Introduction
Glioblastoma multiforme (GBM) is a devastating primary brain tumor that causes death in ~73% of individuals within 2 years of diagnosis despite treatment with surgery, radiation, and chemotherapy [1]. This tumor presents clinically as either primary GBM or progresses from a lower grade (WHO II or III) glioma leading to secondary GBM. Both primary and secondary GBM are WHO grade IV tumors with a similar prognosis [2]. Secondary GBM often arises from WHO grade II astrocytomas that are characterized by low cellularity, low mitotic index and a diffuse pattern of infiltration into normal brain. Due to the disseminated nature of the neoplasm, surgery and adjuvant therapies are frequently inadequate and the tumor evolves into secondary GBM within 5–10 years [2]. Gemistocytic astrocytoma (GemA) is a histological variant of astrocytoma that has been defined in an arbitrary fashion by the presence of at least 20% gemistocytes within the tumor mass [3]. Neoplastic gemistocytes are characterized by their plump appearance, slightly eosinophilic cytoplasm and eccentric nuclei. The classification of GemA has been controversial. Some reports demonstrated an aggressive clinical behavior with a median survival of only 35 months [3], consistent with a malignant astrocytoma (WHO grade III), whereas other studies have suggested these are slow growing tumors consistent with a WHO grade II neoplasm (reviewed in [4]). Regardless, there is clearly a need for targeted therapies for GemA that can delay or prevent progression to GBM. However, until now there has been no useful animal model of GemA available to test adjuvant therapy after surgical debulking as humans are treated. Furthermore, the murine models of glioma have not been predictive of toxicity or efficacy in humans, and this has undoubtedly contributed to the painstakingly slow progress in therapeutic development.
Similarly to humans, dogs develop spontaneous brain tumors that often carry a dismal prognosis. Based on an incidence of primary brain tumors in dogs of 20 per 100,000/year, it has been estimated that 12,000 dogs could be eligible for recruitment into clinical studies in the United States annually [5]. We and others have found many similarities between human and canine glioma such as: overexpression of the epidermal growth factor receptor, mutation of the Tp53 tumor suppressor gene [6], extensive invasion into normal brain, peritumoral edema and necrosis [7, 8], hemorrhage, compression, herniation, and obstructive hydrocephalus [9–11]. Similar to humans, the prognosis for dogs with brain tumors is poor regardless of therapeutic intervention. However, much less is known about treatment outcomes because of a historical lack of treatment options in dogs and because only a small number of studies, each of which includes few dogs, have been reported. The median survival time for dogs with glioma (any grade) that do not receive any type of treatment ranges between 6 and 13 days [9, 10]. In dogs receiving only palliative therapy the range is 60 to 80 days [12, 13]. Radiation therapy may have resulted in an increased survival time in one dog with glioma (176 days) as compared to corticosteroid therapy in three dogs with glioma (18, 40 and 64 days) [12]. The median survival for 9 dogs putatively diagnosed with glioma at our institution based on imaging characteristics of an intra-axial mass was 29 days (range 1 to 128 days). These dogs did not receive any therapy other than corticosteroids and anticonvulsants. The clinical similarities between dogs and humans suggest that dogs may represent an outstanding model for testing targeted therapies; both dogs and humans might benefit from these studies.
We previously developed a dendritic cell culture-free vaccine consisting of glioma cell lysate and CpG ODN, “CpG/Lysate”, that significantly extended survival of glioma-bearing mice [14]. CpG ODN is a potent vaccine adjuvant that signals through toll like receptor nine (TLR9) in dendritic cells and B cells to induce adaptive anti-tumor immune response in murine models and select cancer patients (reviewed in [15]). Subcutaneous CpG/lysate vaccination induced significant increases in activated dendritic cells and tumor-reactive cytotoxic T lymphocytes (CTLs) in lymph nodes draining the vaccination site of mice. The efficacy of CpG/lysate vaccination was dependent on CD4+ T cells, CD8+ T cells, and natural killer cells as shown by depletion of each subset during the priming phase of the immune response [14]. We and others have shown that intratumoral interferon gamma (IFNγ) gene transfer increases recruitment of lymphocytes to the brain tumor site in murine models, but only modestly extends survival when used as a single agent [16, 17]. In addition to enhancing lymphocyte trafficking in situ, IFNγ increases expression of NK cell activating ligands and major histocompatibility complex (MHC) class I and II molecules in human and murine glioma cells [16, 18]. The safety of lysate-based vaccines and in situ IFN gene transfer has been demonstrated in clinical trials [19–22], however as single agents their efficacy has been limited (reviewed in [23]). A more attractive use of in situ cytokine gene transfer might be to precondition the tumor site for an optimal response to vaccination that expands tumor-reactive T cells in the periphery. Indeed, several groups have demonstrated that IFN or CXCL10 cytokine gene transfer synergizes with vaccination in murine glioma models [24, 25]; however, the feasibility and tolerability of the combined use of these potent inflammatory therapies has not been established yet. The present study reports the treatment of a dog with spontaneous GemA using the combination of surgery, CpG/lysate vaccination, and intracavitary IFNγ gene transfer. This is the first demonstration that this therapy is feasible to administer to large animals and provides insight into expected results in humans.
Results
Treatment of spontaneous canine GemA with combination immunogene therapy
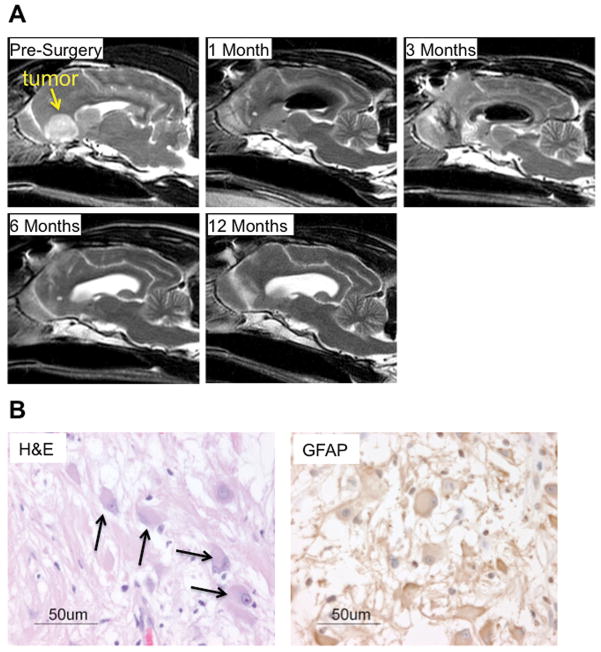
A twelve-year-old German shepherd mix with a history of seizures was diagnosed with a probable glioma in the right frontal lobe by magnetic resonance imaging (MRI) (Figure 1A). Tumor debulking surgery was performed and Ad-IFNγ was administered by 28 injections 1–2 cm deep covering resection cavity. Histological evaluation of the tumor revealed a diffuse astrocytoma, gemistocytic subtype (WHO grade II), which was confirmed by positive immunostaining of the neoplastic cells for glial fibrillary acidic protein (GFAP) (Figure 1B). Steroids were gradually tapered to zero seven days prior to the first vaccination (see methods for steroid use). A total of five CpG/lysate vaccinations were administered on days 37, 51, 65, 84, and 96 following surgery. Tumor cell lysate was prepared from expanded autologous tumor cells by multiple freeze thaw cycles followed by irradiation for the first vaccination. However, the growth of autologous tumor cells was not rapid enough to generate adequate lysate for subsequent vaccinations. To continue vaccinations, we elected to use an allogeneic astrocytoma cell line harvested from a dog with WHO grade III anaplastic astrocytoma to generate subsequent lysates. Serial MRIs were performed in order to determine tumor burden following vaccinations. MRI demonstrated a lack of recurrent tumor up to one year following surgery (Figure 1A).
Figure 1. Magnetic resonance imaging and histology of treated dog.
(a) T1-weighted sagittal images document nearly complete surgical resection of the bulk tumor mass (arrow). Ad-IFNγ was administered at the time of surgery by multiple bolus injections delivered into the brain parenchyma of cavity. Air was notable in the ventricle at 1 and 3 month scans. By seven months CSF replaced air in the ventricle and the resection cavity had closed. No tumor was apparent at one year. (b) Hematoxylin and eosin stain document presence of neoplastic gemistocytes within the tumor mass (arrows). Immunohistochemistry for GFAP confirms a glial origin of the tumor and supports the diagnoses of GemA.
Side effects from combination immunogene therapy
Neurological side effects of this therapy were moderate and resolved within three months. The treated dog experienced transient focal neurologic signs that became more severe with each subsequent vaccine. Specifically, focal seizures, left hemiparesis, and acute blindness as assessed by lack of menace response in the left eye were documented after the fourth and fifth vaccinations. Left hemiparesis and left-sided blindness became apparent three days after the forth and fifth vaccination, and resolved within a week in each instance. In addition, during therapy, the dog exhibited circulating lymphopenia, peripheral lymphadenopathy around the vaccination site, and elevation of gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) that were not present prior to treatment (Table I). Although the elevation of such liver enzymes may have been induced by anti-seizure medication (see methods), the timing of the other neurological symptoms three days after the fourth and fifth vaccination indicate a possible relationship with the vaccines.
Table I.
Blood Chemistry and Cell Counts
| Test | Normal Range | Before Surgery | Day 24 | Day 37 | Day 65 | Day 110 | Day 135 | Day 180 | Day 365 |
|---|---|---|---|---|---|---|---|---|---|
| Alkaline Phosphatase | (8–139) | 14 | 1002 | 179 | 587 | 72 | 62 | 63 | 65 |
| Gamma Glutamyltransferase | (0–6) | 4 | 30 | 10 | 28 | 4 | 4 | 3 | <3 |
| Alanine Aminotransferase | (22–92) | 73 | 289 | 81 | 192 | 172 | 179 | 140 | 58 |
| Lymphocytes | (0.78–3.36) | 0.78 | 0.4 | 0.06 | 0.27 | 1.05 | 0.3 | 0.28 | 0.6 |
| Red Blood Cell (RBC) | (5.44–8.79) | 7.74 | 5.06 | 6.15 | 4.71 | 7.39 | 7.12 | 5.24 | 5.34 |
| Nucleated RBC | no range | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| Hemoglobin | (13.3–20.7) | 17.7 | 11.6 | 14 | 10.5 | 15.9 | 15.3 | 11.4 | 12.7 |
| Hematocrit | (37.5–60.3) | 52.2 | 33.7 | 39.1 | 30.1 | 44.9 | 43.3 | 33.2 | 34.3 |
“Day” pertains to days after surgery and gene therapy
Vaccination induced a polyclonal tumor-reactive IgG response
In order to detect IgG responses specific to the dog’s own tumor, tumor cell lysates from the autologous cells grown in culture were subjected to SDS-PAGE. Autologous serum harvested before or after vaccinations was used as a primary antibody and specific IgG was detected using anti-canine IgG antibody. Western blot analysis revealed that this dog did not have an appreciable pre-existing tumor-reactive IgG response, and there was no signal from normal canine serum used as a control. By two weeks after the first vaccination, IgG reactive to two proteins approximately 50–65 kDa in weight was detected (Figure 2A). This response remained unchanged two weeks later at day 65, but increased in breadth of antigens recognized as subsequent vaccinations were administered. A memory IgG response was induced to three separate tumor antigens, as revealed by three bands on western blot using serum harvested over 100 days following the last vaccination (Figure 2A).
Figure 2. Tumor-reactive IgG and CTL responses induced by therapy.
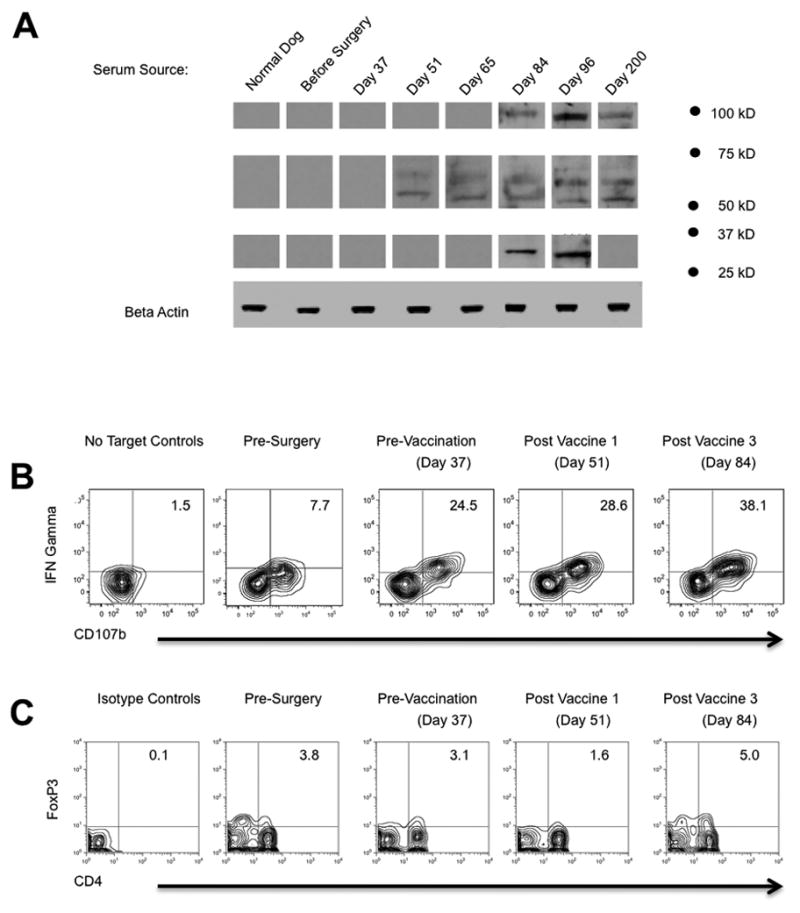
(a) Tumor reactive IgG was detected by subjecting autologous tumor lysates to SDS-PAGE, using serum as a primary antibody, and visualized by anti-canine IgG secondary antibody. No signal was detected using serum from a normal dog or serum from the treated dog harvested before therapy. Each band represents an IgG response against an autologous glioma antigen that was only detected following treatment. (b) Tumor-reactive CTL response was quantified in peripheral blood by flow cytometry (see methods). PBMCs were restimulated with autologous tumor cells or cultured alone to control for spontaneous degranulation and expression of IFNγ. Density plots shown were gated on the CD8+ lymphocyte population. The frequency of CTLs that degranulated (CD107b+) and elaborated IFNγ is noted in the upper right quadrant. The frequency of CD107b+IFNγ+ CTLs increased following surgery/gene therapy, and further increased following CpG/lysate vaccination, consistent with the trend in antibody response. (c) Frozen PBMCs were thawed, stained, and analyzed for Treg frequency. Density plots shown were gated on the live lymphocyte gate.
Vaccination enhanced a pre-existing tumor-reactive CTL response
Upon recognition of their cognate antigen, CTLs elaborate IFNγ and release cytotoxic granules; the proteins CD107a, CD107b, and CD63 within the granule mobilize to the cell surface during degranulation (reviewed in [26]). Accordingly, CD107 cell surface mobilization measured by flow cytometry demonstrates a linear correlation with tumor cell lysis [27], and CD107 has been used to monitor CTL responses in melanoma patients treated by vaccination [28]. Because the number of peripheral blood mononuclear cells (PBMCs) obtained from this dog was limited, we elected to employ this flow cytometry-based assay to detect CTL degranulation and IFNγ production. PBMCs frozen at various time points before and after surgery were thawed and analyzed identically and simultaneously to eliminate interexperiment variability. PBMCs were co-cultured with autologous tumor cells or cultured alone as a control for spontaneous degranulation. Five hours later, PBMCs were harvested and analyzed for CD107b and IFNγ by flow cytometry. There was a minimal background (<2%) in spontaneous CD107b cell surface mobilization and IFNγ expression (Figure 2B). In contrast, 7.7% of CD8+ cells harvested before surgery degranulated and elaborated IFNγ in response to autologous tumor cells, revealing a pre-existing CTL response against the tumor. The frequency of IFNγ+CD107b+ CTLs increased to 24.5% by 37 days following surgery and intracavitary IFNγ gene transfer. The frequency of tumor-reactive CTLs increased with subsequent vaccinations, peaking at a 38% IFNγ+/CD107b+ CTLs measured 14 days after the third vaccination (Figure 2B). In contrast to the CTL response, vaccination was not associated with any clear trend in the percentage of CD4+Fox3P+ regulatory T cells in the peripheral blood (Figure 2C)[29].
Discussion
The majority of GemA patients will ultimately develop GBM and succumb to their disease despite surgery and adjuvant therapy [4]. Compared to the more aggressive GBM that has a median time to progression of 6.9 months [2], we propose that GemA is an attractive target for immunological therapies that may work more slowly and, potentially, more effectively in this earlier and less aggressive form of astrocytoma to induce tumor regression and anti-tumor immunity. This case report is not sufficient to make firm conclusions about the ability of the combination of IFNγ gene transfer and CpG/lysate vaccination to prevent progression of GemA to GBM, however the data do demonstrate that the therapy is feasible in a large animal model. Our results raise several interesting points that warrant attention.
In the present study, the autologous tumor cells grew too slowly to generate adequate lysate after the first vaccination; therefore, we administered allogeneic anaplastic astrocytoma lysate for the remaining four vaccinations. Interestingly, the first vaccination induced an IgG response specific to two antigens in the autologous tumor sample that were approximately 50–65 kDa in molecular weight, as seen at day 51 (Figure 2A). Vaccination with allogeneic lysate apparently primed a polyclonal IgG response to several other autologous antigens. While the identity of these IgG epitopes (or the T cell epitopes) was not determined, our results demonstrate that CpG/lysate vaccination is a feasible method to break immunological tolerance to multiple glioma antigens. Although preliminary, our data indicate that autologous tumor cell lysate production may not always be feasible in WHO II grade gliomas, but allogeneic WHO III grade lysates could be used as a scalable “off the shelf” antigen source. We are currently treating additional dogs to better define the logistics, efficacy, and safety of this therapy.
The relevance of tumor-reactive IgG that was induced by vaccination in our canine subject is unclear, due in part to the fact that antibody permeability is restricted by the blood brain barrier (BBB) [30]. The BBB is relatively intact beyond the surgical resection cavity [31], where invasive tumor cells have been documented several centimeters deep in the normal brain parenchyma [32, 33]. Due to limited permeability of antibody into the normal brain and a focus on cell-mediated immunity, antibody response has largely been ignored in brain tumor immunotherapy literature. However, Daga et al. reported that efficacious vaccination with syngeneic glioma cells transduced with IL-21 failed in B cell deficient mice [34]. Further, a spontaneous antibody response specific to several glioma antigens is associated with significantly longer survival in GBM patients [35]. We have recently demonstrated that CpG/lysate vaccination induces plasma cell infiltration of brain that circumvents the BBB in a murine glioma model (Murphy et al., submitted). Glioma-reactive antibody has been documented to occur in murine models cured by immunostimulatory gene therapy approaches [36]. Additionally, plasma cells that secrete self-reactive antibody have been documented in the cerebral spinal fluid of patients with autoimmune disorders of the brain [37]. Together, such studies implicate tumor-reactive antibody as a plausible mechanism for the neurological side effects and uncharacteristically long survival of the treated dog in this study. Specifically, we noted the appearance of two new bands on the western blot at ~100 kDa and ~30 kDa that correlated with the induction of left sided hemiparesis and blindness in the left eye (Figure 2A). The fact that these symptoms occurred on the left side only is suggestive of an inflammatory response adjacent to the resection cavity in the right cerebral hemisphere which controls left sided vision and motor function.
In addition, steroids, anti-seizure medication, or CpG ODN may have caused some side effects in the treated dog. Corticosteroids induce hepatic changes that can include increased fat and glycogen deposits within hepatocytes resulting in increased ALT and GGT serum levels. Significant increases can be seen in serum alkaline phosphatase levels after corticosteroid administration due to direct induction of the enzyme [38]. Increases in liver enzymes are well described for Phenobarbital in dogs, as well, and are not necessarily indicative of liver dysfunction. Mild anemia in this dog may have been due to the use of CpG ODN as an adjuvant. Mice treated with CpG ODN developed anemia that was attributed to erythropoiesis suppression and shortened red cell survival [39].
Ideally cancer vaccines will initiate expansion of CTLs that secrete multiple effector cytokines, traffic to tumor sites in sufficient number, and release cytotoxic granules to kill tumor cells. However cancer vaccines have had little clinical efficacy to date, suggesting that the quality and quantity of the responding T cells is inadequate. Control of viral infection and response to melanoma immunotherapy was positively correlated with a subset of antigen-experienced T cells that secrete IFNγ, TNFα, IL-2 or MIP-1β, and degranulate; so called “polyfunctional” T cells endowed with truly adequate function [40–43]. It is noteworthy that prior to any therapy, an appreciable fraction of the CD8+ T cells in the treated dog degranulated as shown by CD107b cell surface mobilization, yet failed to make IFNγ (IFNγ−CD107b+ cells in “before surgery panel”; Figure 2B). Surgery and IFNγ gene therapy apparently increased the frequency of dual functional IFNγ+CD107b+ CTLs, and vaccination further increased their frequency to at least 38% of the total CTLs in the blood (Figure 2B). While our studies did not address the ability of CTLs to elaborate TNFα, IL-2 or MIP-1β, properties required to call them polyfunctional, our data reveal that the quality and quantity of tumor-reactive CTLs changed as a function of gene therapy and vaccination. It is likely that this tumor-reactive CTL response also played a role in the neurological side effects observed.
This study is the first documented treatment of a canine with spontaneous glioma to determine the toxicity and immune responses that occur following immune-based therapy. To our knowledge, dogs represent the only naturally occurring large animal model of glioma with a tumor incidence that is frequent enough to be useful for translational investigation. Studies of surgery, radiation, chemotherapy, and experimental therapy in dogs are more likely to provide meaningful data that is predictive of human responses than are similar studies in rodent species. Although the incidence and prognosis for canine GemA has not been adequately defined, canine tumors in general progress approximately seven times faster than their human counterparts (reviewed in [44]). Therefore, the progression-free survival of greater than 450 days (~1/10 of lifespan) in our canine subject may be considered equivalent to 7 years in a human (~ 1/10 of lifespan). As such, we are very encouraged by our data and believe these results warrant further study in additional dogs with spontaneous glioma. Treatment of dogs with low-grade glioma using “immuno prevention” strategies such as the therapy employed in the present study represents an outstanding opportunity to achieve meaningful outcomes in one-seventh the time required for similar data in human patients. Thus, this comparative oncology paradigm could be an important translational approach to justify treatment of human patients with low-grade gliomas using novel therapies. It remains to be proven how predictive of human responses the canine model really is; however the poor predictability of murine glioma models suggests that improvement in this area is needed. The canine model clearly represents an unexplored opportunity to improve the process of translational medicine in the area of brain tumor biology and treatment.
Materials and Methods
Surgery, gene therapy, imaging, and supportive medical care
A German shepherd mix was treated for glioma after obtaining owner consent according to an approved protocol from the University of Minnesota IACUC. Brain MRI was performed before, 1, 3, 6 and 12 months after surgery with the dog under general inhalant anesthesia using a GE Signa HDx 3/0T scanner. A sagittal localizer series (TR = 400 msec/time and TE = 20 msec) was performed to delineate subsequent transverse images. The following MR images were acquired precontrast: sagittal T2 (TE = 105, TR = 2967, 2.5 mm slice thickness, 0.2 mm slice spacing), axial T2 (TE = 102, TR = 3000, 2.5 mm slice thickness, 0.2 mm slice spacing), dorsal T2 (TE = 102, TR = 3017, 3.0 mm slice thickness, 0.2 mm slice spacing), axial T2 flair (TE = 120, TR = 8000, 2.5 mm slice thickness, 0.2 mm slice spacing), axial gradient (TE = 13.5, TR = 800, 2.5 mm slice thickness, 0.2mm slice spacing), axial T1 flair (TE = min full, TR = 2500, 2.5 mm slice thickness, 0.2 mm slice spacing), DWI (TE = min, TR = 10000, 2.4 mm slice thickness), DTI (TE = min, TR = 10000, 3.0 mm slice thickness, 0.3 mm slice spacing), 3DT of MTFS (TE = min, TR = min, 1.6 mm slice thickness with 2 overlap locations). Further images were aquired after gadolinium-diethylenetriamine pentaacetic acid (DTPA) bismethylamide at 0.1-mmol/kg body weight (BW) (Omniscan, GE Healthcare Inc, Princeton, NJ): axial T1 flair (TE = min full, TR = 2500, 2.5 mm slice thickness, 0.2 mm slice spacing), sagittal TI (TE = min full, TR = 700, 2.5 mm slice thickness, 0.2 mm slice spacing), dorsal T1 (TE = min full, TR = 700, 3.0 mm slice thickness, 0.2 mm slice spacing). The scans were evaluated for tumor location, signal intensity, gadolinium enhancement pattern, peritumoral edema and tumor volume.
For the surgical procedure, the dog was placed in sternal recumbency with the head elevated and secured in a craniotomy head stand to prevent jugular vein occlusion. Intravenous catheters were aseptically placed in peripheral veins to administer propofol (6 mg/kg/min continuous intravenous infusion) to maintain general anesthesia and lactated Ringers solution (10 mL/kg/hr) throughout the procedure and for administration of other drugs. Antibiotic prophylaxis was given using cefazolin (22 mg/kg BW IV), 20 minutes prior to surgery, every 90 minutes during surgery. A cuffed endotracheal tube was placed to administer oxygen to induce mild hypocapnia (PaCO2 25 to 35 mmHg). Capnometry (Datex 254 Airway Gas Analyzer, Puritan-Bennett Corp., Wilmington, MA) and arterial blood gas measurements (AVL 995 pH/blood gas analyzer, AVL Scientific Corp., Roswell, GA) were performed to verify maintenance of a hypocapnic state. A catheter was placed in a dorsal pedal artery and connected to an electronic pressure transducer (Transpac II pressure transducer, Abbott Critical Care System, North Chicago, IL) and a pressure monitor (Vital Signs Monitor, PhysioControl VSMI, PhysioControl Inc., Redmond, WA) to directly measure mean arterial blood pressure. Mannitol (1 g/kg BW) over 10 minutes, followed by furosemide (1 mg/kg BW), then methylprednisolone sodium succinate (30 mg/kg BW) were administered intravenously prior to the craniotomy. A modified bilateral transfrontal sinus approach [45] was made with an air-powered high-speed drill (Hall Micro 100 drill 5053-09, Zimmer-Hall, Warsaw, IN) and oscillating saw cooled by continuous lavage with isotonic saline solution. The dura was sharply incised and reflected to expose the right frontal lobe. Pial vessels were cauterized with bipolar electrocoagulation and the neoplastic tissue was excised using blunt and sharp dissection and suction aspiration. Tumor samples were placed in culture media in preparation for isolation and culture of brain tumor cells for vaccine production and in 10% formalin for processing for histopathology.
Immediately following tumor debulking, 6.0 × 108 infectious units of Ad-IFNγ were administered into the resection cavity by 28 injections (2 μl/site, 1–2 cm deep) covering the circumference of the cavity. Ad-IFNγ, encoding human IFNγ regulated by the CMV promoter, was produced as previously described [46]. The dura was closed. Gelatin sponges (Gel Foam, Upjohn Co., Kalamazoo, MI) were placed over the dura, and the bone flap was replaced. Then the subcutaneous tissues and skin were closed over the craniotomy. The dog recovered from anesthesia in the intensive care unit and was monitored for seizure activity until discharged from the hospital. The dog received hydromorphone (0.05 μg/kg SC QID) for analgesia, methylprednisolone sodium succinate (15 mg/kg IV 12 h and 7.5 mg/kg IV 24 h after surgery), and phenobarbital (1.5 mg/kg IV BID) as an anticonvulsant in the ICU. After discharge, phenobarbital (1.5 mg/kg PO BID) was continued, a tapering dose of prednisolone (1 mg/kg PO BID × 3 d, 0.5 mg/kg PO BID × 3 days, 0.5 mg/kg PO EOD × 3 days), and morphine sulfate SR (1 mg/kg PO BID × 3 days) were administered.
Vaccine production and administration
Autologous and allogeneic canine astrocytoma cells used for vaccination were grown in DMEM/F12 media supplemented with N2, B27 (Invitrogen, Carlsbad, CA) and 20 ng/ml of human epidermal growth factor and basic fibroblast growth factor (Peprotech, Rocky Hill, NJ). The allogeneic cells were derived from a French bulldog. The tumors used to make cell cultures were dissociated as previously described [18]. Cells were cultured at 37°C, 5% O2, 5% CO2 in a humidified incubator in 10 cm dishes or 75 cm2 flasks. All vaccinations were prepared as follows. Cells were harvested, washed thrice in PBS, and resuspended in 200 μl PBS. Lysates were generated by the freeze thaw method as previous described [14] and lysates were further irradiated at 200 Gy to ensure complete tumor cell death. Each vaccine administered consisted of an average of 536 μg of protein lysate (range 230–641 μg) mixed with 2 mg of phosphorothioated-unmethylated type-B CpG ODN 685 (5′-tcgtcgacgtcgttcgttctc-3′; SBI Biotech, Japan). Frozen vials of CpG ODN and tumor lysate were thawed at room temperature, mixed with a syringe, and injected intradermally into two sites (200 μl/site) in the neck area on the indicated days following surgery.
Flow cytometry assays
For the CTL assay, frozen PBMC samples from each time point were thawed and cultured for 24 hours prior to use in complete medium consisting of RPMI 1640 (Invitrogen) supplemented with nonessential amino acids (Invitrogen), penicillin/streptomycin (Invitrogen) and 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) at 37°C, in a 5% CO2 incubator. Autologous tumor cells were maintained in a 6 well plate coated with matrigel matrix (BD bioscience, San Jose, CA) in DMEM (Invitrogen) supplemented with penicillin/streptomycin and 10% FBS. The day of the assay, effector cells were incubated with target cells in complete RPMI 1640 media in 12 × 75 mm Facs tubes (BD bioscience) for 5 hours at 37°C in 5% CO2. The effector/target cell ratio used was 10:1 with 1 × 105 PBMCs. Effector cells from each time point were cultured alone (no targets) as control for spontaneous degranulation and IFNγ elaboration. A representative background degranulation response is shown in Figure 2B, left panel. FITC conjugated anti-CD107b antibody (AbD Serotec, Oxford, UK) or IgG1 isotype control (AbD Serotec) was added at the beginning of the co-culture. After a one-hour coincubation, Monensin (1:100 dilution; BD Biosciences) and Brefeldin A (3 μg/ml final concentration; eBioscience) were added for the last 4 hours of incubation [26]. Following incubation, cell suspensions were washed with ice-cold PBS and stained for with anti-CD8 antibody conjugated to Alexa Fluor 700 (AbD Serotec) for 30 min at 4 °C. Samples were then fixed and permeabilized using BD Cytofix/Cytoperm kit (BD bioscience) and stained for intracellular IFNγ with the cross-reactive anti-bovine IFNγ antibody conjugated to PE (AbD Serotec).
For detection of Tregs, frozen PBMCs were thawed and added at 1×105 in 12 × 75 mm Facs tubes. Cell surface staining was done using Pacific Blue-conjugated anti-dog CD4 antibody (AbD Serotec) or IgG1 isotype control (AbD Serotec) at 4 °C for 30 min. Following incubation, cell suspensions were washed with cold PBS and resuspended in fixation permeabilization working solution (Foxp3 staining buffer set, eBioscience) overnight. The next day cells were washed with permeabilization buffer (Foxp3 staining buffer set, eBiosceince) followed by intracellular staining with a cross reactive anti-mouse Foxp3 PeCy-5 conjugated antibody (eBioscience) at 4 °C for 30 min [29]. Samples were then washed and resuspended in PBS for flow cytometric analysis. Analysis gates were set on the live lymphocyte population based on forward and side scatter characteristics. All flow cytometric analysis was performed on a FACS Canto II flow cytometer (BD Biosciences). A total of 20,000 events were acquired and analyzed using FlowJo software (Tree Star, Ashland, OR).
Western blot and histological analyses
Cultured autologous tumor cells were washed, pelleted, and lysed in RIPA buffer (25 mM Tris-HCl, 0.1% SDS, 1% Triton X-100, 1% sodiumdeoxycholate, 0.15 M NaCl, 1mM EDTA) at a ratio of 1 × 106 cells: 200 μl buffer. Protease and phosphatase inhibitors (Calbiochem, San Diego, CA) were added to RIPA buffer at 1:100 for a final concentration of 0.1%. Protein concentrations were determined using the BCA colorimetric method against known concentrations of BSA (Pierce, Rockford, IL). For SDS PAGE, lysates were made 2 mg/ml with laemmli reducing sample buffer, heated at 95°C for 5 minutes, centrifuged at 15,000 × g for 1 minute and left on the bench to come to room temperature. Protein standards (BioRad, Hercules, CA) were loaded next to each 40 μg of lysate and resolved on NuPAGE 4–12% Bis/Tris gels (Invitrogen). Gels were equilibrated for 30 minutes and proteins were then transferred to nitrocellulose (Amersham, Uppsala, Sweden) at 5 volts constant voltage overnight in Towbins Transfer Buffer using semi-dry transfer (BioRad). The membranes were blocked in 5% NFDM/TTBS at room temperature for one hour with constant rocking. Membranes were then cut down into eight identical blots each with a molecular weight standard (BioRad) run adjacent to 40 μg of lysate. Each membrane was incubated at room temperature for one hour in normal, pre or post vaccination sera diluted 1:1000 in 5% NFDM/TTBS. Membranes were washed six times for 10 minutes each in TTBS. Membranes were then incubated at room temperature for one hour in rabbit anti-canine IgG HRP-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA) at 1:50,000 in 5% NFDM/TTBS and washed as described above. Immunoreactive bands were then detected using ECL Western Blotting Detection System (Amersham) by exposing membranes to HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ).
Histology
Sections were cut at 5 μm using a microtome, mounted onto CapGap slides, and rehydrated according to standard protocols. Mounted slides were pretreated with a citrate buffer, 6.0 pH, in a Black & Decker (Hampstead, MD) steamer for 30 min, with a 10 min cool down. Standard 2D immunostaining procedures using peroxidase-labeled streptavidin and DAB chromagen on an automated TechMate 500 capillary gap immunostainer (Ventana Medical Systems, Tucson, AZ) were used. Hematoxylin counterstaining was used to provide cytological detail. Rabbit anti-bovine GFAP antibody was used at a 1:20,000 dilution (Dako, Carpenteria, CA). The tumor was negative for neuronal markers (NeuN and synaptophysin). Two M.D. neuropathologists and 5 veterinary pathologists concurred that the neoplasm was a diffuse astrocytoma, gemistocytic subtype (WHO grade II) based on the histological and immunohistochemistry results.
Acknowledgments
This work was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) (NIH IR21-NS055738 (JRO), American Cancer Society RSG-09-189-01-LIB (JRO), Randy Shaver Cancer Research and Community Fund (JRO), Children’s Cancer Research fund (JRO and GEP). MGC’s and PRL’s research program on brain tumor biology and therapeutics is supported by NIH/NINDS Grant 1UO1 NS052465; 1RO1-NS 057711; 1R21-NSO54143; 1 RO1 NS 054193; RO1 NS 42893. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to PRL and MGC, respectively, The Drown Foundation; The Linda Tallen & David Paul Kane Foundation and the Board of Governors at CSMC. The authors thank the Chunyan Liu at Cedars Sinai Medical Center/UCLA for the preparation of the Ad-IFNγ and the Comparative Pathology Shared Resource of the University of Minnesota Masonic Cancer Center for preparation of the histological sections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Krouwer HG, Davis RL, Silver P, Prados M. Gemistocytic astrocytomas: a reappraisal. J Neurosurg. 1991;74:399–406. doi: 10.3171/jns.1991.74.3.0399. [DOI] [PubMed] [Google Scholar]
- 4.Tihan T, Vohra P, Berger MS, Keles GE. Definition and diagnostic implications of gemistocytic astrocytomas: a pathological perspective. J Neurooncol. 2006;76:175–183. doi: 10.1007/s11060-005-4897-2. [DOI] [PubMed] [Google Scholar]
- 5.Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer research. 2007;67:4541–4544. doi: 10.1158/0008-5472.CAN-06-3792. [DOI] [PubMed] [Google Scholar]
- 6.Stoica G, Kim HT, Hall DG, Coates JR. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41:10–19. doi: 10.1354/vp.41-1-10. [DOI] [PubMed] [Google Scholar]
- 7.Candolfi M, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipsitz D, et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40:659–669. doi: 10.1354/vp.40-6-659. [DOI] [PubMed] [Google Scholar]
- 9.Foster ES, Carrillo JM, Patnaik AK. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J Vet Intern Med. 1988;2:71–74. doi: 10.1111/j.1939-1676.1988.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 10.Heidner GL, Kornegay JN, Page RL, Dodge RK, Thrall DE. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med. 1991;5:219–226. doi: 10.1111/j.1939-1676.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 11.LeCouter RA. Brain Tumors of Cats and Dogs. Vet Med Rep. 1990;2:332–342. [Google Scholar]
- 12.Turrel JM, Fike JR, LeCouteur RA, Higgins RJ. Computed tomographic characteristics of primary brain tumors in 50 dogs. J Am Vet Med Assoc. 1986;188:851–856. [PubMed] [Google Scholar]
- 13.LaRue SM, Gillette EL. Small Animal Clinical Oncology. WB Saunders Co Publishing; Philadelphia: 2001. pp. 119–137. [Google Scholar]
- 14.Wu A, et al. In vivo vaccination with tumor cell lysate plus CpG oligodeoxynucleotides eradicates murine glioblastoma. J Immunother (1997) 2007;30:789–797. doi: 10.1097/CJI.0b013e318155a0f6. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehtesham M, et al. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 17.Wu A, et al. Transposon-based interferon gamma gene transfer overcomes limitations of episomal plasmid for immunogene therapy of glioblastoma. Cancer Gene Ther. 2007;14:550–560. doi: 10.1038/sj.cgt.7701045. [DOI] [PubMed] [Google Scholar]
- 18.Wu A, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med. 2008;10:329–339. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 20.Chiocca EA, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 21.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer research. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka R, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selznick LA, Shamji MF, Fecci P, Gromeier M, Friedman AH, Sampson J. Molecular strategies for the treatment of malignant glioma--genes, viruses, and vaccines. Neurosurg Rev. 2008;31:141–155. doi: 10.1007/s10143-008-0121-0. discussion 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito R, et al. Vaccination with tumor cell lysate-pulsed dendritic cells augments the effect of IFN-beta gene therapy for malignant glioma in an experimental mouse intracranial glioma. International journal of cancer. 2004;111:777–782. doi: 10.1002/ijc.20331. [DOI] [PubMed] [Google Scholar]
- 25.Jiang XB, Lu XL, Hu P, Liu RE. Improved therapeutic efficacy using vaccination with glioma lysate-pulsed dendritic cells combined with IP-10 in murine glioma. Vaccine. 2009;27:6210–6216. doi: 10.1016/j.vaccine.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Suni MA, Maino VC, Maecker HT. Ex vivo analysis of T-cell function. Curr Opin Immunol. 2005;17:434–440. doi: 10.1016/j.coi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Rubio V, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nature medicine. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 28.Zaritskaya L, Shafer-Weaver KA, Gregory MK, Strobl SL, Baseler M, Malyguine A. Application of a flow cytometric cytotoxicity assay for monitoring cancer vaccine trials. J Immunother. 2009;32:186–194. doi: 10.1097/CJI.0b013e318197b1b2. [DOI] [PubMed] [Google Scholar]
- 29.Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. 2007;116:69–78. doi: 10.1016/j.vetimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine RL, et al. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12:5770–5776. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 32.Kuratsu J, Itoyama Y, Uemura S, Ushio Y. Regrowth patterns of glioma--cases of glioma regrew away from the original tumor. Gan No Rinsho. 1989;35:1255–1260. [PubMed] [Google Scholar]
- 33.Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997;86:525–531. doi: 10.3171/jns.1997.86.3.0525. [DOI] [PubMed] [Google Scholar]
- 34.Daga A, et al. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. International journal of cancer. 2007;121:1756–1763. doi: 10.1002/ijc.22901. [DOI] [PubMed] [Google Scholar]
- 35.Pallasch CP, et al. Autoantibodies against GLEA2 and PHF3 in glioblastoma: tumor-associated autoantibodies correlated with prolonged survival. International journal of cancer. 2005;117:456–459. doi: 10.1002/ijc.20929. [DOI] [PubMed] [Google Scholar]
- 36.Ghulam Muhammad AK, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Budingen HC, Gulati M, Kuenzle S, Fischer K, Rupprecht TA, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of patients with central nervous system autoimmune demyelination produce “oligoclonal bands”. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Solter PF, Hoffmann WE, Chambers MD, Schaeffer DJ, Kuhlenschmidt MS. Hepatic total 3 alpha-hydroxy bile acids concentration and enzyme activities in prednisone-treated dogs. Am J Vet Res. 1994;55:1086–1092. [PubMed] [Google Scholar]
- 39.Thawani N, Tam M, Chang KH, Stevenson MM. Interferon-gamma mediates suppression of erythropoiesis but not reduced red cell survival following CpG-ODN administration in vivo. Exp Hematol. 2006;34:1451–1461. doi: 10.1016/j.exphem.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Yuan J, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciuffreda D, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 42.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. The Journal of experimental medicine. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. The Journal of experimental medicine. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 45.Glass EN, Kapatkin A, Vite C, Steinberg SA. A modified bilateral transfrontal sinus approach to the canine frontal lobe and olfactory bulb: surgical technique and five cases. J Am Anim Hosp Assoc. 2000;36:43–50. doi: 10.5326/15473317-36-1-43. [DOI] [PubMed] [Google Scholar]
- 46.Southgate T, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci. 2008;Chapter 4(Unit 4):23. doi: 10.1002/0471142301.ns0423s45. [DOI] [PMC free article] [PubMed] [Google Scholar]