Abstract
Objective
To systematically investigate the effect of lack of adherence to the recommended change in insulin pump infusion line use beyond 48 hrs and determine whether the type of insulin made a difference.
Research design and methods
This was a double-blind, randomized, cross over trial with 20 patients with DM I using Insulins Aspart and Lispro without a line change for up to 100 hrs. Using retrospective continuous glucose monitoring, we analyzed the average glucose over the day. Changes in serum 1,5-anhydroglucitol, carboxymethyllysine, and Free 15-F2t Isoprostane were also studied.
Results
From day 2 to day 5 of the pump line use the daily avg. glucose level increased from 122.7 to 163.9 mg/dL (P < 0.05), fasting glucose from 120.3 to 154.5 mg/dL (P < 0.05): post prandial glucose from 114.6 to 172.1 (P < 0.05): and the daily maximum glucose from 207.7 to 242.8 (P < 0.05 for the trend). Time period that the glucose was > 180 mg/dL increased from 14.5 % to 38.3 (P < 0.05). Loss of control occurred despite increase in total daily insulin dose from 48.5 ± 11.8 units to 55.3 ± 17.9 units (P = 0.05). There was no difference in loss of control between insulin types and biomarkers measured did not change significantly.
Conclusions
The insulin pump infusion should be changed every 48 hours in patients using CSII, to avoid loss of glycemic control. In the short term, this loss of glycemic control has no impact on oxidative stress and glycation.
Key terms: CSII, Type 1 Diabetes, pump infusion line, glycemic control
Introduction
The Diabetes Control and Complications (DCCT) trial demonstrated the value of intensive glycemic control(1). Continuous subcutaneous insulin infusion (CSII) was an important component of intensive therapy in the DCCT and is an established treatment modality for diabetes mellitus. Despite logical arguments regarding the ability of CSII to deliver insulin in a more physiologically appropriate manner than multiple daily injections (MDI)(2;3), it has been hard to demonstrate the long term superiority of this treatment over MDI. In trials of short duration, CSII therapy with insulin analogs has resulted in lower glycemic exposure without increased risk of hypoglycemia as compared to MDI. In practice many patients treated with CSII remain poorly controlled. The reasons for the lack of success of CSII in such patients are unclear.
The recommended duration of needle use or pump infusion line use is 48–72 hours. In 1983, Morbidity and Mortality Weekly Report (4) published a case of an 11 year old girl with type 1 diabetes using CSII who frequently did not change the pump infusion site for ten days. She had staphylococcus aureus abscess at needle insertion site and was diagnosed with toxic shock syndrome. Infection at the infusion site is among the most frequently encountered complications of CSII (5;6). Since no specific procedural guidelines are available to minimize the risk of subcutaneous infections in users of CSII, the recommendation by The Food and Drug Administration (FDA) is based on Center for Disease Control (CDC)’s Guideline for Prevention of Intravascular Infections(4;7). This however, has not been tested in a randomized controlled trial. Factors affecting the stability of the insulin have also been studied in vitro(8). We have observed that many patients do not adhere to this recommendation. Instead, they wait for the self monitoring blood glucose (SMBG) levels to rise or the insulin in the pump to run out before changing the pump infusion line. Since the consequences of such delays have never been systematically investigated we conducted a study to document the effect of lack of non adherence with this recommendation, and thereby determine the optimal time interval between pump infusion line changes in CSII. The primary objective of our study was to determine the optimal duration of pump infusion line use without loss of glycemic control and its effect on glycemic control and biomarkers of long term complications. In addition we also tested whether there was a difference in the optimal duration of pump infusion line use without loss of glycemic control with use of Insulin Aspart and Lispro, which are two of the three FDA approved insulins in use in CSII in external pumps.
Research Design and Methods
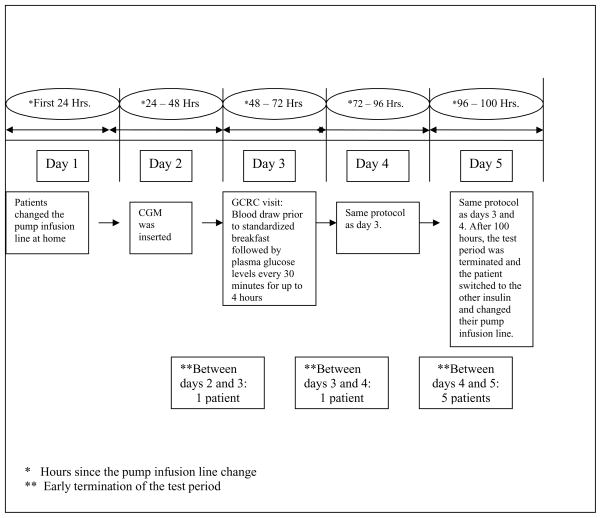
This was a prospective, double blind, cross over, randomized controlled trial. Patients with DM I using CSII between the ages of 18 and 75 with HbA1c ≤ 8.0%, serum creatinine ≤ 1.2 mg/dL were recruited from outpatient clinics. Pregnancy was an exclusion criteria. Please see fig. 1 for a schematic of the test period schedule and patient characteristics. All patients gave informed consent. 24 patients were screened and 20 patients participated in and completed the study of whom, 16 were female and 4 were male. Patients continued to use their own insulin pump during the study. If they qualified for the study after screening, they were randomized to either insulin Lispro or Aspart as insulin 1. They used the insulin that they were randomized to for up to a week. Test period one then commenced with their pump infusion line change. On the second day, patients came to the Clinical and Translation Research Center (CTRC) for insertion of the retrospective Continuous Glucose Monitor (CGM). Continuous Glucose Monitor Model MMT-1702 by Minimed Inc., CA was used for 3 days and patients did not have access to their glucose level while in the study. On days, 3, 4, and 5 the patients came to the CTRC in a fasting state. Blood was drawn prior to being given a standardized breakfast after which they had plasma glucose level done every half an hour for four hours while at CTRC. Serum from the blood samples was stored at −70 degree Celsius. Samples from five patients were lost due to hurricane Katrina.
Figure 1.
Schematic Representation of the protocol.
During this four hour post - prandial period, patients were allowed to give themselves correction boluses of insulin if they needed to. They also maintained a diary of their daily total insulin dose requirements during the study test period. On day 5, after the 4 hour blood draw was over (i.e. at the end of 100 hours), patients were asked to change their pump infusion line and switch to insulin number 2. They used the second cross over insulin for up to a week and then entered test period 2 following the same protocol as test period 1. If the SMBG or plasma glucose was > 300 mg/dL at any time between 48 to 100 hours after the pump infusion line change, the test period was terminated and the patients were advised to change their pump infusion line.
Serum samples from days 3 to 5 of the test period were analyzed for 1,5-anhydroglucitol (1,5-AG). 1,5-AG was assayed by using the enzymatic method kit, Glycomark, in Pharmaceutical Research Laboratories of Nippon Kayaku (Gunma, Japan) the carboxymethyllysine (CML) measurement was performed using the ELISA kit with anti-CML antibody by SRL, Inc. (Tokyo, Japan). Free 15-F2t isoprostane was quantified in EDTA plasma using a fully validated highly sensitive, automated LC/LC-MS/MS assay(9).
Main outcome measures
The main outcome measures were changes in various CGMS and blood glucose parameters from 24 to up to 100 hours after pump infusion line change. The parameters analyzed from the CGMS data were daily avg. glucose, average pre meal glucose, avg. 2 hour post prandial glucose, daily maximum glucose and the percentage of time the glucose was > 180 mg/dL in a 24 hour period. Changes in peak blood glucose were analyzed from the data gathered from the patient’s stay at the GCRC during the protocol. The duration of use of the pump infusion line without loss of glycemic control up to 100 hours since the last pump infusion line change was another outcome measure that was compared between Insulins Aspart and Lispro. Serum 1,5-anhydroglucitol (1,5-AG), carboxymethyllysine and Free 15-F2t isoprostane levels were measured from samples collected from days 3 to 5 of the test period.
Statistical Analysis
All data was analyzed on an intention to treat basis. Repeated measures, paired t-test and Pearson’s correlation on SPSS 14.0 for windows and statistical R-package were used to compare the various variables. Data was downloaded from the CGM using The Solutions™ Software (MMT-7310) for each patient and for each test period.
Results
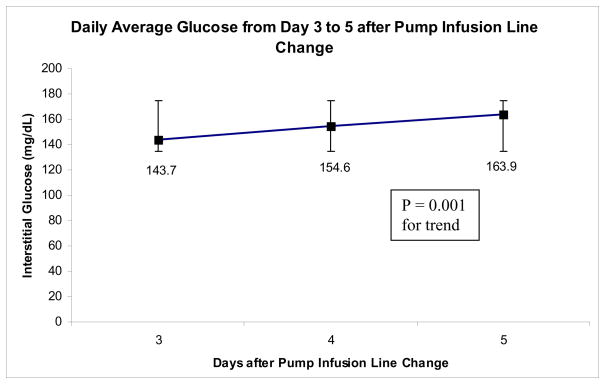
Results are summarized in table 1. The mean age of our patients was 45.5 + 14.3 years, while the mean HbA1c and serum creatinine were 7.4 ± 0.4 % and 0.84 ± 0.14 mg/dL respectively. The daily avg. glucose level increased from day 2 to day 5 of the pump line use from 122.7 mg/dL to 163.9 mg/dL (P < 0.05 for the trend). The avg. pre meal glucose showed a progressive increase from 120.3 mg/dL on day 2 to 154.5 mg/dL on day 5 (P < 0.05 for the trend). This was in parallel to a significant rise in the 2 hour post prandial glucose from 114.6 mg/dL on day 2 to 172.1 mg/dL on day 5 (P < 0.05 for the trend) and the daily maximum glucose (mean daily maximum glucose was 207.7 mg/dL on day 2 and 242.8 mg/dL on day 5, P < 0.05 for the trend). The amount of time that the glucose was > 180 mg/dL in a 24 hour period increased from 14.5 % on day 2 to 38.3 % on day 5 (P < 0.05 for the trend). These values from the CGMS data reflected changes in the interstitial fluid glucose. The peak blood glucose was the maximum blood glucose during the 4 hour post prandial period after breakfast while the patients were in CTRC between days 3 to 5. The peak blood glucose on day 3 was 269 ± 34.6 mg/dL and increased to 212.9 ± 40.2 mg/dL on day 5, being non statistically significant.
Table 1.
Results of the various biomarkers and variables from the CGMS data. All values are expressed as means ± standard deviation. To convert mg/dL of glucose to mmol/L multiply by 18 or divide by 0.055
| Interstitial Fluid Glucose values and Biomarkers | Day 2 (24–48 Hrs.) | Day 3 (48–72 Hrs.) | Day 4 (72–96 Hrs.) | Day 5 (96–100 Hrs.) | P value for the Trend |
|---|---|---|---|---|---|
| Avg. daily glucose (mg/dL) | 122.7 ± 21.8 | 143.7 ± 19.4 | 154.6 ± 26.3 | 163.9 ± 31.8 | 0.001 |
| Avg. fasting glucose (mg/dL) | 120.3 ± 15.1 | 135.5 ± 5.2 | 142.8 ± 7.2 | 154.5 ± 33.4 | 0.038 |
| Avg. 2 hour post-prandial glucose (mg/dL) | 114.6 ± 24.7 | 149.3 ± 18.9 | 153.2 ± 15.1 | 172.1 ± 37.1 | 0.000 |
| Avg. daily maximum glucose (mg/dL) | 207.7 ± 44.6 | 249.8 ± 37.1 | 258.3 ± 34.7 | 242.8 ± 37.8 | 0.002 |
| Percentage of time glucose > 180 mg/dL | 14.5 ± 12.8 | 24.3 ± 13.2 | 31.8 ± 18.6 | 38.3 ± 24.1 | 0.003 |
| 1,5 anhydrodiglucitol (microgram/ml) | 5.8 ± 3.8 | 5.9 ± 3.8 | 6.2 ± 3.8 | .021 | |
| Carboxymethyllysine (microgram/ml) | 5.7 ± 1.1 | 5.8 ± 1.2 | 5.9 ± 1.3 | .517 | |
| Isoprostane (picogram/ml) | 6.9 ± 2.5 | 6.1 ± 2.2 | 6.5 ± 2.2 | .55 |
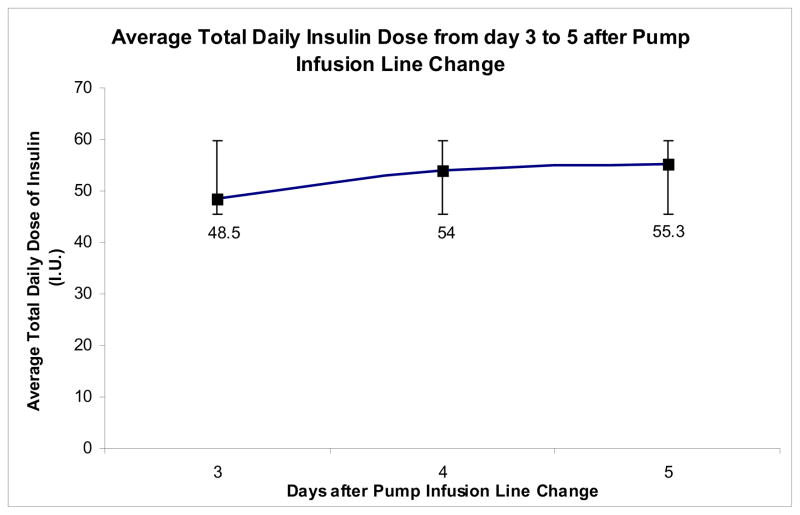
Since the patients were allowed to give themselves additional correction boluses in accordance to their SMBG or the plasma glucose values, we computed the daily avg. glucose rise in context of the total daily insulin doses. Figures 2 and 3 summarize the progressive increase in the total daily dose of insulin starting 48 hours after the line change, with concomitant increase in the daily avg. glucose values seen. The average total daily insulin dose was 48.5 ± 11.8 units on day 3 which progressively increased to 55.3 ± 17.9 units on day 5 (P = 0.05 for trend). The administration of the correction boluses may also explain the non significant rise in the peak blood glucose in the post prandial period. There was no significant difference between the length of time of use of the pump infusion line up to 100 hours between Insulins Aspart and Lispro (96.4 ± 8.5 and 98.1 ± 6.8 hours respectively, P = 0.52). 7 patients underwent early termination of either one or both of their test period (i.e. before 100 hours from pump infusion line change) due to loss of glycemic control (defined as blood glucose > 300 mg/dL). Figure 1 summarizes the details of these patients. One patient’s test period was terminated early as the patient forgot to reconnect the insulin pump after temporary discontinuation during the test period which resulted in a blood glucose level of >300 mg/dL. The data on that patient for that particular day was not included in the analysis.
Figure 2.
The average total daily insulin dose from days 3 to 5 of the test period.
Figure 3.
The average daily interstitial glucose levels from days 3 to 5 of the test period. To convert mg/dL of glucose to mmol/L multiply by 18 or divide by 0.055
Serum 1,5-AG was 5.1 ± 4.13 μg/ml on day 3 and 5.6 ± 4.2 μg/ml on day 5 (P = 0.15 for trend). There was a statistically non-significant increase in serum CML (5.6 ± 0.9 μg/ml on day 3 and 5.8 ± 0.7 μg/ml on day 5, P = 0.42) and Free 15-F2t isoprostane (6.9 ± 2.5 pg/ml on day 3 and 6.5 ± 2.2 pg/ml on day 5, P = 0.55).
Conclusions
Our study clearly demonstrates that glycemic control deteriorates after 48 hours of insulin pump line use and further prolongation of line use leads to additional incremental loss of glycemic control. There is no prospective data to support the FDA’s recommendation to change the pump infusion line after 48 to 72 hours of use. Our study investigated the consequences of use of the pump infusion line up to 100 hours since the last line change. After 48 hours of pump infusion line change, the mean daily glucose, the fasting glucose, the 2 hour post prandial glucose, and the percentage of time spent above a blood glucose > 180 mg/dL increase significantly. This deterioration occurred despite an increase in insulin doses. However, we did not observe any deterioration in biomarkers of oxidative stress and glycation over this time period.
Evidence suggests that CSII has the ability to improve glycemic control(10–13). Several factors affect glycemic control in patients using CSII(14). Since this was a cross over study, the various factors remained the same for both arms of the study, as each patient served as their own control. In a recent study(15), the authors have speculated that the trend toward fewer catheter occlusions, though not significant, with insulin glulisine in comparison to insulin aspart maybe due to the absence of zinc and the presence of polysorbate 20 in the formulation. The subjects changed the pump infusion line every 2 days. A study done by Burdick et al(16) has shown that missed meal time insulin boluses were associated with sub-optimal glycemic control in youths using CSII. There are other factors that may affect the stability of insulin and have been studied in vitro(8). However, we believe that the frequency of pump infusion line change is also an important factor to be considered as cause of suboptimal glycemic control. Our study suggests that the optimal duration of pump infusion line use is 48 hours from the last line change.
Hyperglycemia and glycemic variability are associated with oxidative stress. This is true for type 1 and type 2 diabetes(17;18). The use of a single anatomic site for the continuous infusion of insulin contributes to a more consistent effect of insulin(19). CSII has been shown to decrease glucose variability(20;21). Our hypothesis was that this advantage may be abrogated by prolonged use of the pump infusion line. This is important as glycemic excursions, including post prandial hyperglycemia contribute to a higher HbA1c and increases the risk for cardiovascular disease(22;23). 1,5 – AG is the 1-deoxy form of glucose whose reabsorption is competitively inhibited by glucose in the renal tubule causing urinary loss of 1,5-AG in presence of even transient hyperglycemia. Markers of glucose control such as 1,5-AG that reflect short term, post prandial glycemic changes are a useful adjunct tool to assess the glycemic control as HbA1c reflects changes over the past 2–3 months. A decrease in plasma 1,5-AG levels with hyperglycemia as would be expected and has been demonstrated by others(24) was not seen in our study perhaps related to the small sample size.
Non-enzymatic glycation of proteins and their end products (advanced glycation end products, AGE) have been implicated in the pathogenesis of diabetic complications(25). Accumulation of CML-modified proteins have been shown to be significantly greater in the cerebral vessels of the diabetic patients than their age-matched controls besides being significantly increased in patients with simple retinopathy(26). Whether such changes seen in the short term are unclear. Our study failed to demonstrate such an increase.
F2-Isoprostanes (F2-IsoPs)3, the stable isomers of prostaglandin F2a (PGF2a), are considered a reliable index of in vivo oxidative stress(27). Evidence has suggested that oxidative stress (OS) may play a role in the pathogenesis of diabetic complications, although the relationship between hyperglycemia and OS is inconsistent in diabetic clinical studies. Statistically significant reduction in 8-epi-PGF(2 alpha) values has been demonstrated in patients with type I diabetes upon improvement in metabolic control(28). However, the rise in isoprostane levels was not statistically significant in our study. Oxidative stress has been shown to be more closely related to glucose excursions rather than HbA1c(17) in cross-sectional studies. However, in our prospective study we have failed to demonstrate increased oxidative stress with poor short term control. It is possible that this risk was minimized by patients giving themselves larger boluses of insulin following meals, which we had to allow for safety. Recently though, short term glycemic dysregulation in patients with type 1 diabetes has been shown not to modulate cardiac function(29) which is compatible with no significant rise in markers of oxidative stress with short term hyperglycemia.
Given the importance of achieving the goal of glycemic control and the various determinants of glycemic control, it is imperative that the insulin pump infusion be changed every 48 hours in patients treated with CSII.
Acknowledgments
Acknowledgements and Disclosure summary
Ajay Rao, MD, Haytham Kawji, MD, Lillian Yau, PhD, have nothing to disclose. This study was funded by Novonordisk through an unrestricted grant to Tulane University. This study was also supported in part by NIH Grants # 5M01RR05096 and RR-00827 in support of the General Clinical Research Center from the Division of Research Resources, National Institutes of Health (GCRC) and NIH/NIDDK P30 DK048520 (Mass Spectrometry Core). Diabetes research at Tulane University Health Sciences Center is supported in part by Susan Harling Robinson Fellowship in Diabetes Research and the Tullis-Tulane Alumni Chair in Diabetes. Dr. Fonseca is funded in part by NIH (ACCORD, TINSAL-2D), ADA and the Tullis-Tulane Chair of Diabetes. Dr. Vivian Fonseca has served as a consultant to Minimed, Novonordisk, and Eli Lilly. Dr Thethi and the project described was supported by Award Number K12HD043451 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health. We would like to express our gratitude to John Mastrototaro and colleagues for their help in managing the software and Dr. Soraly Severa for all the support. We would also like to acknowledge the support of Eric Button and colleagues of the Biomarker Group.
Footnotes
Clinical Trials Study No: NCT00461331
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group 1. N Engl J Med. 1993 Sep 30;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes 1. Diabetes Care. 2000 Sep;23(9):1232–5. doi: 10.2337/diacare.23.9.1232. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII 1. Diabetes Care. 2005 Mar;28(3):533–8. doi: 10.2337/diacare.28.3.533. [DOI] [PubMed] [Google Scholar]
- 4.Toxic-Shock Syndrome in a Patient Using a Continuous Subcutaneous Insulin Infusion Pump -- Idaho. MMWR. 1983 August;1232(31):404–406. [PubMed] [Google Scholar]
- 5.Mecklenburg RS, Benson EA, Benson JW, Jr, Fredlund PN, Guinn T, Metz RJ, et al. Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients 1. JAMA. 1984 Dec 21;252(23):3265–9. [PubMed] [Google Scholar]
- 6.Pietri A, Raskin P. Cutaneous complications of chronic continuous subcutaneous insulin infusion therapy 1. Diabetes Care. 1981 Nov;4(6):624–6. doi: 10.2337/diacare.4.6.624. [DOI] [PubMed] [Google Scholar]
- 7.Simmons BP, Hooton TM, Wong ES, Allen JR. Guidelines for prevention of intravascular infections. Infect Control. 1982;(3):61–72. [Google Scholar]
- 8.Senstius J, Poulsen C, Hvass A. Comparison of in vitro stability for insulin aspart and insulin glulisine during simulated use in insulin pumps 1. Diabetes Technol Ther. 2007 Dec;9(6):517–21. doi: 10.1089/dia.2007.0233. [DOI] [PubMed] [Google Scholar]
- 9.Haschke M, Zhang YL, Kahle C, Klawitter J, Korecka M, Shaw LM, et al. HPLC-atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t-isoprostane in human urine and plasma 1. Clin Chem. 2007 Mar;53(3):489–97. doi: 10.1373/clinchem.2006.078972. [DOI] [PubMed] [Google Scholar]
- 10.DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control 1. Diabetes Care. 2002 Nov;25(11):2074–80. doi: 10.2337/diacare.25.11.2074. [DOI] [PubMed] [Google Scholar]
- 11.Lenhard MJ, Reeves GD. Continuous subcutaneous insulin infusion: a comprehensive review of insulin pump therapy 1. Arch Intern Med. 2001 Oct 22;161(19):2293–300. doi: 10.1001/archinte.161.19.2293. [DOI] [PubMed] [Google Scholar]
- 12.Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002 Mar;25(3):593–8. doi: 10.2337/diacare.25.3.593. [DOI] [PubMed] [Google Scholar]
- 13.Tamborlane WV, Bonfig W, Boland E. Recent advances in treatment of youth with Type 1 diabetes: better care through technology 1. Diabet Med. 2001 Nov;18(11):864–70. doi: 10.1046/j.1464-5491.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosilio M, Cotton JB, Wieliczko MC, Gendrault B, Carel JC, Couvaras O, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. The French Pediatric Diabetes Group 1. Diabetes Care. 1998 Jul;21(7):1146–53. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 15.Hoogma RP, Schumicki D. Safety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetes 1. Horm Metab Res. 2006 Jun;38(6):429–33. doi: 10.1055/s-2006-944549. [DOI] [PubMed] [Google Scholar]
- 16.Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatis AK, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy 1. Pediatrics. 2004 Mar;113(3 Pt 1):e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 17.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes 1. JAMA. 2006 Apr 12;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 18.Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes 2. Antioxid Redox Signal. 2007 Jul;9(7):879–89. doi: 10.1089/ars.2007.1631. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds LR. Reemergence of insulin pump therapy in the 1990s 1. South Med J. 2000 Dec;93(12):1157–61. [PubMed] [Google Scholar]
- 20.Bischof F, Meyerhoff C, Pfeiffer EF. Quality control of intensified insulin therapy: HbA1 versus blood glucose 1. Horm Metab Res. 1994 Dec;26(12):574–8. doi: 10.1055/s-2007-1001762. [DOI] [PubMed] [Google Scholar]
- 21.Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion 1. Diabetologia. 1983 May;24(5):326–9. doi: 10.1007/BF00251817. [DOI] [PubMed] [Google Scholar]
- 22.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia 1. Arch Intern Med. 2003 Jun 9;163(11):1306–16. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- 23.Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability 1. Diabetes Metab Res Rev. 2006 May;22(3):232–7. doi: 10.1002/dmrr.614. [DOI] [PubMed] [Google Scholar]
- 24.Bonora E, Corrao G, Bagnardi V, Ceriello A, Comaschi M, Montanari P, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus 1. Diabetologia. 2006 May;49(5):846–54. doi: 10.1007/s00125-006-0203-x. [DOI] [PubMed] [Google Scholar]
- 25.van Deutekom AW, Niessen HW, Schalkwijk CG, Heine RJ, Simsek S. Increased Nepsilon-(carboxymethyl)-lysine levels in cerebral blood vessels of diabetic patients and in a (streptozotocin-treated) rat model of diabetes mellitus 1. Eur J Endocrinol. 2008 May;158(5):655–60. doi: 10.1530/EJE-08-0024. [DOI] [PubMed] [Google Scholar]
- 26.Miura J, Yamagishi S, Uchigata Y, Takeuchi M, Yamamoto H, Makita Z, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes 1. J Diabetes Complications. 2003 Jan;17(1):16–21. doi: 10.1016/s1056-8727(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 27.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo 1. Free Radic Biol Med. 2000 Feb 15;28(4):505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 28.Flores L, Rodela S, Abian J, Claria J, Esmatjes E. F2 isoprostane is already increased at the onset of type 1 diabetes mellitus: effect of glycemic control 1. Metabolism. 2004 Sep;53(9):1118–20. doi: 10.1016/j.metabol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Hammer S, Jonker JT, Lamb HJ, van der Meer RW, Zondag W, Sepers JM, et al. Short-term Hyperglycemic Dysregulation in Patients with Type 1 Diabetes Does Not Change Myocardial Triglyceride Content or Myocardial Function 1. Diabetes Care. 2008 May 5; doi: 10.2337/dc08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]