Abstract
Gastric bypass surgery (GBP), in addition to weight loss, results in dramatic remission of type 2 diabetes (T2DM). The mechanisms by which this remission occurs are unclear. Besides weight loss and caloric restriction, the changes in gut hormones that occur after GBP are increasingly gaining recognition as key players in glucose control. Incretins are gut peptides that stimulate insulin secretion postprandially; the levels of these hormones, particularly glucagon-like peptide-1, increase after GBP in response to nutrient stimulation. Whether these changes are causal to changes in glucose homeostasis remain to be determined. The purpose of this review is to assess the evidence on incretin changes and T2DM remission after GBP, and the possible mechanisms by which these changes occur. Our goals are to provide a thorough update on this field of research so that recommendations for future research and criteria for bariatric surgery can be evaluated.
Keywords: Incretin, GLP-1, GIP, Diabetes, Gastric bypass, Insulin, Weight loss
Introduction
The prevalence of type 2 diabetes (T2DM) has increased rapidly over the last several decades, both in the US and many developing countries. Obesity is a strong risk factor for T2DM, lending to the term “diabesity,” or obesity with accompanying T2DM. In the last several years, bariatric surgery has become an increasingly preferred option for weight loss and treatment of obesity-related metabolic complications in morbidly obese individuals. In 2007, 170,000 bariatric surgery operations were performed in the US, a >9-fold increase from 1999 [1, 2]. In the emergence of the diabesity epidemic, bariatric surgery not only results in significant and sustained weight loss but in many patients also puts T2DM in remission. Compared to conventional diet and pharmacological weight loss treatments, which induce a modest 10% weight loss of short duration and are often followed by weight regain [3], bariatric surgery results in 50% excess weight loss with control of T2DM in 76% of patients [4, 5]. Using bariatric surgery as a treatment for T2DM independently of morbid obesity, as suggested by clinical studies [6, 7], and recently publicized by the lay press [8], is an emerging concept; however, there is a need for more well-designed trials to clearly determine how bariatric surgery elicits these effects and, more importantly, the clinical applicability of these procedures as a treatment option for T2DM. The goal of this review is to evaluate the currently proposed mechanisms by which T2DM is controlled after gastric bypass surgery (GBP), principally in relation to the role of the incretins, based on the available evidence from clinical trials and experimental data from human and animal studies. Several reviews have addressed changes in gut hormones after bariatric surgery in relation to T2DM [9-12]; nevertheless, updates such as these may provide clinicians, researchers, and the public a comprehensive understanding of the current status of the bariatric surgery field. These assessments may eventually help develop less invasive or safer alternatives to surgery.
Background
Pathophysiology of T2DM: Role of Incretins
T2DM is characterized by defects in multiple organs, including decreased glucose uptake into skeletal muscle, pancreatic deficiency (illustrated by defects in β-cell function with impaired insulin secretion and defects in α-cell function with increased glucagon secretion), increased liver glucose output, and the reduced effect of incretins, gut hormones that stimulate insulin secretion after a meal [13, 14].
The two major gut hormones that have been identified as incretins are gastric inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1). GIP is secreted from the K cells located mainly in the duodenum, while GLP-1 is secreted from the L cells found mainly in the ileum [15, 16]. The incretins are rapidly secreted during a meal, circulate in the blood, and have a relatively short half-life (3–7 min), as they are rapidly inactivated by the enzyme dipeptidyl peptidase-IV (DPP-IV) [17, 18]. Incretin hormones increase insulin secretion in response to glucose [19, 20]. The incretin effect on insulin secretion was originally described by Creuzfeldt and Ebert [21] as the greater insulin response from an oral glucose load compared to that after an equivalent rise in blood glucose from an intravenous glucose load.
The effects of incretins on glucose homeostasis have been well reviewed [15, 22, 23]. The main function of incretins is to stimulate glucose-dependent insulin secretion. Both in vivo and in vitro studies showed that GLP-1 in pancreatic β-cells stimulates insulin biosynthesis [24, 25]. In addition to its insulinotropic effects, GLP-1 exerts its glucose-lowering effects through inhibition of gastric emptying [26-29], restoration of insulin sensitivity [26], and inhibition of glucagon secretion [29, 30], which may result in the decrease of hepatic glucose production [31, 32]. GLP-1 agonists also have been shown to increase β-cell mass and pancreas islet size in rodents [20, 33]. GLP-1 receptors have been identified on β-cells of both rats and humans [34, 35], and studies have demonstrated that disruption of the GLP-1 receptor results in enhanced apoptosis of β-cells [36].
In patients with T2DM, the incretin effect is diminished [37]. Plasma measurements (fasting and postprandial) of GIP are normal compared to patients without T2DM, but administration of exogenous GIP does not increase insulin secretion [38], suggesting GIP resistance in T2DM, although the GIP response is restored if glycemia is normalized [39]. GLP-1 levels are generally [40] but not always [41] found to be lower in T2DM. In contrast to GIP administration, patients with T2DM respond to exogenous GLP-1 [42].
Clinical Use of Incretins in T2DM
Most patients with T2DM require a combination of oral antidiabetic agents, followed eventually by insulin treatment. Weight gain and fear of hypoglycemia are often barriers to treatment compliance and glycemic control. Incretin mimetics that are resistant to the effect of DPP-IV as well as DPP-IV inhibitors have recently been developed and are currently in use as treatment for T2DM (as reviewed in [43]). Similar to conventional oral hypoglycemic agents and insulin therapy, incretin mimetics and DPP-IV inhibitors significantly lower hemoglobin A1C (HbA1C) and postprandial glucose excursions in patients with T2DM and often without the added weight gain. Nevertheless, the durability of these effects and their potential long-term benefits are still largely unknown.
Bariatric Surgeries
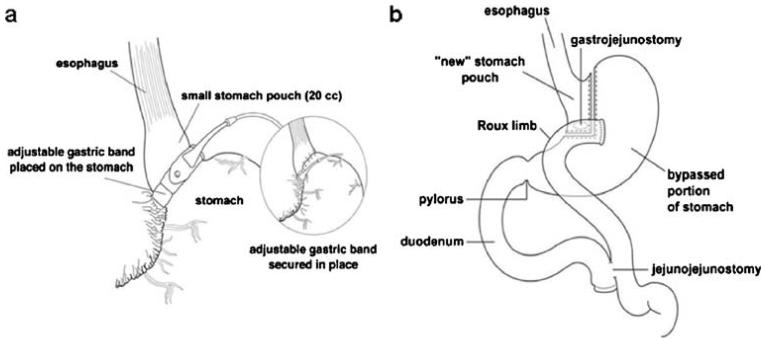
There are three main categories of bariatric surgery: restrictive, malabsorptive, and a combination of the two. Restrictive bariatric surgeries are based on a reduction of the stomach size to increase satiety and subsequently reduce food intake. Vertical banded gastroplasty (VBG) staples the stomach vertically using a synthetic material sutured around the stomach [44]. Laparoscopic adjustable gastric banding (GB), currently the most common restrictive bariatric procedure, consists of a constricting silicone band that is 10–12 mm in diameter, inserted laparoscopically. This band contains a volume-adjustable compartment on its inner surface connected to a subcutaneous port device. The size of the band is controlled by saline infusion to this port [9, 45]. The size of the proximal gastric pouch is about 20–30 ml (Fig. 1a). GB results in about 40.7–54.2% excess weight loss [4].
Fig. 1.
Schematic representation of gastric banding (a) and gastric bypass (b). Graphics courtesy of Packard Children's Hospital, Palo Alto, CA, USA
Malabsorptive surgeries are based on the principle of bypassing certain portions of the intestine so that food is not absorbed. Strictly, malabsorptive procedures include jejunoileal bypass (JIB) and biliopancreatic diversion (BPD); however, these surgeries currently are not frequently performed due to several undesirable side effects, such as severe macronutrient and vitamin deficiencies. The most commonly performed bariatric procedure in the US is Rouxen-Y GBP [1], which is a combination malabsorptive–restrictive surgery. GBP surgery entails division of the stomach into a small proximal pouch that holds about 20–30 ml and a larger distal portion that is bypassed. The small pouch is then anastomosed to the distal part of the ileum (alimentary limb). The remaining larger portion of the stomach, the duodenum, and the jejunum are reattached to the distal part of the ileum (below the gastroileal anastomosis) to allow for excretion of gastrointestinal and pancreatic juices (biliopancreatic limb; Fig. 1b) [9, 45, 46]. GBP results in about 56.7–66.5% excess weight loss [4].
Effect of Weight Loss on T2DM Control
Diet-Induced Weight Loss
T2DM is often associated with overweight and/or obesity and two thirds of patients with T2DM have a body mass index (BMI) of 27 kg/m2 or greater [47]. Although patients with T2DM often require a combination of medications, oral and insulin; the cornerstone of treatment is weight loss. There are many short-term studies showing improvement of T2DM control by diet or diet and exercise, with or without pharmacological treatment [48-52]. Both caloric restriction and the weight loss itself account for the major ameliorating effects of dietary intervention on T2DM [53, 54]. However, diet-induced weight loss is often of short duration and is usually followed by weight regain [3].
Surgical Weight Loss: Clinical Outcome in T2DM
In contrast, bariatric surgery results in weight loss of great magnitude (up to 33% weight reduction) sustained over time [55]. In addition to its substantial weight loss effect, bariatric surgery has been shown to result in T2DM remission. A meta-analysis in 2004 reported that GBP resulted in remission of T2DM in 83.7% of its cases, while GB produced T2DM remission in 47.9% of patients [4]. Many studies report decreases in fasting glucose, insulin, hemoglobin A1c (HbA1c), markers of insulin resistance, and medication usage as a result of surgery (Table 1). Although the spectacular effect of malabsorptive procedures such as GBP or BPD on T2DM remission is well known, some studies have also reported positive results on the effects of GB on remission of T2DM [56, 57]. A recent randomized clinical trial showed dramatic effects of GB, reporting 73% T2DM remission (defined as HbA1c levels <6.2%, fasting glucose <126 mg/dl, and cessation of T2DM medication usage) at 2 years [5]. These remarkable results on T2DM remission after surgical weight loss may have a broad clinical impact, on both the quality of life for patients, who often are required to take multiple medications for T2DM and related conditions, and also on the cost of health care.
Table 1.
Resolution of T2DM after bariatric surgery
| Reference | Surgery type | Follow-up duration | Diabetes outcome |
|---|---|---|---|
| Pories et al. [137] | Greenville gastric bypass (n=101 with T2DM) | 1–10 years | ↓ FBG, insulin, HbA1c, ↑ insulin release, glucose disappearance by 1 year |
| Poulos et al. [138] | GBP (n=29 with T2DM) | >1 year | ↓ FBG, insulin, HbA1c |
| Sjostrom et al. [55] | GBP, GB, or VBG (n=195 with diabetes) | 6–24 months | ↓ Incidence of diabetes as defined by ↓ FBG and no medication usage |
| Pontirolli et al. [57] | GB (n=46 with T2DM) | 1–3 years | ↓ FBG, insulin, HbA1c, HOMA-IR, insulin resistance, glucose tolerance by 1 year |
| Schauer et al. [139] | GBP (n=191 with T2DM) | 20 months | ↓ FBG, HbA1c, diabetes medication usage by 6 months |
| Polyzogopoulou et al. [85] | BPD-GBP (n=12 with T2DM) | 3–12 months | ↓ FBG, fasting insulin, ↑insulin sensitivity, AIR by 3 months |
| Diniz et al. [140] | GBP (n=31 with T2DM) | 27 months | ↓ FBG, HbA1c |
| Clements et al. [63] | GBP (n=20 with T2DM) | 2–12 weeks | ↓ FBG by 2 weeks |
| Rubino et al. [61] | GBP (n=6 with T2DM) | 3 weeks | ↓ FBG, insulin |
| Wickremesekera et al. [86] | GBP (n=31 with T2DM) | 6 days to 12 months | ↓ FBG, ↓ HOMA-IR by 6 days |
| Guidone et al. [66] | BPD (n=10 with T2DM) | 4 weeks | ↓ FBG, insulin, ↑glucose tolerance, insulin sensitivity, β-cell glucose sensitivity |
| Mari et al. [87] | BPD (n=11 with T2DM) | 5 months | ↑ glucose tolerance, insulin secretion, insulin sensitivity, β-cell glucose sensitivity |
| Morinigo et al. [68] | GBP (n=11 with T2DM) | 6–12 months | ↓ FBG, HbA1c, ↑ HOMA-IR,and insulin sensitivity |
| Alexandrides et al. [141] | GBP (n=26 with T2DM) | 27 months | ↓ FBG |
| Alexandrides et al. [141] | BPD-RYGBP (n=111 with T2DM) | 2 years | ↓ FBG |
| DePaula et al. [6] | Ileal interposition with sleeve gastrectomy (n=23 with T2DM) | 7 months | ↓ FBG, fasting insulin, HOMA-IR, HbA1c, ↑ glucose tolerance |
| DePaula et al. [6] | Ileal interposition with diverted sleeve gastrectomy (n=16 with T2DM) | 7 months | ↓ FBG, fasting insulin, HOMA-IR, HbA1c, ↑ glucose tolerance |
| Briatore et al. [96] | BPD (n=9 with T2DM) | 1 month | ↓ FBG, HOMA-IR, ↑AIR |
| Dixon et al. [5] | GB (n=30 with T2DM) | 2 years | ↓ FBG, insulin, HOMA-IR, HbA1c |
| Brancatisano et al. [56] | GB (n=78 with T2DM) | 1 year | ↓ FBG, HbA1c, diabetes medication usage |
T2DM type 2 diabetes mellitus, FBG fasting blood glucose, HbA1c hemoglobin A1c, GBP gastric bypass, GB gastric banding, VBG vertical banded gastroplasty, HOMA-IR homeostasis model assessment of insulin resistance, BPD biliopancreatic diversion, AIR acute insulin response
Potential Mechanisms for T2DM Remission after GBP
Changes in Incretins
Many studies have examined changes in gastrointestinal hormones, including incretins, after surgery, in relation to both glucose homeostasis and satiety effects. The major longitudinal studies that have studied incretin levels after bariatric surgery are summarized in Table 2. Early studies showed increased fasting and postprandial enteroglucagon (a previously used marker for GLP-1) levels after both GBP and JIB [58, 59]. In 1998, a cross-sectional study by Naslund et al. [60] reported dramatic increases in GIP and GLP-1 levels in JIB patients 20 years postoperatively compared to nonoperated obese and lean control patients.
Table 2.
Longitudinal studies on the effects of bariatric surgery on incretin levels
| Reference | Population | Surgery type | Follow-up duration | Outcome |
|---|---|---|---|---|
| Barry et al. [58] | >300 lb, n=12 | JIB | 3–6 weeks | ↑ Fasting EG by 3 weeks ↑ Postprandial EG by 6 weeks |
| Jorde et al. [77] | n=5 | JIB | 2–6 weeks | ↓ Postprandial GIP |
| Schrumpf et al. [142] | n=9 | GBP | 3–12 months | No change in GIP levels at 3 or 12 months |
| Sirinek et al. [76] | n=12 | GBP | 3–4 months | ↓ Fasting, postprandial GIP |
| Kellum et al. [59] | n=9 | GBP | 11 months | ↑ Postprandial EG |
| Kellum et al. [59] | n=7 | VBG | 11 months | No effect on fasting, postprandial EG |
| Rubino et al. [61] | n=9, 6 with T2DM | GBP | 3 weeks | ↓ Fasting GIP in T2DM patients only No change in fasting GLP-1 |
| Clements et al. [63] | n=20 with T2DM | GBP | 2–12 weeks | ↓ Fasting GIP by 6 weeks No significant effect in fasting GLP-1 |
| Valverde et al. [65] | n=19 | BPD | 1–6 months | ↑ Fasting, postprandial GLP-1by 6 months |
| Valverde et al. [65] | n=12 | VBG | 1–6 months | No effect on fasting, postprandial GLP-1 |
| Guidone et al. [66] | n=10 with T2DM | BPD | 1–4 weeks | ↓ Fasting, postprandial GIP by 1 week ↑ Fasting, postprandial GLP-1 by 1 week |
| Morinigo et al. [69] | n=9 | GBP | 6 weeks | ↑ Postprandial GLP-1 |
| Morinigo et al. [68] | n=34 | GBP | 6 weeks, 12 months | ↑ Postprandial GLP-1 by 6 weeks |
| Borg et al. [70] | IR, n=6 | GBP | 1–6 months | ↑ Postprandial EG, GLP-1 by 6 months |
| le Roux et al. [71] | n=16 | GBP | 2–42 days | ↑ Postprandial GLP-1 by 2 days |
| Laferrere et al. [64] | n=8 with T2DM | GBP | 1 month | ↑ Postprandial GLP-1, GIP ↑ IE |
| Reinehr et al. [67] | n=19 | GBP | 2 years | ↓ Fasting GLP-1 |
| Whitson et al. [143] | n=10, 5 with T2DM | GBP | 6 months | ↑ Nonfasted GLP-1 in T2DM only |
| Laferrere et al. [62] | n=9 with T2DM | GBP | 1 month | ↑ Postprandial GLP-1, GIP ↑ IE |
| Shak et al. [75] | n=24 | GB | 6–12 months | No change in GLP-1, GIP |
JIB jejunoileal bypass, EG enteroglucagon, GIP gastric inhibitory peptide, VBG vertical banded gastroplasty, T2DM type 2 diabetes mellitus, GBP gastric bypass, GLP-1 glucagon-like peptide-1, BPD biliopancreatic diversion, IR insulin resistant, IE incretin effect, GB gastric banding
GLP-1
The changes in GLP-1 levels after bariatric surgery have been extensively studied in the last 10 years. Several studies showed either no change [61-64] or an increase [65, 66] in fasting GLP-1 levels after malabsorptive surgeries, although one longitudinal study recently reported a decrease in fasting GLP-1 levels in obese patients 2 years after GBP [67].
The postprandial GLP-1 response after bariatric surgery, in contrast, has been more consistent, with all studies reporting unanimously an increase of GLP-1 levels during an oral glucose tolerance test (OGTT) or mixed test meal after GBP or BPD in obese subjects [65, 68-70], as well as in patients with T2DM after BPD [66] or GBP [62, 64]. The GLP-1 increase occurs as early as 2 days after GBP [71] and persists at 6 months and 1 year [72]. Purely restrictive procedures do not result in an increase of GLP-1 [65, 73-75].
GIP
Fewer studies have reported the effects of bariatric surgery on GIP levels. Additionally, the results have not been as consistent as those reported for GLP-1. Many studies have observed a reduction [66, 76] or no change [62, 64] in fasting GIP levels after BPD or GBP. No studies to date have reported and increase in fasting GIP after surgery. Stimulated GIP levels decreased after a test meal in obese patients 2 weeks after JIB [77] or after GBP and BPD [66, 76]. In our own study in patients with T2DM, GIP levels increased during an OGTT 1 month after GBP [62, 64], an effect that did not persist over time [72]. This is in agreement with several cross-sectional studies reporting increased postprandial GIP levels after GBP or JIB [60, 77, 78], 6 months to 20 years after surgery. Overall, the variability in GIP levels in these studies may be due to the time after surgery, T2DM status and the overall metabolic control of these patients. Nevertheless, despite this variability, the literature suggests that these effects are distinct from those of purely restrictive surgeries, as studies have reported no effect on GIP after VBG or GB [65, 73, 75].
In addition to the increase of meal- or glucose-stimulated GLP-1 and GIP levels occurring after bypass procedures, we have shown that the incretin effect on insulin secretion, impaired in patients with T2DM, returns to the level of controls 1 month after the surgery [64]. The normalization of the incretin effect, in patients with recently diagnosed T2DM (less than 5 years) persists at 1 year after surgery [72].
Other Changes in Glucose Homeostasis after Bariatric Surgery: Hepatic Glucose Production
There are very few studies that have reported the effect of bariatric surgery on hepatic glucose production (HGP). One recent cross-sectional study observed earlier suppression of HGP during an oral glucose load (as measured by glucose appearance using isotope-labeled glucose tracers) in GBP patients 1–4 years after surgery [74]. Another prospective study using similar methods reported decreased endogenous glucose production 1 year after GBP [79].
Patients with T2DM have hyperglucagonemia which improves with diet-induced weight loss [80, 81]. Additionally, glucagon levels are suppressed after administration with a GLP-1 analog [82]. With weight loss and the increase of GLP-1 observed after GBP, a decrease of glucagon levels following GBP surgery is expected. This has been shown in one cross-sectional study, where GBP patients exhibited decreased glucagon levels 180 min after a test meal compared to nonoperated BMI-matched control subjects [73]. In contrast, we [62] and others [78] have observed an increase of glucagon levels after GBP. The reasons for this are unclear.
Other Changes in Glucose Homeostasis after Bariatric Surgery: Insulin Resistance and Secretion
One major characteristic of T2DM is insulin resistance, normally manifesting in a reduced insulin-mediated glucose uptake. Many studies have shown a decrease of insulin resistance after bariatric surgery, determined either by homeostasis model assessment for insulin resistance (HOMA-IR) [83, 84], quantitative insulin sensitivity check index [85], intravenous glucose tolerance test (IVGTT) [68, 86], or by the gold standard measurement of insulin sensitivity, the euglycemic–hyperinsulinemic clamp [66, 87, 88]. This effect seems to occur rapidly after surgery and often prior to substantial weight loss [85-87]. In longer studies, however, insulin sensitivity appears to be related to the degree of weight loss [57, 89]. Recent comparisons of GBP and GB have shown no significant difference on insulin resistance between the two interventions [83, 84], suggesting that caloric restriction and weight loss following surgery, rather than the nature of the procedure, is a major factor in the improvement of insulin sensitivity after bariatric surgery. However, GLP-1 [26, 29] may improve insulin sensitivity, but its role and that of other gut peptides such as peptide YY (PYY) [90, 91] and ghrelin [92] in increasing insulin sensitivity, above and beyond caloric restriction and weight loss, require further study.
T2DM is also characterized by a defect in early-phase insulin secretion after oral stimulus and/or first-phase insulin secretion during IV glucose challenge [93]. The biphasic insulin response to a rapid IV glucose challenge is abnormal in T2DM; the first-phase insulin secretion, seen over the first 10 min, is absent [94].
Commonly used tools to assess insulin secretion in patients include IVGTT and insulin response to glucose. Weight loss by dietary restriction improves insulin secretion, possibly by decreasing glucose and free fatty acid toxicity to the β-cell [50, 95]. Surgical weight loss has been shown to restore first-phase insulin secretion in obese subjects with T2DM [85, 96] as measured by IVGTT. This is also demonstrated in patients with impaired glucose tolerance, as shown by frequently sampled intravenous glucose tolerance test (FSIVGTT) [97] or insulin response to arginine [98]. Infusion of the GLP-1 or a GLP-1 analog increased first-phase insulin secretion in patients with T2DM [99] and in normoglycemic subjects [100]; the increase of postprandial incretins after malabsorptive surgeries may participate in the improvement of insulin secretion, although this has not been extensively studied. Nevertheless, the insulin secretory capacity of patients with T2DM is highly variable [101, 102]. Accordingly, the effects of weight loss on insulin secretion will be mainly dependent upon the β-cell capacity and the degree of hyperglycemia [103].
Mechanisms of Increased Incretins After GBP
There are several proposed mechanisms for the increase in incretin levels after GBP, although to date none have been clearly established, and the results of many studies often conflict with one another.
Weight Loss
In 2001, Verdich et al. [104] reported a 9.2% increase in meal-stimulated GLP-1 area under the curve (AUC) over 3 h after a 6-month dietary weight loss program (~20-kg weight loss) in a group of obese nondiabetic patients. However, we recently showed that GLP-1 levels during an OGTT did not change significantly after a 10-kg diet-induced weight loss in obese subjects with T2DM [62]. The reasons for these discrepancies could be attributable to the difference in weight loss amount and/or T2DM status. Additionally, we found that an equivalent weight loss 1 month after GBP increased GLP-1 AUC during a 3-h OGTT by >300% [62]. These data suggest that it is unlikely that weight loss contributes to increased incretin levels; the surgical nature of GBP appears to play a much greater role in this increase than weight loss per se.
Gut Exposure to Nutrients
Rapid Hindgut Delivery Hypothesis
One proposed hypothesis regarding the mechanisms of increased incretins following GBP is the rapid exposure of the lower small intestine to nutrients. Several groups have examined this hypothesis. In 2005, Strader et al. [105] reported the effects of ileal interposition (IT) in high-fat-fed obese nondiabetic Long-Evans rats. This procedure, where a segment of the ileum is relocated to the proximal small intestine, was able to specifically assess the effect of rapid ileal exposure without any other gastric restriction or intestinal rerouting. IT increased plasma GLP-1 levels during an OGTT 3 weeks after surgery compared to sham-operated rats. However, the surgery did not affect glucose or insulin levels, which were difficult to assess in these nondiabetic rats with essentially normal glucose levels. A study in a model of nonobese diabetic Goto-Kakizaki (GK) rats showed that glucose tolerance improved during an OGTT 30 days after IT [106]. Plasma GLP-1 levels during the first 15 min of the OGTT, measured 45 days after IT, were significantly increased compared to sham-operated controls. The same group later demonstrated an improvement in glucose tolerance with a decreased glucose AUC during an OGTT in GK rats 45 days after IT compared to sham-operated or nonoperated rats [107]. The authors also reported increased insulin levels during the OGTT with increased insulin sensitivity 5 months after IT compared to control groups. There was no effect of IT on glucose-stimulated GLP-1 levels (as measured by GLP-1 AUC) by 6 months postsurgery, although IT rats exhibited a prolonged GLP-1 response during the OGTT and increased proglucagon mRNA expression in the ileum compared to those of the sham-operated or nonoperated controls.
Recently, one group examined the clinical effects of IT in remission of T2DM [6]. In this study, IT to the proximal jejunum followed by a sleeve gastrectomy was performed on 39 T2DM patients with a presurgery BMI of 30.1 kg/m2. Seven months after surgery, patients lost an average of 22% of their presurgery body weight (postsurgery BMI 24.9 kg/m2) and showed significantly decreased HbA1c (by 28%), fasting (by 44.6%) and postprandial glucose (by 45.3%) levels, and HOMA-IR (by 50%). Incretin levels were not reported in this study.
Foregut Exclusion Hypothesis
A second proposed hypothesis regarding the mechanisms of increased incretin levels after GBP is the exclusion of the foregut from nutrient exposure. This concept was first proposed when Hickey et al. [108] observed in a cross-sectional study that glucose tolerance and insulin sensitivity in a group of patients who had undergone GBP were significantly improved compared to a group of weight-matched (postsurgery) controls. In 2004, Rubino and Marescaux [109] reported the effects of a gastrojejunal bypass (GJB) procedure on glucose homeostasis in diabetic GK rats. Fasting glucose was significantly decreased over the 32-week period following GJB, and glucose tolerance was improved (AUC during a 3-h OGTT) 1 week after GJB compared to sham-operated controls. Insulin sensitivity was also improved (as measured by glucose disappearance during an insulin tolerance test) 20 weeks after surgery in GJB rats compared to controls. In addition, there was an increase in fasting GIP levels in GJB rats 2 weeks postsurgery compared to preoperative levels [109]. These observations were made in the absence of any significant difference in body-weight change or food intake between the GJB and the sham-operated controls.
In an effort to distinguish foregut exclusion from rapid hindgut exposure in the T2DM-related effects after GBP, Rubino et al. [110] designed a study where GK rats received either a duodenal-jejunal bypass surgery (DJB) or a gastrojejunostomy (GJ) to allow for rapid ileal exposure to nutrients without foregut bypass. DJB rats showed improved oral glucose tolerance (as measured by decreased glucose AUC during an OGTT) compared to both GJ and sham-operated control rats 10 days after surgery. In addition, a subsequent reoperation where the GJ was converted to a DJB significantly improved glucose tolerance compared to before the conversion procedure. In contrast to the effects observed in GK diabetic rats, DJB performed on normoglycemic Wistar rats resulted in a significant decrease in glucose tolerance compared to sham-operated control Wistar rats. The authors concluded that there may be a factor present in the proximal intestine that contributes to the T2DM phenotype, and bypass of this portion of the small intestine may ameliorate T2DM. However, this procedure may disrupt glucose homeostasis in nondiabetic conditions. Incretin levels, incidentally, were not reported in this study. A recent study compared the effects of IT and DJB on glucose, insulin, and GLP-1 levels in GK rats [111]. This study reported similar effects between DJB and IT on improved tolerance (as measured by glucose AUC during an OGTT) 4 weeks postsurgery. Mean GLP-1 levels 30 min after an oral glucose load were lower in the DJB group compared to the IT group by 1 week after surgery, but both surgery groups exhibited higher GLP-1 levels compared to sham-operated controls by 4 weeks after surgery. These results suggest that both foregut exclusion and rapid hindgut exposure equally improve glucose tolerance; both principles may be key players in T2DM remission after GBP. However, these effects on glucose tolerance may be mediated through different mechanisms. Rapid hindgut exposure to nutrients may be related to increased GLP-1 levels after surgery, while duodenal exclusion may mediate some of its effects independent of changes in GLP-1.
Still, others report no effect of foregut exclusion or rapid ileal exposure to nutrients during GBP on changes in incretin levels, despite the improvement in glucose tolerance. A study by Pacheco et al. [112] also found that duodenal-jejunal exclusion in GK rats, similar to the procedure described in Rubino et al. [61], decreased fasting glucose and glucose levels during an OGTT by 1 week after surgery compared to nonoperated control GK rats. There was no effect of the procedure on glucose-stimulated GLP-1 or GIP levels compared to controls; however, there was a significant decrease in glucagon and leptin levels following the glucose load 1 week after surgery. The authors suggested that the rapid improvement of glucose homeostasis observed by duodenal-jejunal bypass might be mediated by the decrease in leptin levels, which may stimulate insulin secretion, although no changes in glucose-stimulated insulin levels after surgery was observed.
Gut motility–Gastric Emptying
The gastrointestinal tract is increasingly regarded as an important organ in glucose homeostasis, and both gut motility and gastric emptying may play an important role in postprandial glucose control [13]. Few groups, however, have reported the effect of GBP on gut motility and gastric emptying, and results have varied among studies. Horowitz et al. [113] reported in a 1986 cross-sectional study that gastric emptying of solids was slower in GBP patients compared to nonoperated control patients; however, gastric emptying of liquids was faster in GBP patients. Naslund and Beckman [114] later reported in a longitudinal study reduced pouch emptying at 2 months after GBP. Similarly, a small number of studies are available on gut motility after bariatric surgery. Kotler et al. [115] showed increased intestinal transit time and increased enteroglucagon levels after various types of gastric surgery in weight-losing subjects compared to postsurgery weight-stable subjects. A recent study demonstrated accelerated gastric emptying and shortened intestinal transit time in morbidly obese subjects 6 weeks after GBP. This was accompanied by an increased postprandial GLP-1 response, which was significantly correlated with the gastric-emptying response after surgery [69].
Other Proposed Mechanisms of T2DM Resolution After GBP (Ghrelin, Peptide YY, Leptin)
In addition to incretins, there are also a number of other hormones that are altered after GBP which may be integral in the maintenance of glucose homeostasis.
Ghrelin is a hormone produced by the stomach and may play a role in short- and long-term energy balance. Administration of ghrelin or its analogs stimulates food intake [116, 117], and ghrelin levels vary as a function of BMI and weight change. Obese individuals have lower circulating ghrelin levels. Weight loss by diet increases ghrelin levels, increasing food intake [118, 119]. In contrast, ghrelin levels do not rise after GBP in spite of considerable weight loss [118, 120-123], which may influence caloric intake and subsequently glucose homeostasis. Additionally, recent studies have proposed other roles for ghrelin related to T2DM. Ghrelin has been shown to inhibit insulin secretion in humans [124, 125], and a recent study demonstrated that genetic knockout of ghrelin in lean mice reduced fasting glucose levels and endogenous glucose production and increased glucose-stimulated insulin levels compared to wild-type mice [92]. In diabetic ob/ob mice, ghrelin deletion reduced fasting glucose and fasting insulin and improved glucose tolerance [92].
PYY is cosecreted with GLP-1 from intestinal L cells in response to food intake. PYY3-36 has been shown to decrease food intake in humans [126] and regulates body weight in rodents [127]. Cross-sectional [74, 128] and longitudinal [69, 129] studies have reported increased PYY levels after GBP, which may partially explain the reduced caloric intake and improved glucose homeostasis after surgery. A study in our laboratory recently found increased PYY3-36 levels 1 month after GBP; this effect was not observed after a matched weight loss achieved with dietary restriction (Oliván et al. in review). Similar to ghrelin, there are recent studies that have suggested more direct effects of PYY on insulin sensitivity [90]; however, the role of PYY independent of food intake still needs to be confirmed.
Leptin, which is secreted from the adipose tissue, is also involved with food intake and long-term energy regulation [130, 131]. However, in contrast to ghrelin, leptin levels are generally higher in obese individuals. Several studies showed a reduction of leptin levels after GBP [123, 128, 132], some of which indicated this reduction was correlative with weight or BMI [128, 132]. Some studies, however, have shown reduction in leptin levels which were not correlated with weight and fat mass loss [108, 133], suggesting an increase in leptin sensitivity after GBP; this may play a role in glucose homeostasis. A recent study in GK rats showed decreased leptin levels 1 week after duodenal exclusion surgery compared to nonoperated GK rats [112].
These hormones may act in concert with the incretins, or one hormone may potentiate the action of another, although the currently available data implicating these hormones in T2DM remission after surgery are minimal and controversial. There is a need for more carefully designed clinical trials, beyond descriptive studies, that will confirm these hormonal changes and determine whether or not they play a role in T2DM-related changes after GBP.
Conclusions
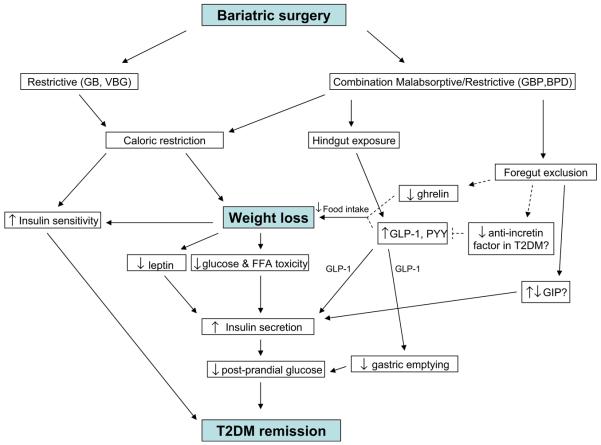
It is well-documented that T2DM remission occurs after bariatric surgery. First and foremost, the effect of caloric restriction and subsequent weight loss clearly plays an important role in this remission. This is evident by the restoration of glucose tolerance, decreased HbA1c levels, and improvement of insulin sensitivity by all categories of bariatric surgery. Alterations in the small intestinal anatomy after GBP may also be integral in T2DM control. Foregut exclusion or rapid delivery of food to the hindgut may be responsible for increased incretin levels, which can promote insulin secretion and also possibly increase insulin sensitivity. GLP-1 appears to be consistently increased after GBP, and these changes are often related to improvement of glucose homeostasis. The role of GIP and how it changes after GBP are less clear and require further research. There also may be changes in ghrelin, PYY, and gastric emptying that occur as a result gastrointestinal tract rerouting after GBP, although these findings in relation to T2DM remission necessitate more investigation. Figure 2 shows a schematic of the potential mechanisms by which T2DM is resolved after bariatric surgery, based on the existing literature.
Fig. 2.
Proposed model for mechanisms of T2DM remission after GBP based on available studies. Dashed lines indicate hypothetical links
Future Directions
Despite the research available, there are significant limitations in the previous studies. A large number of the studies that have examined hormonal changes in relation to glucose homeostasis after surgery have been conducted in nondiabetic subjects. The role of T2DM status and duration needs to be addressed in terms of the effectiveness of bariatric surgery. Long-term studies are needed to test not only the impact of T2DM remission on cardiovascular outcome but also the metabolic consequences of elevated incretin levels on hypoglycemia after GBP surgery.
Mechanistic studies to elucidate the role of caloric restriction versus weight loss, the role of the vagus nerve on gut peptide release, and the duodenal exclusion versus ileal exposure to nutrients are necessary. The mechanisms by which appetite is reduced after GBP or with GB will also need to be studied, as decreased calorie intake is a major component of weight loss. Finally, although most patients benefit from bariatric surgery with sustained weight loss, some patients regain the weight lost initially after surgery [134]. Understanding the predictors and the mechanisms of failure after bariatric surgery will be an important factor in better patient selection for bariatric surgery.
The current recommendations for bariatric surgery in the US are BMI≥35 kg/m2 with comorbidities or BMI≥40 kg/m2 without comorbidities. In view of the multiple benefits of the surgery and the recent report of increased longevity after bariatric surgery [135], these criteria may need to be revised and surgery be offered at lower BMI. Nevertheless, it is premature to advocate experimental bariatric procedures to nonobese patients with T2DM. Recent developments in experimental bariatric surgery such as ileal interposition [6], endoluminal [136], and minigastric bypass surgery [7] will require more careful research trials before becoming clinically applicable on a larger scale. It is imperative to clearly understand how these treatments are improving metabolic conditions such as T2DM.
Acknowledgements
The authors would like to thank Ms. Baani Bawa, Ms. Adrienne O'Reilly, and Ms. Caroline Poryles for their assistance in manuscript preparation. This work was funded by the National Institute of Health training grant #DK07559.
Abbreviations
- T2DM
type 2 diabetes mellitusy
- GBP
gastric bypass surgery
- GIP
gastric inhibitory peptide-1
- GLP-1
glucagon-like peptide-1
- DPP-IV
dipeptidyl peptidase-IV
- HbA1C
hemoglobin A1C
- VBG
vertical banded gastroplasty
- GB
gastric banding
- JIB
jejunoileal bypass
- BPD
biliopancreatic diversion
- BMI
body mass index
- OGTT
oral glucose tolerance test
- HGP
hepatic glucose production
- HOMA-IR
homeostasis model assessment for insulin resistance
- QUICKI
quantitative insulin sensitivity check index
- IVGTT
intravenous glucose tolerance test
- PYY
peptide YY
- IGT
impaired glucose tolerance
- FSIVGTT
frequently sampled intravenous glucose tolerance test
- AUC
area under the curve
- IT
ileal interposition
- GK
Goto-Kakizaki
- GJB
gastrojejunal bypass
- DJB
duodenal-jejunal bypass
- GJ
gastrojejunostomy
Footnotes
Author Disclosures M. Bose, B. Oliván, J. Teixeira, F.X. Pi-Sunyer, and B. Laferrère have no conflicts of interest.
References
- 1.Surgery ASMBS . Press release 8/22/2007: metabolic surgery expected to play bigger role in treating type 2 diabetes and other metabolic diseases. Gainesville, FL: 2007. [updated 2007; cited]; Available from: http://www.asbs.org/Newsite07/resources/press_release_8202007.pdf (2007) [Google Scholar]
- 2.Zhao Y, Encinosa W, Quality AfHRa . Bariatric surgery utilization and outcomes in 1998 and 2004: statistical brief #23. Agency for Healthcare Research and Quality; Rockville: 2007. [updated 2007; cited 2008 May 9]; Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb23.pdf. [PubMed] [Google Scholar]
- 3.Anonymous Methods for voluntary weight loss and control. Ann Intern Med; NIH Technology Assessment Conference Panel. Consensus Development Conference; 30 March to 1 April 1992; 1993. pp. 764–70. [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 6.DePaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008;22:706–16. doi: 10.1007/s00464-007-9472-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic minigastric bypass for type 2 diabetes mellitus: comparison of BMI >35 and <35 kg/m(2) J Gastrointest Surg. 2008;12:945–52. doi: 10.1007/s11605-007-0319-4. [DOI] [PubMed] [Google Scholar]
- 8.Stein R. Surgery shows promise for treatment of diabetes. Washington Post: May 4, 2008. 2008;Sect. 2. [Google Scholar]
- 9.Fetner R, McGinty J, Russell C, et al. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis. 2005;1:589–97. doi: 10.1016/j.soard.2005.09.001. discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 10.Greenway SE, Greenway FL, 3rd, Klein S. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg. 2002;137:1109–17. doi: 10.1001/archsurg.137.10.1109. [DOI] [PubMed] [Google Scholar]
- 11.Patriti A, Facchiano E, Sanna A, et al. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14:840–8. doi: 10.1381/0960892041590818. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Overduin J, Shannon MH, et al. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1:358–68. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 13.Maggs D, MacDonald I, Nauck MA. Glucose homeostasis and the gastrointestinal tract: insights into the treatment of diabetes. Diabetes Obes Metab. 2008;10:18–33. doi: 10.1111/j.1463-1326.2007.00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Quinn L. Mechanisms in the development of type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:1–16. doi: 10.1097/00005082-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 16.Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–4. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 17.Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res. 2004;36:761–5. doi: 10.1055/s-2004-826160. [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–40. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 19.Kjems LL, Holst JJ, Volund A, et al. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–6. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 20.Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeo-domain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–8. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 21.Creutzfeldt W, Ebert R. New developments in the incretin concept. Diabetologia. 1985;28:565–73. doi: 10.1007/BF00281990. [DOI] [PubMed] [Google Scholar]
- 22.D'Alessio DA, Vahl TP. Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab. 2004;286:E882–890. doi: 10.1152/ajpendo.00014.2004. [DOI] [PubMed] [Google Scholar]
- 23.Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–66. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 24.Buteau J, Roduit R, Susini S, et al. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia. 1999;42:856–64. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 25.Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–66. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 26.Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–30. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 27.Naslund E, Gutniak M, Skogar S, et al. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–30. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 28.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulino-tropic effects in healthy humans. Am J Physiol. 1997;273:E981–8. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 29.Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–25. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 30.Creutzfeldt WO, Kleine N, Willms B, et al. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–6. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 31.Hvidberg A, Nielsen MT, Hilsted J, et al. Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism. 1994;43:104–8. doi: 10.1016/0026-0495(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 32.Prigeon RL, Quddusi S, Paty B, et al. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–7. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 34.Moens K, Heimberg H, Flamez D, et al. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes. 1996;45:257–61. doi: 10.2337/diab.45.2.257. [DOI] [PubMed] [Google Scholar]
- 35.Huypens P, Ling Z, Pipeleers D, et al. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–9. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Hansotia T, Yusta B, et al. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–8. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–8. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 38.Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hojberg PV, Vilsboll T, Knop FK, et al. Four weeks of near-normalization of blood glucose restores the insulin response to GIP and improves the insulin response to GLP-1 in patients with type 2 diabetes; American Diabetes Association 2007 Annual Meeting; 2007. American Diabetes Association; 2007. [Google Scholar]
- 40.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 41.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 42.Toft-Nielson M, Madsbad S, Holst JJ. The effect of glucagon-like peptide I (GLP-I) on glucose elimination in healthy subjects depends on the pancreatic glucoregulatory hormones. Diabetes. 1996;45:552–6. doi: 10.2337/diab.45.5.552. [DOI] [PubMed] [Google Scholar]
- 43.Madsbad S, Krarup T, Deacon CF, et al. Glucagon-like peptide receptor agonists and dipeptidyl peptidase-4 inhibitors in the treatment of diabetes: a review of clinical trials. Curr Opin Clin Nutr Metab Care. 2008;11:491–9. doi: 10.1097/MCO.0b013e328302f414. [DOI] [PubMed] [Google Scholar]
- 44.Mason EE. Vertical banded gastroplasty for obesity. Arch Surg. 1982;117:701–6. doi: 10.1001/archsurg.1982.01380290147026. [DOI] [PubMed] [Google Scholar]
- 45.Saber AA, Elgamal MH, McLeod MK. Bariatric surgery: the past, present, and future. Obes Surg. 2008;18:121–8. doi: 10.1007/s11695-007-9308-7. [DOI] [PubMed] [Google Scholar]
- 46.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 47.Anonymous Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 48.Berne C. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabet Med. 2005;22:612–8. doi: 10.1111/j.1464-5491.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda M, Tahara Y, Yamamoto Y, et al. Effects of very-low-calorie diet weight reduction on glucose tolerance, insulin secretion, and insulin resistance in obese non-insulin-dependent diabetics. Diabetes Res Clin Pract. 1989;7:61–7. doi: 10.1016/0168-8227(89)90047-8. [DOI] [PubMed] [Google Scholar]
- 50.Gumbiner B, Polonsky KS, Beltz WF, et al. Effects of weight loss and reduced hyperglycemia on the kinetics of insulin secretion in obese non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1990;70:1594–602. doi: 10.1210/jcem-70-6-1594. [DOI] [PubMed] [Google Scholar]
- 51.Redmon JB, Raatz SK, Reck KP, et al. One-year outcome of a combination of weight loss therapies for subjects with type 2 diabetes: a randomized trial. Diabetes Care. 2003;26:2505–11. doi: 10.2337/diacare.26.9.2505. [DOI] [PubMed] [Google Scholar]
- 52.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 53.Wing RR, Marcus MD, Salata R, et al. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med. 1991;151:1334–40. [PubMed] [Google Scholar]
- 54.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in non-insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–25. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 55.Sjostrom CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–84. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 56.Brancatisano A, Wahlroos S, Matthews S, et al. Gastric banding for the treatment of type 2 diabetes mellitus in morbidly obese. Surg Obes Relat Dis. 2008;4:423–9. doi: 10.1016/j.soard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Pontiroli AE, Pizzocri P, Librenti MC, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab. 2002;87:3555–61. doi: 10.1210/jcem.87.8.8708. [DOI] [PubMed] [Google Scholar]
- 58.Barry RE, Barisch J, Bray GA, et al. Intestinal adaptation after jejunoileal bypass in man. Am J Clin Nutr. 1977;30:32–42. doi: 10.1093/ajcn/30.1.32. [DOI] [PubMed] [Google Scholar]
- 59.Kellum JM, Kuemmerle JF, O'Dorisio TM, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–70. doi: 10.1097/00000658-199006000-00016. discussion 70–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naslund E, Backman L, Holst JJ, et al. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–60. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 61.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clements RH, Gonzalez QH, Long CI, et al. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. discussion 5. [PubMed] [Google Scholar]
- 64.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valverde I, Puente J, Martin-Duce A, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–97. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 66.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 67.Reinehr T, Roth CL, Schernthaner GH, et al. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–7. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 68.Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 69.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 70.Borg CM, le Roux CW, Ghatei MA, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 71.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 72.Laferrere B, Tran H, Egger JR, et al. 2007 The Obesity Society Annual Meeting; 2007 October 20, 2007. Nature Publishing Group; New Orleans, LA: 2007. The increase in GLP-1 levels and incretin effect after Roux-en-Y Gastric Bypass Surgery (RYGBP) persists up to 1 year in patients with Type 2 Diabetes Mellitus (T2DM) p. A7. [Google Scholar]
- 73.Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodieux F, Giusti V, D'Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 75.Shak JR, Roper J, Perez-Perez GI, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1097–8. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sirinek KR, O'Dorisio TM, Hill D, et al. Hyperinsulinism, glucose-dependent insulinotropic polypeptide, and the enteroinsular axis in morbidly obese patients before and after gastric bypass. Surgery. 1986;100:781–7. [PubMed] [Google Scholar]
- 77.Jorde R, Burhol PG, Johnson JA. The effect of jejunoileal bypass on postprandial release of plasma gastric inhibitory polypeptide (GIP) Scand J Gastroenterol. 1981;16:313–9. doi: 10.3109/00365528109181974. [DOI] [PubMed] [Google Scholar]
- 78.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–85. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 79.Klein S, Mittendorfer B, Eagon JC, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–72. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 80.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986;35:990–8. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- 81.Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1988;66:979–86. doi: 10.1210/jcem-66-5-979. [DOI] [PubMed] [Google Scholar]
- 82.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–9. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 83.Ballantyne GH, Farkas D, Laker S, et al. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:1189–97. doi: 10.1381/096089206778392158. [DOI] [PubMed] [Google Scholar]
- 84.Lee WJ, Lee YC, Ser KH, et al. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–25. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 85.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, et al. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 86.Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 87.Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–43. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 88.Pereira JA, Lazarin MA, Pareja JC, et al. Insulin resistance in nondiabetic morbidly obese patients: effect of bariatric surgery. Obes Res. 2003;11:1495–501. doi: 10.1038/oby.2003.200. [DOI] [PubMed] [Google Scholar]
- 89.Geloneze B, Tambascia MA, Pareja JC, et al. The insulin tolerance test in morbidly obese patients undergoing bariatric surgery. Obes Res. 2001;9:763–9. doi: 10.1038/oby.2001.105. [DOI] [PubMed] [Google Scholar]
- 90.van den Hoek AM, Heijboer AC, Voshol PJ, et al. Chronic PYY3-36 treatment promotes fat oxidation and ameliorates insulin resistance in C57BL6 mice. Am J Physiol Endocrinol Metab. 2007;292:E238–245. doi: 10.1152/ajpendo.00239.2006. [DOI] [PubMed] [Google Scholar]
- 91.Vrang N, Madsen AN, Tang-Christensen M, et al. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R367–375. doi: 10.1152/ajpregu.00726.2005. [DOI] [PubMed] [Google Scholar]
- 92.Sun Y, Asnicar M, Saha PK, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–86. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150:97–104. doi: 10.1530/eje.0.1500097. [DOI] [PubMed] [Google Scholar]
- 94.Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–9. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 95.Yoshida Y, Hashimoto N, Tokuyama Y, et al. Effects of weight loss in obese subjects with normal fasting plasma glucose or impaired glucose tolerance on insulin release and insulin resistance according to a minimal model analysis. Metabolism. 2004;53:1095–100. doi: 10.1016/j.metabol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 96.Briatore L, Salani B, Andraghetti G, et al. Restoration of acute insulin response in T2DM subjects 1 month after biliopancreatic diversion. Obesity (Silver Spring) 2008;16:77–81. doi: 10.1038/oby.2007.9. [DOI] [PubMed] [Google Scholar]
- 97.Letiexhe MR, Scheen AJ, Gerard PL, et al. Insulin secretion, clearance and action before and after gastroplasty in severely obese subjects. Int J Obes Relat Metab Disord. 1994;18:295–300. [PubMed] [Google Scholar]
- 98.Guldstrand M, Ahren B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab. 2003;284:E557–565. doi: 10.1152/ajpendo.00325.2002. [DOI] [PubMed] [Google Scholar]
- 99.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first-and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–7. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- 100.Vahl TP, Paty BW, Fuller BD, et al. Effects of GLP-1-(7-36) NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;88:1772–9. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 101.Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–80. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 102.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–87. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 103.Pi-Sunyer FX. Weight and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1996;63:426S–9S. doi: 10.1093/ajcn/63.3.426. [DOI] [PubMed] [Google Scholar]
- 104.Verdich C, Toubro S, Buemann B, et al. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–14. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 105.Strader AD, Vahl TP, Jandacek RJ, et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–53. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 106.Patriti A, Facchiano E, Annetti C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15:1258–64. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 107.Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-Kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142:74–85. doi: 10.1016/j.surg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Hickey MS, Pories WJ, MacDonald KG, Jr, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637–43. doi: 10.1097/00000658-199805000-00004. discussion 43–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247:968–75. doi: 10.1097/SLA.0b013e318172504d. [DOI] [PubMed] [Google Scholar]
- 112.Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg. 2007;194:221–4. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 113.Horowitz M, Collins PJ, Harding PE, et al. Gastric emptying after gastric bypass. Int J Obes. 1986;10:117–21. [PubMed] [Google Scholar]
- 114.Naslund I, Beckman KW. Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol. 1987;22:193–201. doi: 10.3109/00365528708991879. [DOI] [PubMed] [Google Scholar]
- 115.Kotler DP, Sherman D, Bloom SR, et al. Malnutrition after gastric surgery. Association with exaggerated distal intestinal hormone release. Dig Dis Sci. 1985;30:193–9. doi: 10.1007/BF01347882. [DOI] [PubMed] [Google Scholar]
- 116.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–6. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 117.Laferrere B, Hart AB, Bowers CY. Obese subjects respond to the stimulatory effect of the ghrelin agonist growth hormone-releasing peptide-2 on food intake. Obesity (Silver Spring) 2006;14:1056–63. doi: 10.1038/oby.2006.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 119.Kotidis EV, Koliakos GG, Baltzopoulos VG, et al. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16:1425–32. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 120.Chan JL, Mun EC, Stoyneva V, et al. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–8. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 121.Morinigo R, Casamitjana R, Moize V, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–16. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 122.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faraj M, Havel PJ, Phelis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 124.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–6. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 125.Broglio F, Gottero C, Prodam F, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062–5. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 126.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 127.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY (3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 128.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 129.Morinigo R, Vidal J, Lacy AM, et al. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–5. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 130.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–88. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 131.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–71. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 132.Geloneze B, Tambascia MA, Pareja JC, et al. Serum leptin levels after bariatric surgery across a range of glucose tolerance from normal to diabetes. Obes Surg. 2001;11:693–8. doi: 10.1381/09608920160558623. [DOI] [PubMed] [Google Scholar]
- 133.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 134.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127–32. doi: 10.1016/j.soard.2006.12.005. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 135.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 136.Schauer P, Chand B, Brethauer S. New applications for endoscopy: the emerging field of endoluminal and transgastric bariatric surgery. Surg Endosc. 2007;21:347–56. doi: 10.1007/s00464-006-9008-8. [DOI] [PubMed] [Google Scholar]
- 137.Pories WJ, MacDonald KG, Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55:582S–5S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 138.Poulos JE, Leggett-Frazier N, Khazanie P, et al. Circulating insulin-like growth factor I concentrations in clinically severe obese patients with and without NIDDM in response to weight loss. Horm Metab Res. 1994;26:478–80. doi: 10.1055/s-2007-1001737. [DOI] [PubMed] [Google Scholar]
- 139.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diniz Mde F, Diniz MT, Sanches SR, et al. Glycemic control in diabetic patients after bariatric surgery. Obes Surg. 2004;14:1051–5. doi: 10.1381/0960892041975686. [DOI] [PubMed] [Google Scholar]
- 141.Alexandrides TK, Skroubis G, Kalfarentzos F. Resolution of diabetes mellitus and metabolic syndrome following Roux-en-Y gastric bypass and a variant of biliopancreatic diversion in patients with morbid obesity. Obes Surg. 2007;17:176–84. doi: 10.1007/s11695-007-9044-z. [DOI] [PubMed] [Google Scholar]
- 142.Schrumpf E, Bergan A, Djoseland O, et al. The effect of gastric bypass operation on glucose tolerance in obesity. Scand J Gastroenterol Suppl. 1985;107:24–31. doi: 10.3109/00365528509099748. [DOI] [PubMed] [Google Scholar]
- 143.Whitson BA, Leslie DB, Kellogg TA, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–9. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]