Abstract
Adiponectin, an adipocytokine, is secreted by adipocytes and mediates anti-hypertrophic and anti-inflammatory effects in the heart. Plasma concentrations of adiponectin are decreased in obesity, insulin resistance and obesity-associated conditions such as hypertension and coronary heart disease. However, a paradoxical increase in adiponectin levels is observed in human systolic heart failure (HF). We sought to investigate the determinants of adiponectin levels in patients with chronic systolic HF. Total adiponectin levels were measured in 99 patients with stable HF and left ventricular (LV) ejection fraction (EF) <40%. Determinants of adiponectin levels by univariate analysis were included in a multivariate linear regression model. At baseline patients were 62% black, 63% male, mean age of 60±13 years, LVEF of 21±9% and a body mass index (BMI) of 30.6±6.7kg/m2. Mean adiponectin levels were 15.8±15µg/ml. Beta-blocker use, BMI, and blood urea nitrogen (BUN) were significant determinants of adiponectin levels by multivariate analysis. LV mass, structure, and LVEF were not related to adiponectin levels by multivariate analysis. Interestingly, the effect of beta-blocker therapy was most marked in non-obese patients with BMI < 30kg/m2. In conclusion, in chronic systolic HF patients, beta-blocker therapy is correlated with lower adiponectin levels, especially in non-obese patients. This relation should be taken into account when studying the complex role of adiponectin in chronic systolic HF.
Keywords: Adiponectin, chronic systolic heart failure, beta-blockers
Introduction
Adiponectin, an adipocyte-derived cytokine, is abundantly present in human plasma1 and mediates favorable actions in cardiovascular and metabolic-associated diseases. Adiponectin exerts a plethora of effects including anti-hypertrophic and anti-inflammatory effects2 in addition to improving vascular function and modulating pathological cardiac remodeling2;3. Depressed adiponectin levels are evident in obesity, coronary artery disease (CAD), hypertension and insulin resistance3–5 and may reflect greater cardiovascular risk and inflammation. However increased adiponectin levels are seen in patients with systolic heart failure (HF)6–8. The release of adiponectin into the circulation is associated with the severity of HF symptoms9, disease severity and mortality6;7. Elevated adiponectin levels likely reflects an attempt to mitigate pro-inflammatory or impaired metabolic states and demonstrates a balance between protective and harmful pathways in left ventricular (LV) systolic dysfunction and HF. Interactions between various factors may alter adiponectin levels in HF patients, making it difficult to interpret adiponectin levels in the development and progression of HF. We therefore sought to investigate the determinants of adiponectin levels in a cohort of HF patients with chronic systolic dysfunction.
Methods
One hundred and four patients with chronic HF and LVEF <40% were recruited from an ambulatory HF clinic, at Boston Medical Center between 2001–2005. Five patients had end-stage renal disease and were on dialysis. They were excluded because adiponectin levels are positively associated with abnormal renal function10. Data was recorded at enrollment and the analysis reflects the measurements of 99 patients. A medical history was obtained to document etiology, severity of HF symptoms by New York Heart Association (NYHA) functional class and co-morbid disease. Routine laboratory results (e.g., electrolytes and blood count) and concomitant cardiac medications were recorded. Body mass index (BMI) was calculated as the ratio of weight to squared height. The Boston Medical Center Institutional Review Board approved the study. All patients gave written informed consent. Ischemic etiology was defined by a prior history of myocardial infarction (electrocardiogram/positive troponin), results of a positive non-invasive stress test, or cardiac catheterization. Hypertensive etiology was defined by a documented history of pharmacologically treated hypertension and no known CAD (by electrocardiogram, non-invasive stress test, or cardiac catheterization). Idiopathic etiology was defined as having no identifiable cause of the cardiomyopathy and included primary valvular disease, alcohol-induced and familial.
Two-dimensional and Doppler echocardiography were performed at baseline using the Vingmed Vivid Five System (GE Vingmed, Milwaukee, WI) as previously described11. Echocardiograms were performed and analyzed in a blinded manner. As described previously measurements of systolic and diastolic chamber dimensions and wall thickness were obtained from two-dimensional imaging according to the recommendations of the American Society of Echocardiography11. Similarly the standard cube formula was used to calculate LV mass. Relative wall thickness (RWT) was calculated as: (2*PWT)/ LVEDD, where PWT is posterior wall thickness and LVEDD is LV end-diastolic diameter11.
Vital signs were recorded and 35mL of fasting blood samples were collected from the antecubital vein after 10-minutes of rest in the supine position. Plasma/ serum was decanted and immediately stored at −80°C. Adiponectin and BNP levels were determined by ELISA (Otsuka Pharmaceutical Co Ltd) and the ADVIA Centaur assay (Siemans Healthcare Diagnostics) respectively. The minimal detection limit for adiponectin was 0.12µg/mL and the intra- and interassay CVs were both <10%. CRP levels were measured by a commercial laboratory (Quest Diagnostics, Cambridge, MA).
Summary statistics are presented as mean±standard deviation for continuous variables and as number (percentage) for categorical variables. We examined the relationship between each marker and adiponectin using the Pearson’s correlation. In these analyses, the skewed variables were analyzed after log transformation. Adiponectin levels were divided into terciles, and the relationship between adiponectin, clinical characteristics and echocardiography parameters were examined using ANOVA, or chi-square test, as appropriate. For medication use, we compared adiponectin levels between patients "using" or "not using" each class of medication by student t test. We specified a priori that a factor must show a potential association (i.e., P<0.15) with adiponectin by bivariate analysis in order to be tested in a multivariable regression model. A value of P<0.05 was considered statistically significant. All reported P-values are 2-tailed and all confidence intervals are computed at the 95% level. All analyses were conducted using SPSS software, version 11.5 (SPSS Inc, Chicago, Ill).
Results
Ninety-nine chronic, systolic HF patients completed the study. The majority of patients were black, predominantly men and had hypertension (Table 1). Patients were on evidence-based therapy for systolic HF and most were New York Heart Association (NYHA) Class II/III. At the time of enrollment, the mean duration with the diagnosis of HF for these patients was 4 years (range: 1 to 237 months). The majority of patients has a mean BMI >30kg/m2. Mean creatinine was 1.7±2.3mg/dl with the MDRD GFR of 63.3±31ml/min/1.73m2. The mean adiponectin level was 15.8±15µg/ml.
Table 1.
Demographics and Clinical Characteristics of Chronic Heart Failure Patients
| Characteristics | N = 99 |
|---|---|
| Age (years) | 60 ± 13 |
| Men | 62 (63 %) |
| Black | 61 (62 %) |
| White | 35 (35 %) |
| Hispanic | 3 (3 %) |
| Body mass index (kg/m2) | 30.6 ± 6.7 |
| Duration of heart failure (months) at enrollment | 48 ± 50 |
| Systolic blood pressure (mmHg) | 125 ± 23 |
| Diastolic blood pressure (mmHg) | 72 ± 13 |
| Heart rate (beats/minute) | 75 ± 16 |
| Etiology of heart failure | |
| Ischemic | 38 (38 %) |
| Idiopathic | 31 (32 %) |
| Hypertension | 30 (30 %) |
| Diabetes mellitus | 39 (39 %) |
| Hypertension | 84 (85 %) |
| Chronic Renal Insufficiency | 45 (45 %) |
| Gout | 25 (25 %) |
| Alcohol use | |
| Active | 40 (40 %) |
| Inactive | 59 (60 %) |
| Smoker | 22 (22%) |
| New York Heart Association Functional Class | |
| I | 17 (18 %) |
| II | 40 (40 %) |
| III | 34 (34 %) |
| IV | 8 (8%) |
| Medication use | |
| Angiotensin converting enzyme inhibitor / | 87 (88%) |
| Angiotensin receptor blocker | |
| Beta-blocker | 82 (83%) |
| Digoxin | 51 (52%) |
| Spironolactone | 13 (13%) |
| Nitrates | 31 (31%) |
| Hydralazine | 9 (9%) |
| Statin | 50 (50%) |
Continuous variables are described by mean ± SD and categorical variables by percentages (in parenthesis).
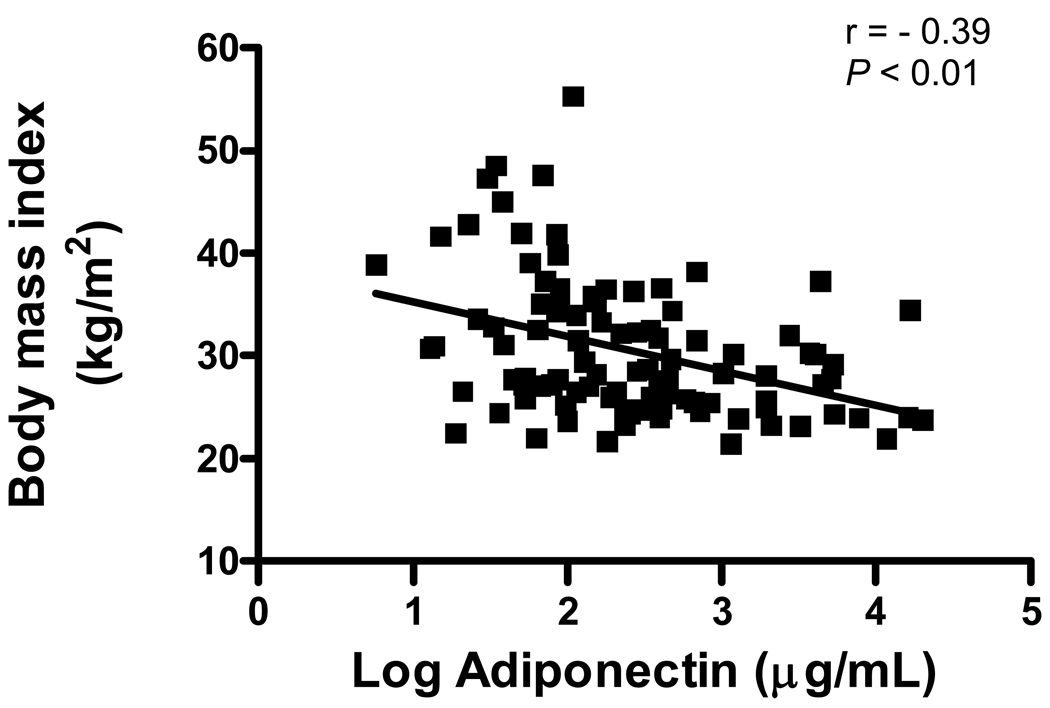
Adiponectin levels and BMI had a significant inverse correlation (Figure 1). Correlations with other clinical and echocardiography variables were nonsignificant, except for LV posterior wall and LV septal thickness (r= −0.27, P= 0.007 and r= −0.24, P= 0.016, respectively), blood urea nitrogen (BUN; r= 0.29, P= 0.003) and creatinine clearance (r= −0.31, P= 0.003). Table 2 shows the variables across adiponectin terciles. The lowest tercile of adiponectin levels was seen in the most obese patients. Similarly, adiponectin levels were significantly associated with BUN across the terciles. The other variables were not significantly associated with adiponectin.
Figure 1.
Relationship between Adiponectin levels and Body Mass Index (BMI) in Chronic Systolic Heart Failure
Table 2.
Clinical, Biochemical and Echocardiography Variables across Adiponectin Terciles (n=99)
| APN terciles | ||||
|---|---|---|---|---|
| Variable | 1 | 2 | 3 | P-value |
| (APN < 7.1 | (APN 7.1–13.6 | (APN >13.6 | ||
| µg/ml) | µg/ml) | µg/ml) | ||
| Age (years) | 57 ± 14 | 60 ± 13 | 64 ± 12 | 0.12 |
| Men | 25 (76%) | 15 (45%) | 22 (67%) | 0.45 |
| Black | 20 (61%) | 15 (45%) | 25 (76%) | 0.21 |
| Body Mass Index (kg/m2) | 34.3 ± 7.6 | 29.5 ± 6.3 | 27.9 ± 4.5 | <0.001 |
| Ischemic etiology | 14 (42%) | 14 (42%) | 10 (30%) | 0.31 |
| Diabetes mellitus | 15 (45%) | 11 (33%) | 10 (30%) | 0.20 |
| Hypertension | 25 (76%) | 26 (79%) | 29 (88%) | 0.21 |
| Creatinine (mg/dl) | 1.3 ± 0.6 | 1.4 ± 1.3 | 1.6 ± 0.9 | 0.58 |
| Creatinine clearance (mL/min) | 90 ± 43 | 87 ± 60 | 70 ± 37 | 0.18 |
| Blood urea nitrogen (mg/dl) | 25 ± 19 | 29 ± 23 | 40 ± 28 | 0.04 |
| Brain natriuretic peptide (pg/ml) | 375 ± 106 | 347 ± 254 | 421 ± 201 | 0.31 |
| Hemoglobin (g/dl) | 12.8 ± 1.6 | 12.5 ± 1.7 | 12.6 ± 1.7 | 0.73 |
| C-reactive protein (mg/l) | 10.8 ± 20 | 5.8 ± 8 | 6.5 ± 8 | 0.70 |
| Systolic blood pressure (mmHg) | 122 ± 22 | 130 ± 19 | 123 ± 28 | 0.33 |
| Diastolic blood pressure (mmHg) | 71 ± 15 | 71 ± 11 | 74 ± 15 | 0.56 |
| Echocardiography parameters | ||||
| Left atrium (mm) | 48 ± 8 | 45 ± 6 | 48 ± 6 | 0.15 |
| Ventricular septal thickness (mm) |
11 ± 2 | 10 ± 2 | 10 ± 2 | 0.14 |
| Posterior wall (mm) | 11 ± 2 | 10 ± 2 | 10 ± 2 | 0.27 |
| Left ventricular end-diastolic diameter (mm) |
64 ± 9 | 61 ± 9 | 64 ± 12 | 0.49 |
| Left ventricular end-systolic diameter (mm) |
50 ± 10 | 47 ± 11 | 53 ± 14 | 0.15 |
| Left ventricular ejection fraction (%) |
21 ± 8 | 23 ± 9 | 19 ± 10 | 0.14 |
| Left ventricular mass (g) | 300 ± 79 | 257 ± 80 | 270 ± 102 | 0.14 |
| Relative wall thickness | 0.17 ± 0.04 | 0.17 ± 0.04 | 0.16 ± 0.04 | 0.54 |
Continuous variables are described by mean ± SD and categorical variables by percentages (in parenthesis).
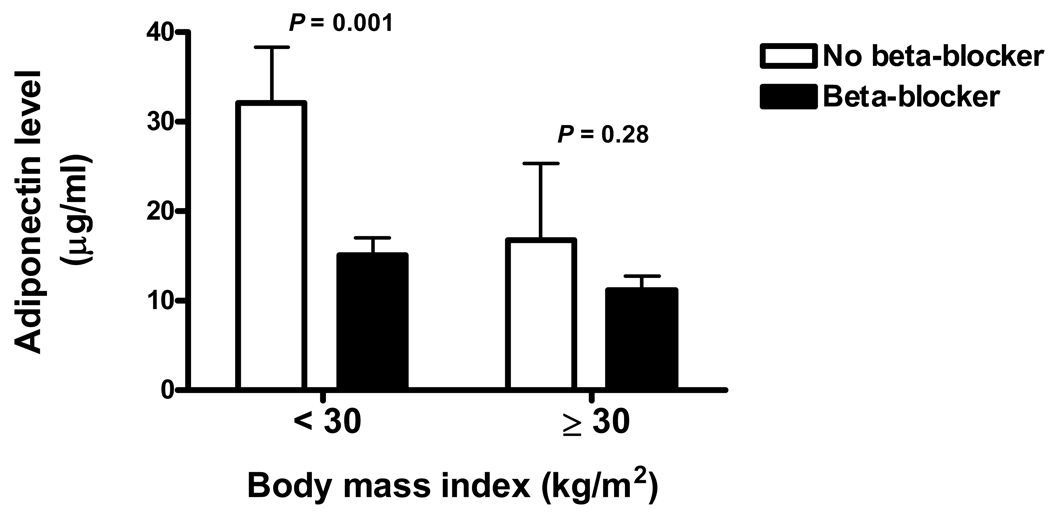
The relation between adiponectin levels and medication used was investigated (Table 3). The use of beta-blockers (metoprolol or carvedilol) was significantly associated with adiponectin levels. Patients on beta-blockers had lower adiponectin levels than those patients not on beta-blockers. As shown in Figure 2, the effect of beta-blocker therapy on adiponectin levels was most marked in non-obese patients (BMI < 30 kg/m2). As expected higher adiponectin levels were seen in non-obese patients (Figure 1).
Table 3.
Adiponectin Levels According To Medication Use
| Medication | Number of Patients | Adiponectin level (µg/ml) |
P-value |
|---|---|---|---|
| ACE-I / ARB | 87 | 15.0 ± 13.7 | 0.44 |
| Beta-blocker | 82 | 13.2 ± 11.6 | 0.02 |
| Spironolactone | 13 | 17.4 ± 20.7 | 0.63 |
| Statin | 50 | 14.3 ± 14.8 | 0.41 |
| Digoxin | 51 | 14.6 ± 12.8 | 0.43 |
Figure 2.
Effects of Beta-blocker therapy on Adiponectin levels according to Body Mass Index (BMI) in Chronic Systolic Heart Failure
In multivariate linear regression, beta-blocker use, BMI, and BUN remained significant determinants of adiponectin levels (Table 4). LV mass, structure, LVEF, gender, age and race were not correlated with adiponectin levels in multivariate analysis.
Table 4.
Determinants of Adiponectin Levels on Multivariate Analysis.
| Independent Variables | B coefficient | 95% confidence interval |
P-value |
|---|---|---|---|
| Beta-blocker use | −11.16 | −17.9, −4.4 | 0.001 |
| Body Mass Index | −.644 | −1.07, −0.22 | 0.003 |
| Blood urea nitrogen | 0.16 | 0.05, 0.27 | 0.005 |
| Left ventricular mass | −0.026 | −0.06, 0.009 | 0.15 |
| Black race | 3.97 | −1.56, 9.49 | 0.16 |
| Left ventricular ejection fraction | −21.30 | −53.28, 10.67 | 0.19 |
| Age | −0.058 | −0.28, 0.16 | 0.59 |
| Men | −0.98 | −6.76, 4.80 | 0.74 |
Discussion
Our study demonstrated that in chronic, stable systolic HF patients, beta-blocker therapy was significantly correlated with lower adiponectin levels, especially in non-obese patients. BMI and renal function were also significant determinants of adiponectin values. The impact of medications, particularly beta-blockers, should be taken into account when evaluating adiponectin levels in patients with HF, as this finding may help to explain some of the divergent results from prior studies.
Normal adiponectin levels vary by race, gender and age12–14. In normal non-obese Japanese subjects the mean value was 8.9±5.4µg/ml12; in African American subjects it was 11.1±5µg/ml13 and in Caucasian subjects the levels were somewhat higher, with value range of 11.7±5.4µg/ml14 to 17.3±10.4µg/ml15. Although adiponectin levels are not standardized and direct comparisons must be interpreted with caution, our diverse HF patient population had adiponectin levels within the normal range (mean levels were 15.8±15µg/ml) and comparable to other HF studies7;8;16. However our study differed in the following manner. The HF patients studied by Kistorp and colleagues6 had a shorter duration of HF (median duration of HF was 6 months) whereas our patients had chronic HF with the median duration of HF of 28 months. Unlike other studies6;7, the patient population in our study was heterogenous (>50% were black, almost 40% were females and were obese) and more reflective of a chronic, ambulatory HF population.
Adiponectin levels are depressed in obesity, diabetes mellitus, insulin resistance and in CAD3–5. However adiponectin levels are elevated in patients with anorexia nervosa17 and in cardiac cachexia6;7. Mean adiponectin levels were not depressed in our chronic HF population although these patients were overweight/obese. We speculate that this is possibly because of a compensatory response to the “stress” of HF – perhaps an attempt to mitigate adverse factors in LV remodeling in HF.
In normal subjects, the adult heart uses fatty acids as an energy source. This substrate is shifted from fatty acids to glucose utilization in the failing human heart. However if this shift is inadequate, the failing heart suffers from a state of chronic energy deprivation. Therefore adiponectin may be released by adipose tissue to compensate for this impaired metabolic state6;7. Adiponectin improves both glucose metabolism and insulin resistance1 via the AMPK signaling pathway18. Use of beta-blockers in HF patients may improve the metabolic profile by improving insulin sensitivity19. Interestingly, beta-blockers decrease adiponectin levels in patients with CAD20, suggesting beta-blockers may mediate effects on adiponectin levels regardless of the presence of chronic HF. However, since adiponectin levels appear to be increased in HF patients, the pathophysiological importance of beta-blockers on adiponectin levels may be accentuated in this context. The lack of correlation between adiponectin and beta-blocker in the study by Kistorp et al, may be because only 31% of the HF patients received beta-blocker and for most of these patients beta-blocker was reportedly being uptitrated6. It would be interesting in this group of patients to determine adiponectin levels in those who achieved maximal tolerated doses of beta-blockers. Yamaji et al, also showed that carvedilol administration in HF patients was accompanied by decreased adiponectin and BNP levels and associated with improved LVEF21. Thus several human studies21;22 have suggested that increased adiponectin levels in human HF reflects an attempt of the body to compensate for impaired metabolism that is evident in human HF.
Chronic exercise may mediate effects on the adrenergic system that are comparable to that of beta-blockade23. Chronic exercise training reduces adiponectin levels in patients with chronic HF and may be due to improved insulin resistance and the anti-inflammatory effects of exercise in chronic systolic HF24. Therefore alterations in adrenergic activation may underlie, at least in part, the increase in adiponectin levels seen in HF patients.
BNP levels correlate with adiponectin levels in human HF6;21 and may modulate adiponectin signaling via a cGMP-mediated pathway in human adipocytes obtained from HF patients25. Our study showed no change in BNP levels across the 3 terciles of adiponectin levels although patients in the highest tercile of adiponectin had the highest BNP levels. Similarly decreased adiponectin levels are correlated with high CRP levels in patients with obesity-linked complications26. There was no association with the adiponectin terciles in our study likely because of small numbers, although the highest CRP levels were found in the lowest tercile of adiponectin.
Adiponectin levels are increased in renal failure and have been associated with increased mortality10. This relates to impaired clearance, because kidney transplantation decreases adiponectin levels. Thus HF, like renal disease, may be a state of “adiponectin-resistance” and reflects the attempt of increased adiponectin to mitigate vascular disease triggered by risk factors27. It is possible that clearance of adiponectin is impaired in HF patients and may be due to relative changes in adiponectin receptors28 but these will require further study. The presence of “non-depressed” adiponectin levels in our study may also reflect a disconnection between adiponectin and/or a dysfunction of adiponectin receptors with a resulting increase in adiponectin secretion in HF as a compensatory response.
Adiponectin resistance has been described by a small number of studies examining human tissue18 and animal models29 but the mechanistic details have not been defined. Another possibility is that situation may be confounded in some chronic human disease states by the counter-regulation of poorly described adiponectin paralogues that may have overlapping functions with adiponectin30.
The present study has several limitations. First, the sample size is relatively small, and additional prospective studies are necessary in a larger population. However, our patient population also represents a stable, chronic HF group, which is a representative HF population. Second, we did not measure pro-inflammatory cytokines such as TNF-α and IL-6, which are associated with adverse cardiac remodeling however CRP was measured. Finally it was not our objective to demonstrate the impact of adiponectin levels or the reduction of these levels by beta-blocker therapy on clinical outcomes and HF progression. We believe that would be an interesting focus for future studies.
Acknowledgments
This work was supported by National Heart Lung and Blood Institute (NHLBI) grants, Bethesda, Maryland, USA, HL-04423 (F.Sam) and HL079099 (F.Sam). Additional support was provided by an American Heart Association (AHA) Postdoctoral Fellowship Awards, Northeast Affiliate, Baltimore, Maryland, USA; (R.Shibata) and an AHA Scientist Development Grant, Northeast Affiliate, Baltimore, Maryland, USA; (N.Ouchi).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 6.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 7.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, Grossman E, Sheps D, Keren G, Roth A. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420–1424. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haugen E, Furukawa Y, Isic A, Fu M. Increased adiponectin level in parallel with increased NT-pro BNP in patients with severe heart failure in the elderly: A hospital cohort study. Int J Cardiol. 2008;125:216–219. doi: 10.1016/j.ijcard.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Takano H, Obata Je, Kodama Y, Kitta Y, Nakamura T, Mende A, Kawabata Ki, Saito Y, Fujioka D, Kobayashi T, Yano T, Sano K, Kugiyama K. Adiponectin is released from the heart in patients with heart failure. International Journal of Cardiology. 2009;132:221–226. doi: 10.1016/j.ijcard.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 11.Biolo A, Ramamurthy S, Connors LH, O'Hara CJ, Meier-Ewert HK, Soo Hoo PT, Sawyer DB, Seldin DS, Sam F. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail. 2008;1:249–257. doi: 10.1161/CIRCHEARTFAILURE.108.788687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adiposespecific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 13.Osei K, Gaillard T, Schuster D. Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes. Obes Res. 2005;13:179–185. doi: 10.1038/oby.2005.23. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61:332–338. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 15.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Funayama H, Kubo N, Yasu T, Kawakami M, Saito M, Momomura S, Ishikawa SE. Association of hyperadiponectinemia with severity of ventricular dysfunction in congestive heart failure. Circ J. 2006;70:1557–1562. doi: 10.1253/circj.70.1557. [DOI] [PubMed] [Google Scholar]
- 17.Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 2003;58:22–29. doi: 10.1046/j.1365-2265.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE, Steinberg GR. Impaired Activation of AMP-Kinase and Fatty Acid Oxidation by Globular Adiponectin in Cultured Human Skeletal Muscle of Obese Type 2 Diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 19.Kirpichnikov D, McFarlane SI, Sowers JR. Heart failure in diabetic patients: Utility of beta-blockade. Journal of Cardiac Failure. 2003;9:333–344. doi: 10.1054/jcaf.2003.36. [DOI] [PubMed] [Google Scholar]
- 20.Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, Sinning C, Tzikas S, Keller T, Genth-Zotz S, Lackner KJ, Munzel TF, Blankenberg S. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J. 2008;29:649–657. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 21.Yamaji M, Tsutamoto T, Tanaka T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of carvedilol on plasma adiponectin concentration in patients with chronic heart failure. Circ J. 2009;73:1067–1073. doi: 10.1253/circj.cj-08-1026. [DOI] [PubMed] [Google Scholar]
- 22.Tsutamoto T, Tanaka T, Sakai H, Ishikawa C, Fujii M, Yamamoto T, Horie M. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 23.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Berendoncks AM, Beckers P, Hoymans VY, Possemiers N, Wuyts FL, Vrints CJ, Conraads VM. Exercise training reduces circulating adiponectin levels in patients with chronic heart failure. Clin Sci (Lond) 2009 Aug 6; doi: 10.1042/CS20090213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic Peptides Enhance the Production of Adiponectin in Human Adipocytes and in Patients With Chronic Heart Failure. Journal of the American College of Cardiology. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Mallamaci F, Panuccio V, Tripepi G, Cutrupi S, Parlongo S, Catalano F, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin is markedly increased in patients with nephrotic syndrome and is related to metabolic risk factors. Kidney Int Suppl. 2003:S98–S102. doi: 10.1046/j.1523-1755.63.s84.49.x. [DOI] [PubMed] [Google Scholar]
- 28.Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, Schultheiss HP, Poller W. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. Eur Heart J. 2008;29:1168–1180. doi: 10.1093/eurheartj/ehn136. [DOI] [PubMed] [Google Scholar]
- 29.Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat- fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 30.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]