Abstract
Study Objectives:
To determine the frequency of sleep disordered breathing (SDB) in ischemic and hemorrhagic stroke and transient ischemic attack (TIA) patients by meta-analysis.
Methods:
A systematic literature search using Medline, EMBASE and CINAHL and a manual review of references through December 2008 was conducted using specific search terms. The frequency of SDB stratified by apnea hypopnea index (AHI) was extracted by the author. Weighted averages using a random-effects model are reported with 95% confidence intervals.
Results:
Twenty-nine articles evaluating patients with auto-CPAP, limited-channel sleep study, or full polysomnography were included in this study. In meta-analysis of 2,343 ischemic or hemorrhagic stroke and TIA patients, the frequency of SDB with AHI > 5 was 72% and with AHI > 20 was 38%. Only 7% of the SDB was primarily central apnea. There was no significant difference in SDB prevalence by event type, timing after stroke, or type of monitoring. Males had a higher percentage of SDB (AHI > 10) than females (65% compared to 48% p = 0.001). Patients with recurrent strokes had a higher percentage of SDB (AHI > 10) than initial strokes (74% compared to 57% p = 0.013). Patients with unknown etiology of stroke had a higher and cardioembolic etiology a lower percentage of SDB than other etiologies.
Conclusions:
SDB is very common in stroke patients irrespective of type of stroke or timing after stroke and is typically obstructive in nature. Since clinical history alone does not identify many patients with SDB, sleep studies should be considered in all stroke and TIA patients.
Citation:
Johnson KG; Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010;6(2):131-137.
Keywords: Stroke prevention, all cerebrovascular disease/strok, sleep apne, prevalence studies, screening in epidemiology
In the US, approximately 795,000 people have strokes and 135,000 people die from stroke complications each year, making it the third most common cause of death. There are an estimated 5.6 million stroke survivors receiving acute and long-term care costing $68.9 billion per year.1 Aggressive treatment of risk factors in stroke patients, including hypertension, atrial fibrillation, and carotid stenosis, is widely accepted. Obstructive sleep apnea (OSA) has been shown to increase the risk of these and other stroke risk factors.2 Evaluation for OSA is not routinely recommended as part of a stroke workup3 despite growing evidence that OSA is an independent risk factor for stroke, that treating OSA improves recovery from stroke, and that treating severe OSA decreases cardiovascular morbidity and mortality.2
BRIEF SUMMARY
Current Knowledge/Study Rationale: Studies of the prevalence of sleep disordered breathing (SDB) in stroke and transient ischemic attack (TIA) patients use varying methods and have wide variations ranging form 30-80%. There is growing evidence that obstructive sleep apnea affects stroke morbidity and mortality, so a more precise estimate of this stroke risk factor is important.
Study Impact: This study provides a meta-analysis of such studies, finding that obstructive sleep apnea is very common among patients with TIA and stroke, and is more common among patients with strokes of unknown origin and those with recurrent strokes. The findings of this study support screening for SDB in all stroke and TIA patients.
The community prevalence of sleep disordered breathing (SDB) has been reported to range from 8% to 57% in men and 9% to 35% in women (30-70 years) and between 12% to 81% in the elderly (65–100 years).4 In studies of frequency of OSA in stroke patients, there similarly has been a wide variability in the reported frequencies of OSA ranging from about 30% to 80%.5 Approximately 30% of stroke patients with severe OSA would be missed if clinical history alone were used to screen for OSA.6 There are currently no guidelines about whether stroke patients should be routinely evaluated for the presence of OSA or how they should be evaluated.3 The aim of this study is to provide an estimate of the frequency of OSA in stroke patients, and to determine what factors may influence its frequency. A meta-analysis of studies of patients with acute strokes or TIAs who were tested for OSA with sleep studies was performed to generate this information.
RESEARCH DESIGN AND METHODS
Data Extraction
A Medline, EMBASE, and CINAHL search was performed through December 2008 using the terms apnea, obstructive, sleep, TIA, and stroke. Further articles were found by reviewing bibliographies of these studies. Non-English language articles and abstracts were excluded. If there was a question of duplicative data, authors were contacted to determine whether there was overlap of patients. Studies were excluded if oximetry alone or snoring history was used to define SDB instead of sleep studies. The first author performed the searches and reviewed the initial citations. The second author reviewed all studies that involved testing for sleep apnea in stroke patients to confirm the exclusions and review the methodology.
The percentage of patients with sleep disordered breathing determined by AHI criteria of > 5, > 10, > 20, > 30, and > 40, as well as the percentage of patients with primarily central apnea was determined. The studies were analyzed for age, BMI, gender differences, differences between TIA, ischemic stroke and hemorrhagic stroke patients, initial stroke compared to recurrent stroke events, time from stroke onset to testing, inpatient compared to rehab setting, effect of stroke localization, and presenting symptoms. When appropriate, average characteristics of studies were reported as mean ± standard deviation.
The studies were analyzed for sleep study type including full polysomnography (PSG) with EEG, limited-channel without EEG or auto-CPAP in diagnostic mode. The method of testing included thermistor, pressure transducer, effort band, or auto-CPAP device. Most studies defined apnea as > 10 sec of no airflow, with 2 studies also using > 10 sec of no airflow associated with a 4% oxygen desaturation. Hypopnea was variably defined with most studies using a 50% decrease in airflow and/or effort either alone or 30% to 50% decrease with arousal and/or 3% or 4% oxygen desaturation. AHI is the number of apneas and hypopneas per hour of sleep for full PSG and per hour of study duration for limited-channel studies in which sleep stage was not determined.
Statistical Analysis
Composite estimates of the percentage of patients with SDB (effect size) were computed for all studies combined, and separately for studies grouped according to several criteria. The criteria included event type, first or recurrent event, sex, study location, study timing, stroke etiology, study type, testing method, and hypopnea criteria. The Q-test was used to test for significant heterogeneity of percentage of patients with SDB according to AHI cut-off points among studies. In the presence of significant heterogeneity of variance among groups, a random effects model was used.7 It gives a more conservative estimate of the variability in treatment effect (larger standard error and wider 95% confidence intervals) than the fixed effects method. A funnel plot was done to evaluate for possible publication bias.
All analyses were conducted using Comprehensive Meta-Analysis software.8 Analyses were performed to determine if there were differences among subgroups of studies according to various criteria. A random effects model was used to combine studies within each subgroup. A variant of the Q-statistic was used to assess the difference in percentage of patients with AHI > 10 between subgroups of studies. The study-to-study variance (tau-squared) was not assumed to be the same for all subgroups—this value was computed within subgroups and not pooled across subgroups. Composite estimates of percentage of patients with AHI > 10 and heterogeneity statistics were derived for each subgroup. Meta-regression using a random effects model was used to test whether age or BMI correlated with the proportion of patients with AHI > 10. Chi square with Yate's correction was used to compare groups.
RESULTS
The initial search identified 1,239 possible papers. Fifty-two articles reported prevalence of SDB in stroke or TIA patients. Exclusions included eleven studies with duplicative data, 2 non-English language studies, 3 studies testing with oximetry, 5 studies defining OSA by snoring, one abstract, and one study which did not report AHI cut-offs. Twenty-nine studies including 2,343 patients evaluating patients with full PSG with EEG (n = 11), limited channel sleep study (n = 13), or auto-CPAP in diagnostic mode (n = 5) were included.4–6,9–35 A funnel plot (data not shown) did not suggest publication bias.
Table 1 summarizes the study characteristics. Twenty studies evaluated patients who were admitted to an acute stroke unit, 7 evaluated patients admitted to rehabilitation units, one to either, and one study evaluated a stroke cohort. The timing of sleep study after stroke onset was within one week in 16 studies, 7-28 days in 8 studies (some patients in this group were studied within a week), and over 28 days in 5 studies, including one study approximately 3 years after stroke onset. A total of 2,151 patients had ischemic strokes, 104 had TIAs, and 87 had hemorrhagic strokes. Of the 26 studies reporting the percentage of females studied, on average 35.4% ± 11.3% (range 14% to 58%) were female. The average age was 65 ± 6 years and average BMI was 26.4 ± 1.7 kg/m2. Using a linear meta-regression, there was a trend toward increasing frequency of AHI > 10 with increasing average age (p = 0.056). Average BMI had no effect (p = 0.14).
Table 1.
Study characteristics
| Study & Year, Country | N (% female) | Stroke Type (% recurrent) | % of Eligible | Location | Study Type/ Test method | Hypopnea Criteria | Time After CVA | Age y | BMI kg/ m2 |
|---|---|---|---|---|---|---|---|---|---|
| Bassetti 1999, USA | 80 (38) | I, T (11) | 63% | Stroke Unit | F/Thermistor | Arousal | 9 d | 60 | 29 |
| Bassetti 2006, Switzerland | 152 (32) | I (-) | - | Stroke Unit | A/Pressure | Flow | 3 ± 2 d | 56 | 26 |
| Broadley 2007, Australia | 55 (42) | I, H (-) | 68% | Stroke Unit | L/ Pressure | Flow | 2 d | 71 | 27 |
| Brown 2008, USA | 30 (33) | I (27) | - | Stroke – Rehab | F/ Pressure | Arousal | < 7 d | 67 | - |
| Cadhilac 2005, Australia | 78 (32) | I, H (19) | 29% | Home | L/ Pressure | Flow | 3 y | 64 | 28 |
| Disler 2002, Australia | 38 (-) | I, H (-) | - | Rehab Unit | A/ Pressure | Flow | 7-28 d | 65 | - |
| Dyken 1996, USA | 24 (46) | I, H (-) | 56% | Stroke Unit | F/ Pressure | Desaturation | 14-35 d | 65 | 28 |
| Dziewas 2005, Germany | 102 (33) | I (25) | - | Stroke Unit | L/ Thermistor | Effort | < 3 d | 65 | 26 |
| Dziewas 2007, Germany | 214 (34) | I (0) | - | Stroke Unit | L/ Thermistor | Effort | < 3 d | 66 | 26 |
| Dziewas 2008, Germany | 55 (24) | I (0) | - | Stroke Unit | L/ Thermistor | Effort | < 3 d | 65 | 26 |
| Harbison 2003, UK | 78 (49) | I, H (18) | - | Stroke Unit | A/ Pressure | Flow | 7-14 d | 73 | - |
| Hermann 2007, Switzerland | 31 (36) | I (0) | - | Stroke Unit | F/ Thermistor | Arousal | 5 d | 50 | - |
| Hsu 2006, UK | 66 (32) | I, H (9) | 10% | Stroke Unit | L/ Pressure | Flow-50 | 14-19 d | 72 | 26 |
| Hui 2002, China | 51 (45) | I (-) | 80% | Stroke Unit | F/ Pressure | Arousal | 3 d | 64 | 24 |
| Iranzo 2002, Spain | 50 (40) | I (0) | - | Stroke Unit | F/ Thermistor | Arousal-E | 1 d | 67 | 26 |
| Kaneko 2003, Canada | 60 (39) | I, H (18) | 98% | Rehab Unit | F/ Effort Band | Effort | 44 ± 3 d | 66 | 28 |
| Martinez 2005, Spain | 95 (-) | I, T (-) | 68% | Rehab Unit | A/ Pressure | Flow | 64 ± 11 d | 73 | 27 |
| Mohsenin 1995, USA | 10 (20) | I (-) | 59% | Rehab Unit | F/ Thermistor | Desaturation | < 1 y | 56 | 26 |
| Nachtmann 2003, Germany | 235 (30) | I (25) | 59% | Rehab Unit | F/ Thermistor | Arousal | 27 d | 66 | - |
| NorAdina 2006, Malaysia | 28 (29) | I (11) | 31% | Stroke Unit | A/ Pressure | Flow | 7-28 d | 60 | 23 |
| Palombini 2006, USA | 21 (-) | I (-) | 42% | Stroke Unit | F/ Pressure | Desaturation | < 30 d | 62 | 23 |
| Parra 2000, Spain | 161 (49) | I, T, H (0) | - | Stroke Unit | L/ Thermistor | Desaturation | 2-3 d | 72 | 27 |
| Rola 2007, Poland | 70 (14) | I, T (-) | - | Stroke Unit | L/ Pressure | Flow-50 | < 7 d | 66 | 28 |
| Sandberg 2001, Sweden | 133 (59) | I, H (39) | 88% | Rehab Unit | L/ Thermistor | Des-body-pulse | 23 d | 77 | 24 |
| Selic 2005, Switzerland | 41 (20) | I (-) | 91% | Stroke Unit | L/ Pressure | Flow-50 | 1 d | 63 | 27 |
| Siccolli 2008, Switzerland | 74 (34) | I (0) | - | Stroke Unit | L/ Pressure | Flow-50 | < 3 d | 63 | 28 |
| Turkington 2002, UK | 120 (58) | I (27) | 82% | Stroke Unit | L/ Thermistor | Desaturation | 1 d | 79 | 24 |
| Wessendorf 2000, Germany | 147 (35) | I (0) | 86% | Rehab Unit | F/ Thermistor | Arousal | 46 ± 20 d | 61 | 28 |
| Wierzbicka 2006, Poland | 43 (19) | I, T (-) | - | Stroke Unit | L/ Pressure | Desaturation | < 7 d | 69 | 28 |
- refers to results not available. Stroke type: H, Hemorrhagic; I, Ischemic; T, Transient ischemic attack. Study Type: F, Full PSG (including EEG); L, Limited (without EEG); A, Auto-CPAP diagnostic mode. Test method: Pressure, nasal pressure transducer. Hypopnea Criteria: Arousal, flow reduction with arousal or oxygen desaturation (of at least 3-4%); Arousal-E, thoracic/abdominal reduction with arousal or desaturation; Desaturation, flow reduction with desaturation; Des-body-pulse, flow reduction with desaturation, body movement or pulse change; Effort, effort band reduction or thoracic/abdominal reduction alone; Flow, flow reduction alone; Flow-50, flow reduction at least 50% or flow reduction at least 30% with desaturation or arousal (if full PSG). Time: d, day; y, year.
In most studies, patients were excluded if there was a serious medical condition, dementia or confusion, or if consent could not be obtained. Eight studies excluded patients with known OSA12,22,24,26,29 or prior CPAP use.9,11,23 Of the 17 studies which listed how many patients were excluded, the average inclusion rate was 64% ± 26% (range 10% to 100%).
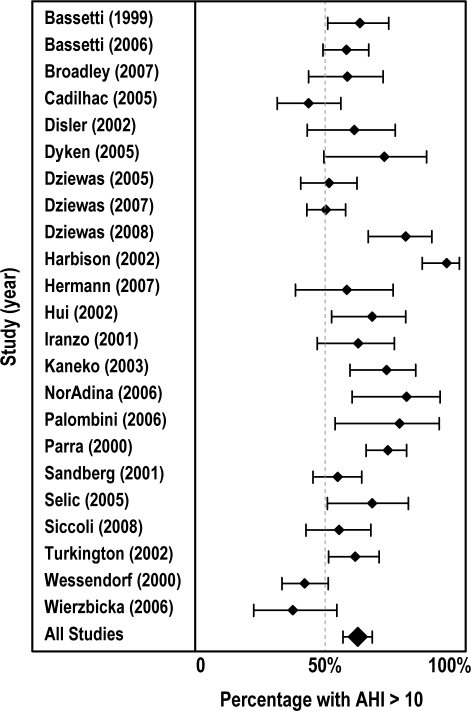
Table 2 summaries the frequency of SDB in each individual study. Figure 1 shows a forest plot (mean with 95% confidence intervals) of all studies showing the proportion of patients with an AHI > 10. There was significant heterogeneity among studies (Q = 94.38, df = 22, p < 0.001), validating the choice of a random effects model for the meta-analysis. Table 3 summarizes the frequency of SDB after stroke or TIA according to different AHI cut-off points. The frequency of SDB in stroke and TIA patients ranged from 72% (95% CI 60–81) for AHI > 5 to 14% (95% CI 7–25) for AHI > 40. Seventeen of the studies reported the percentage of patients with primarily central apneas or Cheyne Stokes respiration (7% [95% CI 4.5–12.0]).
Table 2.
Percentage of patients with SDB by AHI cut-off points
| Study – Year | > 5 | > 10 | > 20 | > 30 | > 40 |
|---|---|---|---|---|---|
| Bassetti 1999 | 63 | ||||
| Bassetti 2006 | 58 | 31 | 17 | ||
| Broadley 2007 | 58 | ||||
| Brown 2008 | 73 | ||||
| Cadhilac 2005 | 81 | 45 | 18 | 8 | |
| Disler 2002 | 61 | ||||
| Dziewas 2005 | 52 | ||||
| Dziewas 2007 | 51 | ||||
| Dziewas 2008 | 78 | ||||
| Dyken 1996 | 83 | 71 | 46 | 42 | 29 |
| Harbison 2003 | 92 | 67 | 46 | ||
| Herman 2007 | 58 | ||||
| Hsu 2006 | 50 | ||||
| Hui 2002 | 67 | 49 | 31 | ||
| Iranzo 2002 | 62 | 46 | |||
| Kaneko 2003 | 72 | ||||
| Martinez 2005 | 54 | ||||
| Mohsenin 1995 | |||||
| Nachtmann 2003 | 43 | 25 | |||
| NorAdina 2006 | 93 | 79 | 39 | ||
| Palombini 2006 | 76 | ||||
| Parra 2000 | 72 | 47 | 28 | 11 | |
| Rola 2007 | 66 | ||||
| Sandberg 2001 | 77 | 55 | 37 | 21 | 8 |
| Selic 2005 | 69 | 29 | |||
| Siccolli 2008 | 55 | ||||
| Turkington 2002 | 80 | 61 | 30 | ||
| Wessendorf 2000 | 61 | 43 | 22 | ||
| Wierzbicka 2006 | 63 | 40 | 21 | ||
Figure 1.
Forest plot of percentage of patients with AHI > 10 with 95% confidence intervals.
Table 3.
Percentage of stroke or TIA patients with SDB stratified by AHI
| Cutpoint | # Studies (# patients) | % (95% CI) |
|---|---|---|
| AHI > 5 | 9 (908) | 72 (60–81) |
| AHI > 10 | 24 (1980) | 63 (58–68) |
| AHI > 20 | 15 (1405) | 38 (31–46) |
| AHI > 30 | 10 (865) | 29 (21–37) |
| AHI > 40 | 3 (318) | 14 (7–25) |
| Central* | 17 (1286) | 7 (5–12) |
Percentage of patients who had primarily central apnea
Table 4 shows the results of the group comparisons according to nine dimensions: event type, first or recurrent stroke, sex, patient location, timing of sleep study after stroke, stroke etiology, sleep study type, testing method, and hypopnea criteria. There were significant differences in percentage of patients with AHI > 10 in 4 dimensions: initial or recurrent stroke, sex, patient location, and stroke etiology. The percentage of patients with AHI > 10 was higher in patients with recurrent stroke (73%) than first stroke (57%) (p = 0.013). More males than females had AHI > 10 (65% vs 48% p = 0.001). The percentage of patients with AHI > 10 was higher in the studies of stroke units (64%) and rehabilitation units (57%) than the one stroke cohort (45%) (p = 0.009).
Table 4.
Percentage of patients with AHI > 10 by subgroups
| Dimension | Group | # Studies | % AHI > 10 (95% CI) | p-value |
|---|---|---|---|---|
| Overall | All Studies | 23 | 62 (57–67) | |
| Event type | Ischemic Stroke | 3 | 61 (42–78) | 0.489 |
| TIA | 3 | 52 (30–73) | ||
| Hemorrhagic Stroke | 4 | 71 (50–85) | ||
| Prior event | First | 9 | 57 (49–64) | 0.013 |
| Recurrent | 3 | 74( 63–82) | ||
| Sex | Female | 12 | 48 (40–56) | 0.001 |
| Male | 12 | 65 (59–71) | ||
| Patient location | Cohort | 1 | 45 (34–56) | 0.009 |
| Rehab Unit | 4 | 57 (45–68) | ||
| Inpatient Unit | 18 | 64 (58–69) | ||
| Timing of study after CVA | < 7 days | 13 | 60 (55–65) | 0.128 |
| 7-28 days | 7 | 72 (60–81) | ||
| > 28 days | 3 | 53(37–69) | ||
| Stroke etiology | Cardioembolic | 3 | 48 (41–56) | 0.005 |
| Macrovascular | 3 | 71 (49–86) | ||
| Microvascular | 3 | 61 (46–74) | ||
| Other | 3 | 66 (31–90) | ||
| Unknown | 3 | 82 (66–91) | ||
| Study type | Full PSG (with EEG) | 8 | 63 (54–71) | 0.226 |
| Limited (without EEG) | 11 | 58 (51–64) | ||
| AutoCPAP | 4 | 75 (54–88) | ||
| Testing method | Pressure Transducer | 8 | 58 (50–66) | 0.319 |
| Thermistor | 10 | 59 (53–66) | ||
| Auto-CPAP | 4 | 75 (54–88) | ||
| Hypopnea criteria | Arousal or desat with reduced flow | 5 | 58 (48–68) | 0.860 |
| Desaturation with reduced flow | 5 | 63 (51–75) | ||
| Effort reduction | 4 | 63 (50–74) | ||
| Flow reduction alone | 6 | 67 (52–79) | ||
| Flow-50 | 2 | 60 (49–70) | ||
CI, confidence interval. The p-values indicate whether any of the subgroups had a statistical effect on the percentage of patients with AHI > 10 determined with a random effects model.Hypopnea criteria: Flow-50, flow reduction at least 50% or flow reduction at least 30% with desaturation or arousal (if full PSG).
There were limited data available to assess whether the frequency of SDB was affected by stroke etiology or stroke location. Three studies reported the frequency of SDB according to stroke etiology including macrovascular, microvascular, cardioembolic, unknown, and other.9,13,14 There was a significantly higher percentage of patients with AHI > 10 if the cause was unknown (82%) and lower percentage (48%) if the cause was cardioembolic (p = 0.005)
The type of stroke event did not affect the percentage of patients with an AHI > 10. Only 3 of the 5 studies including TIA patients6,28,29 and 4 of 9 studies including patients with hemorrhagic stroke patients10,22,28,30 reported the proportion of patients with AHI > 10. There was no difference in AHI frequency among event types: 71% of hemorrhagic stroke patients, 61% of ischemic stroke patients, and 52% of TIA patients had AHI > 10 (p = 0.548).
Only 3 studies reported the frequency of SBD differentiating brainstem and hemispheric location.16,22,28 There was no difference in percentage of patients with AHI > 10 in the 23 patients with brainstem strokes (83%) compared to the 109 patients with hemispheric strokes (73%) (p = 0.449). Five additional studies also found no difference in SDB frequency when stroke location was evaluated but did not report the data.20,21,30,33,35
The prevalence of snoring in stroke patients was reported by 6 studies.6,9,21,23,26,34 Fifty-three percent of 231 patients without SDB snored, while 73% of 371 patients with SDB had a history of snoring (p < 0.001). The relative risk of snoring in SDB patients was 1.39.
DISCUSSION
This meta-analysis found that SDB is present in up to 72% of ischemic and hemorrhagic stroke and TIA patients. The SDB was primarily obstructive in nature, with only 7% of patients having primarily central apneas. SDB was more common in male patients, those with recurrent strokes and those with strokes of unknown etiology. SDB was less common among those with strokes of cardioembolic etiology. Other factors, including stroke event type, patient location, timing, and testing methods after stroke, did not affect the percentage of patients with OSA.
The SDB frequency was high in stroke patients despite a relatively low average BMI of 26.4 kg/m2. The frequency of OSA found in this study was very similar to rates found in a community-based study of adults with age greater than 65 (62% with AHI > 10)36; however, it was significantly higher than the rates of OSA found in younger adults.4 This meta-analysis found a trend toward increasing average age in the study population being associated with increasing rates of SDB. Several of the individual studies reported a significant relationship between increasing age and OSA frequency.9,13,14,15,35 There are no studies of the frequency of OSA in stroke patients broken down by age groups. However, it is likely that younger stroke patients would have a higher OSA frequency than a similar aged non-stroke population.
This analysis found no significant difference between the different stroke types, although there was limited data on hemorrhagic stroke and TIA patients. There was no difference in OSA frequency with the timing of the sleep study after stroke onset. In 2 studies that found a decrease in SDB with time, there was either a decrease in central apneas28 or there was improvement in OSA only in patients with dysphagia.37 One factor that may increase the frequency of OSA in acute stroke patients is the increase in supine positioning.11,15 Because the stability of OSA after stroke and the similar rates in TIA and stroke patients, it has been previously proposed that OSA precedes stroke onset.28 Another argument that OSA precedes stroke onset is that brainstem and hemispheric strokes have no differences in rates of OSA.
In this meta-analysis, unknown cause for stroke was associated with higher frequencies of SDB and cardioembolic cause a lower frequency of SDB. In other studies which did not provide data, but commented on whether stroke etiology was a factor, most reported no relationship between etiology and the frequency of SDB. Given that OSA has multiple mechanisms2 by which it can increase stroke risk including causing endothelial damage, increasing hypertension, increasing right to left shunting through patent foramen ovale,38 increasing hypercoagulability, and increasing cardiac arrhythmias, it is not surprising that there is no difference in frequency in patients with microvascular, macrovascular, or other causes of stroke. That patients with an unknown cause of stroke had increased rate of SDB suggests that OSA may be the cause in some of these patients. That patients with a cardioembolic cause of stroke had a lower rate of SDB suggests that many of the emboli were not related to OSA, but to other factors such as atrial fibrillation.
The variability in the rates of SBD in the studies may be related to several factors including whether the patients were in inpatient or rehabilitation settings, exclusion criteria, testing method, scoring method, type of stroke, sex, age, and BMI. However only male sex and recurrent strokes were associated with statistically higher frequencies of SDB. The studies were done in 12 different countries, which may be another factor. One study with higher rates of SDB may be related to the use of auto-CPAP and an older mean age.17 However in the subanalyses, the use of auto-CPAP for diagnosis and age did not affect SDB frequency.
While there was no evidence of publication bias affecting the overall percentages, some of the subanalyses are likely affected. For example, there was a difference in the 3 studies that reported the frequency of SDB in patients with first compared to recurrent stroke, while 2 studies that did not report data commented that there was no significant difference found.22,33
The reported frequencies likely underestimate the actual prevalence of SDB in stroke patients. Most of the studies included patients consecutively admitted to stroke or rehabilitation units, but excluded patients if they had severe medical conditions, died prior to sleep study, or were unable to consent. Eight studies excluded patients with known OSA. One study with lower rates of SDB used a stroke cohort 3 years after stroke onset and only included 29% of the original cohort.4 It is possible that increased morbidity and mortality caused by SDB in stroke patients caused fewer of these patients to be included.
While some symptoms such as snoring, daytime sleepiness and witnessed apneas may increase the likelihood of the patient having OSA, a significant number of patients will be missed if history alone is used for screening. In this meta-analysis, over 25% of patients with SDB did not snore while over 50% of patients without SDB did snore. Bassetti et al. found that only 70% patients with AHI > 30 and 32% of patients with AHI 10–30 had clinically probable OSA.6
While large randomized trials of the effects of OSA on stroke morbidity and mortality are not available, there is a growing body of literature. OSA has been shown to increase the risk of stroke and multiple stroke risk factors including hypertension and atrial fibrillation.2 Morbidity after stroke is worsened with more depression, delirium and ADL dependence30 and lower functional capacity and longer hospitalization.22 In a 10-year follow-up of stroke patients, there was a higher risk of death in patients with OSA (adjusted hazard ratio 1.76).39
Several studies have evaluated the use of CPAP in stroke patients with OSA. One randomized trial did not find any functional improvement after stroke, but it was limited by poor CPAP compliance.19 Another study found a benefit of CPAP in reducing cardiovascular events after stroke.23 A third study found treatment with CPAP in stroke patients with AHI > 20 reduced 5-year mortality from stroke and all cardiovascular and noncardiovascular causes compared to the 70% of patients intolerant to CPAP. 40 Similar benefit was found in preventing cardiovascular events in primary prevention.41
More studies are needed to clarify the relationship between OSA and stroke and TIA patients. There have been no studies specifically studying the frequency of OSA in younger stroke patients, especially with unknown causes of stroke, to see if there is a higher prevalence of OSA that might explain the stroke etiology. Further randomized trials of CPAP in acute stroke patients are needed to clarify the benefits on recovery from the initial event. Studies in both mild-moderate as well as severe sleep apnea are needed. CPAP trials in TIA patients are needed to assess the benefits in secondary prevention. The challenge of these studies will be to determine the optimal methods of diagnosing OSA and quickly treating patients since the risk of stroke is the highest in the first 30 days.
This meta-analysis found that obstructive sleep apnea is very common in patients with TIA and stroke, is more common in patients with recurrent stokes and those with strokes of unknown etiology, and is less common in patients with strokes of cardioembolic etiology. Multiple testing methods including full polysomnography with EEG, limited-channel devices and auto-CPAP devices can be used to diagnose sleep apnea in stroke patients. Given the high frequency of OSA in stroke patients, the effects of OSA on stroke morbidity and mortality, and the improvements with CPAP therapy, screening for OSA in all stroke and TIA patients may be warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank Jane Garb for providing statistical analysis.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Bradley TD, Floras JS. Obstructive sleep apnea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 3.Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient. A scientific statement from the American Heart Association. Stroke. 2009;40:2911–44. doi: 10.1161/STROKEAHA.109.192362. [DOI] [PubMed] [Google Scholar]
- 4.Cadilhac DA, Thorpe RD, Pearce DC, et al. Sleep disordered breathing in chronic stroke survivors. A study of the long term follow-up of the SCOPES cohort using home based polysomnography. J Clin Neurosci. 2005;12:632–37. doi: 10.1016/j.jocn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Hui DS, Choy DK, Wong LK, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in Chinese patients with first-ever ischemic stroke. Chest. 2002;122:852–60. doi: 10.1378/chest.122.3.852. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trails. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Borenstein M, Hedges L, et al. Comprehensive meta-analysis. Englewood NJ: 2005. Biostat. [Google Scholar]
- 9.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–72. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 10.Broadley SA, Jorgensen L, Cheek A, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–33. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Brown D, Lisabeth L, Zupancic M, et al. High prevalence of supine sleep in ischemic stroke patients. Stroke. 2008;39:2511–4. doi: 10.1161/STROKEAHA.107.513572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disler P, Hansford A, Wright P, et al. Diagnosis and treatment of obstructive sleep apnea in a stroke rehabilitation unit: A feasibility study. Am J Phys Med Rehab. 2002;81:622–5. doi: 10.1097/00002060-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Dziewas R, Humpert M, Homann B, et al. Increased prevalence of sleep apnea in patients with recurring ischemic stroke compared with first stroke victims. J Neurol. 2005;252:1394–8. doi: 10.1007/s00415-005-0888-7. [DOI] [PubMed] [Google Scholar]
- 14.Dziewas R, Ritter M, Usta N, et al. Atherosclerosis and obstructive sleep apnea in patients with ischemic stroke. Cerebrovasc Dis. 2007;24:122–6. doi: 10.1159/000103611. [DOI] [PubMed] [Google Scholar]
- 15.Dziewas R, Hopmann B, Humpert M, et al. Positional sleep apnea in patients with ischemic stroke. Neurol Res. 2008;30:645–8. doi: 10.1179/174313208X289598. [DOI] [PubMed] [Google Scholar]
- 16.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 17.Harbison J, Gibson GJ, Birchall D, Zammit-Maempel I, Ford GA. White matter disease and sleep-disordered breathing after acute stroke. Neurology. 2003;61:959–63. doi: 10.1212/01.wnl.0000086818.57992.b8. [DOI] [PubMed] [Google Scholar]
- 18.Hermann D, Siccoli M, Kirov P, et al. Central periodic breathing during sleep in acute ischemic stroke. Stroke. 2007;38:1082–4. doi: 10.1161/01.STR.0000258105.58221.9a. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–9. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iranzo A, Santamaria J, Berenguer J, Sanchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58:911–6. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Garcia MA, Galiano-Blancart R, Roman-Sanchez P, Soler-Cataluna JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128:2123–9. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- 24.Mohsenin V, Valor Sleep apnea in patients with hemispheric stroke. Arch Phys Med Rehabil. 1995;76:71–6. doi: 10.1016/s0003-9993(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 25.Nachtmann A, Stang, A, Wang, Y, et al. Association of obstructive sleep apnea and stenotic artery disease in ischemic stroke patients. Atherosclerosis. 2003;169:301–7. doi: 10.1016/s0021-9150(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 26.Noradina AT, Hamidon BB, Roslan H, Raymond AA. Risk factors for developing sleep-disordered breathing in patients with recent ischaemic stroke. Singapore Med J. 2006;47:392–9. [PubMed] [Google Scholar]
- 27.Palombini L, Guillemault C. Stroke and treatment with nasal CPAP. Eur J Neuro. 2006;13:198–200. doi: 10.1111/j.1468-1331.2006.01169.x. [DOI] [PubMed] [Google Scholar]
- 28.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–80. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 29.Rola R, Wierzbicka A, Wichniak A, et al. Sleep related breathing disorders in patients with ischemic stroke and transient ischemic attacks: respiratory and clinical correlations. J Physiol Pharmacol. 2007;58(Pt 2) Suppl 5:575–82. [PubMed] [Google Scholar]
- 30.Sandberg O, Franklin KA, Bucht G, Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Soc. 2001;49:391–7. doi: 10.1046/j.1532-5415.2001.49081.x. [DOI] [PubMed] [Google Scholar]
- 31.Selic C, Siccoli MM, Hermann DM, Bassetti CL. Blood pressure evolution after acute ischemic stroke in patients with and without sleep apnea. Stroke. 2005;36:2614–8. doi: 10.1161/01.STR.0000189689.65734.a3. [DOI] [PubMed] [Google Scholar]
- 32.Siccoli M, Valko P, Hermann D, et al. Central periodic breathing during sleep in 74 patients with acute ischemic stroke- Neurogenic and cardiogenic factors. J Neurol. 2008;255:1687–92. doi: 10.1007/s00415-008-0981-9. [DOI] [PubMed] [Google Scholar]
- 33.Turkington PM, Bamford J, Wanklyn P, Elliott MW. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke. 2002;33:2037–42. doi: 10.1161/01.str.0000023576.94311.27. [DOI] [PubMed] [Google Scholar]
- 34.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 35.Wierzbicka A, Rola R, Wichniak A, Richter P, Ryglewicz D, Jernajczyk W, et al. The incidence of sleep apnea in patients with stroke or transient ischemic attack. J Physiol Pharmacol. 2006;57(Suppl 4):385–90. [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Garcia MA, Galiano-Blancart R, Soler-Cataluna JJ, Cabero-Salt L, Roman-Sanchez P. Improvement in nocturnal disordered breathing after first-ever ischemic stroke: role of dysphagia. Chest. 2006;129:238–45. doi: 10.1378/chest.129.2.238. [DOI] [PubMed] [Google Scholar]
- 38.Beelke M, Angeli S, Del Sette M, et al. Obstructive sleep apnea can be provocative for right-to-left shunting through a patent foramen ovale. Sleep. 2002;25:21–7. [PubMed] [Google Scholar]
- 39.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 41.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]