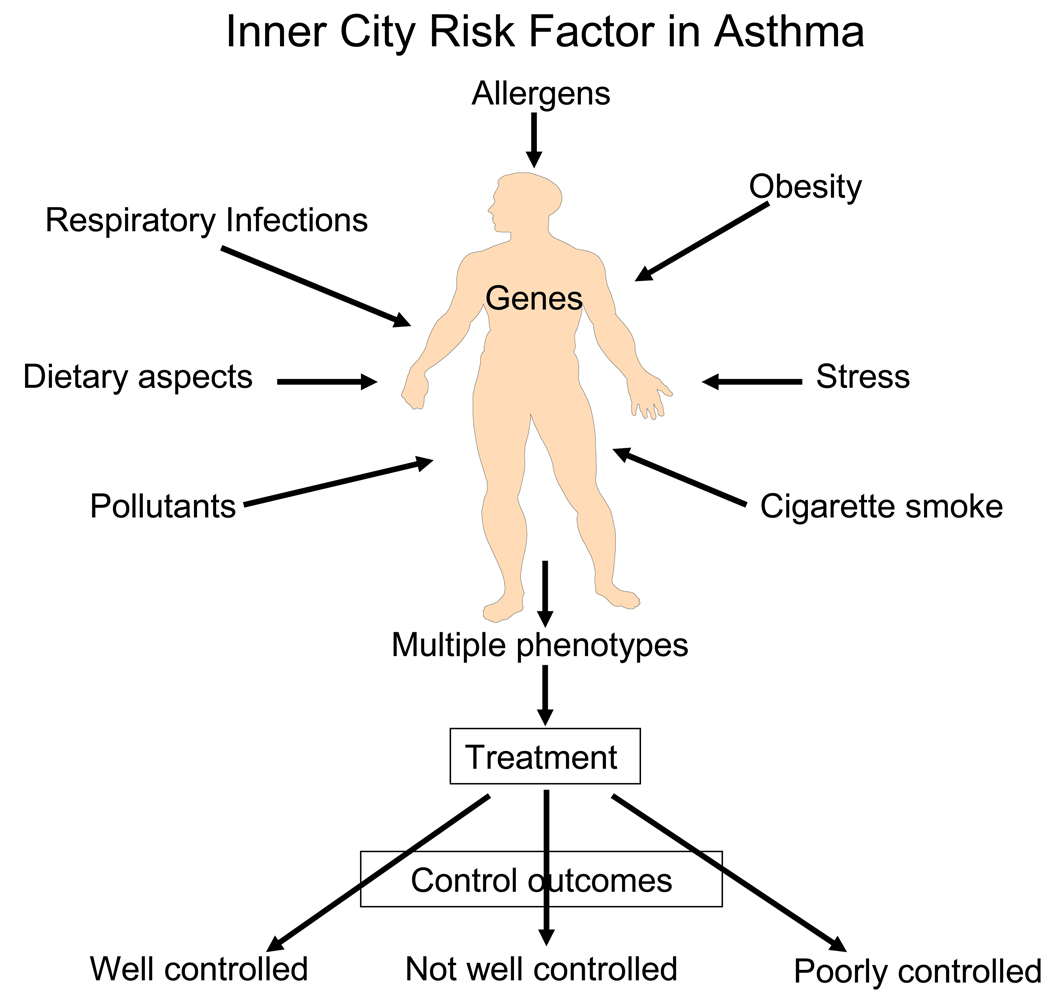
These findings from the ACE trial in ICAC-1 have been, nonetheless, helpful in directing what future assessments of asthma are needed to define existing phenotypes, and from these data to be able to more fully determine and predict responsiveness to treatment. We know, and now appreciate, that asthma is influenced by a wide variety of environmental factors, including allergens, obesity, nutrition, and stress, for example, and these stimuli likely interact differently within individual patients (Fig 3.). Each of these external factors has the capacity to generate an immune inflammatory pattern, which may, in turn, determine the clinical features, or phenotype, of asthma, the response to treatment, and, possibly, the inherent potential for impairment and future risks.
Figure 3.
A multiplicity of environmental factors influence the development of asthma and resulting phenotype. The resulting phenotypes will also have variable levels of severity, which, in turn, will influence the response to treatment and eventual asthma control.
A critical “next” step to define inner city asthma phenotypes, cockroach-sensitive, obesity associated, viral respiratory tract infection prone, and/or cigarette smoke exposed, for example, and to determine how these phenotypes may determine the patient’s response to treatment as well as risk for loss of asthma control, is the need to link assessments of immune and inflammatory response patterns to the existing clinical profiles. It is likely that such a “fingerprint” will be a “pattern” of inflammation and not necessarily a single cellular marker, cytokine or chemokine. Moreover, it is also likely that there will be additional variabilities in these inflammatory response patterns which will be dependent upon developmental aspects of the patient. The peeling back of the multiple features that eventually constitute the clinical expression, or phenotype, of an individual asthma patient is likely to reveal a complex picture which is influenced, to a large degree, by the genetic background and broad description of the environment. Therefore, to more fully understand the asthmatic portraits of patients living in the inner cities will require a careful and systematic approach to define the unique features, both clinical and immunological, of asthma in this population and from this information more effectively design management approaches, including a selection of medications most likely to lead to improved disease control.
Conclusions
For children living in an inner city environment, asthma continues to pose a major burden to the patient, family, and society. As a consequence of these outcomes, and possibly features of underlying asthma, the NIAID has developed research networks whose efforts are directed towards identifying the major effects of environmental allergens on their asthma and developing treatments to achieve control. Ongoing efforts of the current network, ICAC2, promise to further our understanding of the potentially unique contribution of environmental allergens, and other co-existing factors, to asthma in the inner city and to evaluate immune-based therapies that will improve disease control in this population and to identify the mechanisms that may limit the response to current treatment, including corticosteroids.
Acknowledgments
Supported by NIH Inner City Asthma Consortium, Contract HHSN272200900052C
Abbreviations
- ATS
American Thoracic Society
- FeNO
Fraction of exhaled Nitric Oxide
- ICAC
Inner City Asthma Consortium
- ICAS
Inner City Asthma Study
- IT
Immunotherapy
- NCICAS
National Cooperative Inner City Asthma Study
- NIAID
National Institutes of Allergy and Infectious Diseases
- SARP
Severe Asthma Research Program
- URECA
Urban Environment and Childhood Asthma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busse WW, Mitchell H. Addressing issues of asthma in inner-city children. J Allergy Clin Immunol. 2007;119:43–49. doi: 10.1016/j.jaci.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Galea S, Vlahov D. Urban health: evidence, challenges, and directions. Annu Rev Public Health. 2005;26:341–365. doi: 10.1146/annurev.publhealth.26.021304.144708. [DOI] [PubMed] [Google Scholar]
- 3.Smith LA, Hatcher-Ross JL, Wertheimer R, Kahn RS. Rethinking race/ethnicity, income, and childhood asthma: racial/ethnic disparities concentrated among the very poor. Public Health Rep. 2005;120:109–116. doi: 10.1177/003335490512000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aligne CA, Auinger P, Byrd RS, Weitzman M. Risk factors for pediatric asthma. Contributions of poverty, race, and urban residence. Am J Respir Crit Care Med. 2000;162:873–877. doi: 10.1164/ajrccm.162.3.9908085. [DOI] [PubMed] [Google Scholar]
- 5.Szefler SJ, Gergen P, Mitchell H, Morgan W. Achieving Asthma Control in the Inner City: Do the NIH Asthma Guidlines Really Work? Journal of Allergy & Clinical Immunology. doi: 10.1016/j.jaci.2010.01.025. In press. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 7.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 8.Gern JE. The Urban Environment and Childhood Asthma Study. Journal of Allergy & Clinical Immunology. doi: 10.1016/j.jaci.2010.01.037. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang BC, Johnson J, Morgan C, Chang JL. The role of immunotherapy in cockroach asthma. J Asthma. 1988;25:205–218. doi: 10.3109/02770908809071367. [DOI] [PubMed] [Google Scholar]
- 10.Sublingual Immunotheapy for Adults. Blue Cross and Blue Shield. 2003;18:1–45. [Google Scholar]
- 11.Wilson DR, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD002893. [DOI] [PubMed] [Google Scholar]
- 12.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008;358:2259–2264. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 13.Cox LS, Larenas LD, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–1035. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102:157–164. doi: 10.1016/s0091-6749(98)70079-x. [DOI] [PubMed] [Google Scholar]
- 15.Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 16.Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 17.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122:662–668. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120:526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008;122:741–747. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. doi: 10.1016/j.jaci.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eder W, Ege MJ, Von Mutius E. The Asthma Epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 26.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 27.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 29.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 31.The Lancet. A plea to abandon asthma as a disease concept. The Lancet. 2006;368:705. doi: 10.1016/S0140-6736(06)69257-X. [DOI] [PubMed] [Google Scholar]
- 32.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzel SE, Fahy JV, Irvin C, Peters SP, Spector S, Szefler S American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 36.van VI, Ten BA, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 37.Gruchalla RS, Sampson HA, Matsui E, David G, Gergen PJ, Calatroni A, et al. Asthma morbidity among inner-city adolescents receiving guidelines-based therapy: role of predictors in the setting of high adherence. J Allergy Clin Immunol. 2009;124:213–221. doi: 10.1016/j.jaci.2009.05.036. 221. [DOI] [PMC free article] [PubMed] [Google Scholar]