Abstract
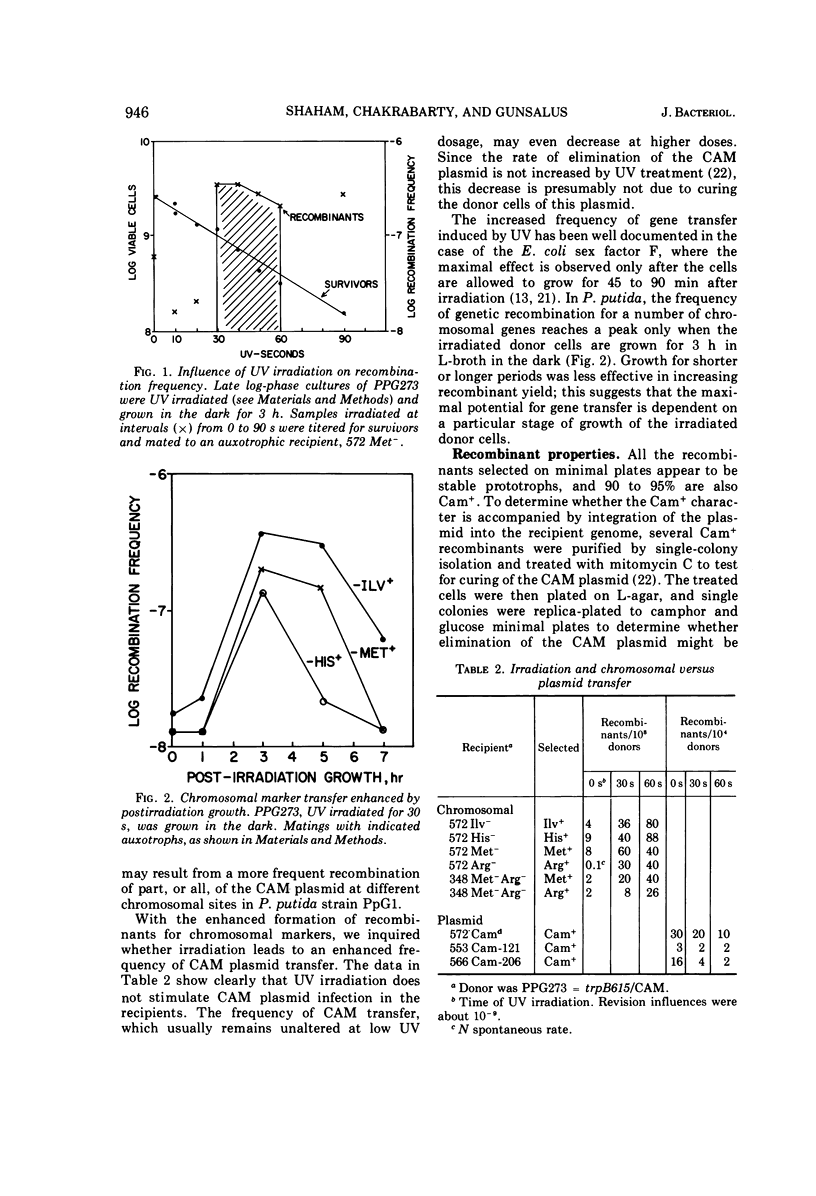
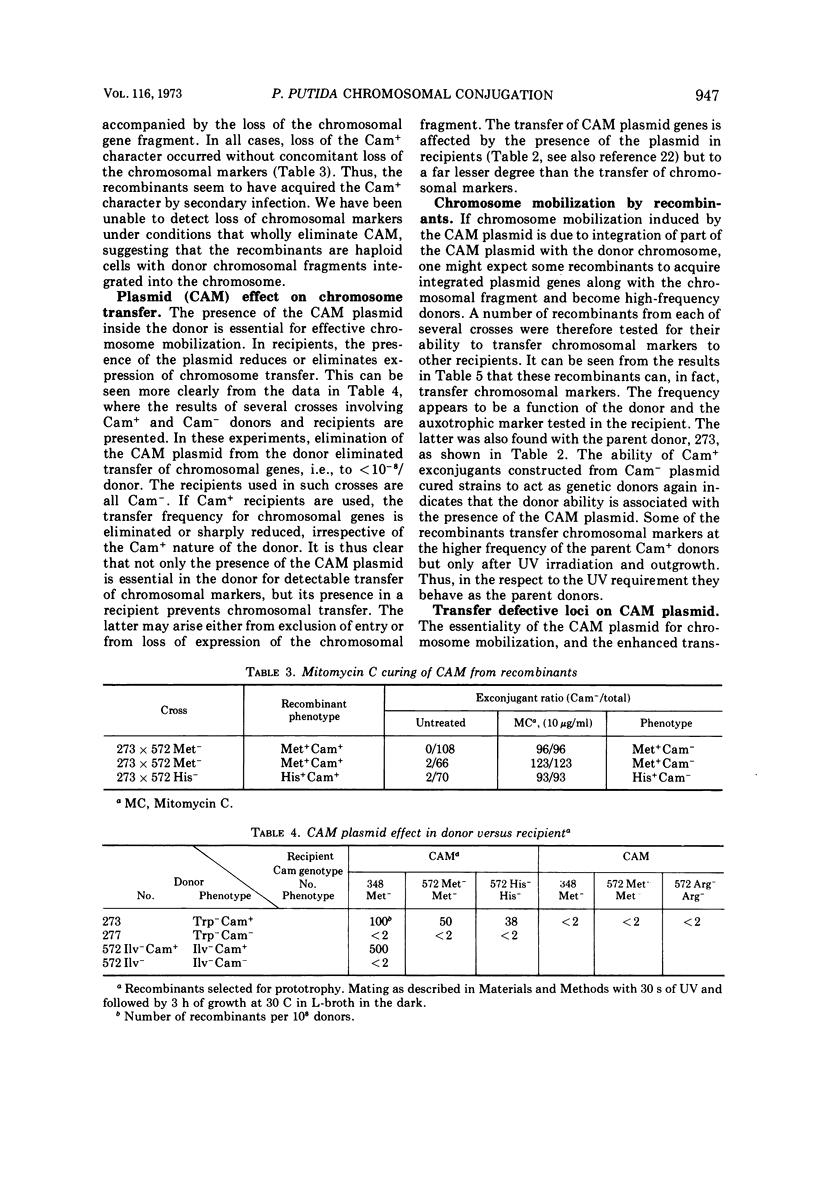
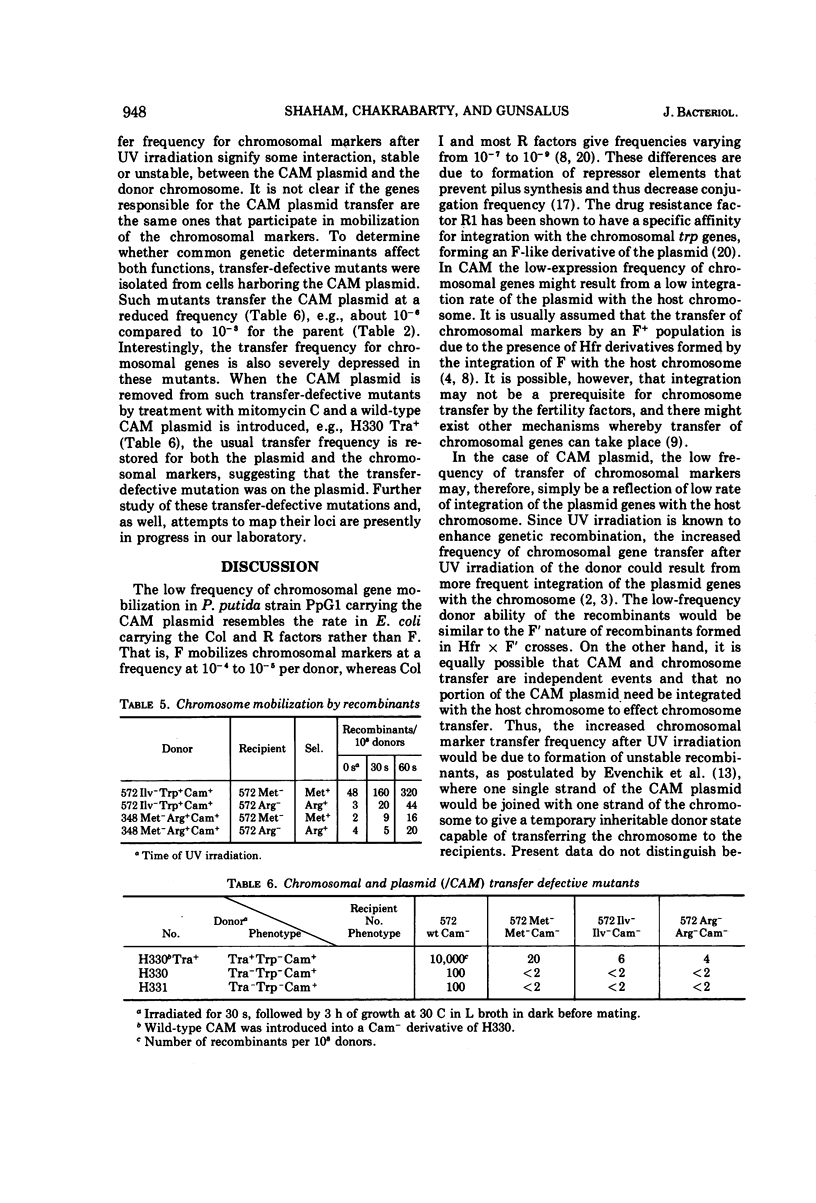
Camphor-utilizing strains of Pseudomonas putida have been shown to carry the genetic information required for camphor degradation on a plasmid. The plasmid-carrying strains can serve as donors of both plasmid-borne and chromosomal genes. As recipients, plasmid-deleted strains are much superior to those carrying the camphor pathway genes. The transfer frequency of chromosomal, but not plasmid-borne, genes is markedly enhanced if the donor cells are irradiated with ultraviolet light followed by 3-h of growth on a rich medium in the dark. Recombinants selected for prototrophy are stable and most acquire the camphor (CAM) plasmid concomitantly; only a few of the Cam+ recombinants inherit the donor's ability to transfer chromosomal genes at a high frequency. Transfer-defective mutations occur on the CAM plasmid, affecting both CAM and chromosomal gene transfer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENZINGER R., HARTMAN P. E. Effects of ultraviolet light on transducing phage P22. Virology. 1962 Dec;18:614–626. doi: 10.1016/0042-6822(62)90064-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Defective phage and chromosome mobilization in Pseudomonas putida. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1217–1223. doi: 10.1073/pnas.64.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Renshaw J. Kinetics of f transfer and recombinant production in F+ times F- matings in Escherichia coli K-12. Genetics. 1969 Sep;63(1):39–52. doi: 10.1093/genetics/63.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Parker C. D., Wohlhieter J. A., Baron L. S. Isolation and characterization of the fertility factor P of Vibrio cholerae. J Bacteriol. 1973 Feb;113(2):763–771. doi: 10.1128/jb.113.2.763-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc-Nguyen H., Rosenblum E. N., Wivel N. A., Smith M. V. Malignant transformation by Rauscher strain murine leukaemia virus. Nature. 1967 May 20;214(5090):815–817. doi: 10.1038/214815a0. [DOI] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenchik Z., Stacey K. A., Hayes W. Ultraviolet induction of chromosome transfer by autonomous sex factors in Escherichia coli. J Gen Microbiol. 1969 Apr;56(1):1–14. doi: 10.1099/00221287-56-1-1. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- KAHN P., HELINSKI D. R. RELATIONSHIP BETWEEN COLICINOGENIC FACTORS E1 AND V AND AN F FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1573–1579. doi: 10.1128/jb.88.6.1573-1579.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Meynell G. G. Exclusion, superinfection immunity and abortive recombinants in I+ X I+ bacterial crosses. Genet Res. 1969 Feb;13(1):113–115. doi: 10.1017/s0016672300002809. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Specific chromosomal affinity of a resistant factor. J Gen Microbiol. 1968 Jan;50(1):159–172. doi: 10.1099/00221287-50-1-159. [DOI] [PubMed] [Google Scholar]
- Rajchert-Trzpil M., Dobrzański W. T. Influence of mutagenic agents on the integration of the F episome into the chromosome of Escherichia coli K12F+. J Gen Microbiol. 1968 Nov;54(1):47–57. doi: 10.1099/00221287-54-1-47. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. Chromosome transfer in Pseudomonas aeruginosa mediated by R factors. Genet Res. 1971 Apr;17(2):169–172. doi: 10.1017/s0016672300012179. [DOI] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. The genetics of transmissible plasmids. Annu Rev Genet. 1972;6:257–268. doi: 10.1146/annurev.ge.06.120172.001353. [DOI] [PubMed] [Google Scholar]


