Abstract
We report the case of an 88-year-old man with Alzheimer's disease (AD) of 8 years duration (emerging shortly after the de novo onset of sleeptalking) who developed REM sleep behavior disorder (RBD) after increasing the nightly dose of rivastigmine, an acetylcholinesterase inhibitor, from 1.5 mg to 3 mg (total daily dose, 4.5 mg), as therapy for his dementia. His family then became aware of recurrent nocturnal episodes arising from sleep of his leaving bed, and he sustained multiple abrasion injuries from falling down. Polysomnography (PSG), utilizing a seizure montage with fast paper speed, conducted with the patient taking rivastigmine 3 mg at bedtime, documented 3 abrupt episodes of bilateral arm-waving with moaning and shouting that emerged exclusively during each of the 3 REM sleep periods, with the duration of the episodes lasting 8 to 25 seconds. No epileptiform discharge appeared with the onset of these REM sleep behaviors. Therapy with clonazepam, 0.5 mg at bedtime (with ongoing 3 mg bedtime and 4.5 mg total daily rivastigmine therapy), fully suppressed the sleep-related events, with prompt relapse whenever clonazepam was not taken. This is the second reported case (both males with AD) of rivastigmine-induced RBD, and the oldest reported case of RBD; and it represents reversible, medication-induced, acute RBD.
Citation:
Yeh SB; Yeh PY; Schenck CH. Rivastigmine-induced rem sleep behavior disorder (RBD) in a 88-year-old man with alzheimer's disease. J Clin Sleep Med 2010;6(2):192-195.
Keywords: REM sleep behavior disorder, RBD, acute RBD, parasomnia overlap disorder, sleep injury, parasomnia, dementia, Alzheimer's disease, rivastigmine, cholinergic therapy, polysomnography, clonazepam
RBD is usually a chronic parasomnia affecting older males,1 but can emerge acutely, triggered by medications, drug/alcohol withdrawal states, relapsing multiple sclerosis, and severe stress.2 Although cholinergic therapy of Alzheimer's disease (AD) can induce RBD,3 cholinergic therapy of idiopathic RBD with the acetylcholinesterase inhibitors (AIs) donepezil or rivastigmine can be therapeutic.4,5 RBD in a 62-year-old man was induced by rivastigmine (SDZ-ENA 713) during a phase III clinical trial, at a dose of 8 mg daily.3 In contrast, donepezil was effective in treating RBD in 3 male cases aged 29–72 y (2 with PSG) at doses of 10-15 mg, with follow-up ≥ 1 year in 2 cases.4 One case had AD; none had parkinsonism. A series of 10 RBD cases (5 males), mean age 63.9 y, range, 48–70 y, without dementia or parkinsonism used AIs as first-line therapy of PSG-confirmed RBD.5 Donepezil up to 20 mg at bedtime, or rivastigmine up to 6 mg at bedtime was utilized (mean doses and numbers for each therapy, not reported). All 10 patients had substantial improvement in the severity and frequency of RBD during a mean 11.5 month follow-up (range, 4–18 months).
REPORT OF CASE
An 88-year-old Taiwanese farmer developed a slowly progressive, non-fluctuating memory impairment and executive dysfunction for 7–8 years that forced him to retire. He no longer could plan or organize his farm work; he did not know when he should go seeding and or weeding, and no longer knew how to do fertilization or harvesting. His family did not observe any depressive symptoms, unusual or strange waking behavior, visual or auditory hallucinations, mood instability, or delusions. There were no falls, loss of consciousness, or autonomic symptoms. He ate and slept well.
Neurologic exam was abnormal for mental status, with prominent memory impairment, poor judgment, poor calculations, and other deficits. Apraxia was present, as he showed lost skills, such as not being to mimic brushing his teeth. There were no parkinsonian signs. His comprehension was normal. Neither aphasia nor agnosia was detected. The patient had spontaneous, fluent language without errors. Mini Mental State Exam score: 6/30 (orientation: time 0/5, location 2/5; registration 0/3; attention and calculation 0/5; recall 0/3; language: naming 2/2, repeat 1/1; write a sentence 0/1; draw and copy 0/1; follow a 3 stage command 1/3). CASI (Cognitive Assessment Screening Instrument) score was 22 (normal score minimum, 32.2; maximum, 100). CDR (Clinical Dementia Rating) score was 2 (moderate dementia; range 0.5–3.0). Hachinshi Ischemic Scale score: 2 (indicating degenerative dementia). Waking EEG showed mild diffuse slowing. Brain CT showed moderate, age-related cortical atrophy and marked Sylvain fissure widening. Brain MRI was negative for cerebrovascular disease, hydrocephalus, subdural hematoma, brain tumor, or other cerebral lesion. Blood chemistries ruled out hypothyroidism, vitamin B12 and folic acid deficiency, hypercalcemia, and syphilis. A lumbar puncture was not performed. A SPECT brain and cMRT were not ordered, since they are non-reimbursable clinical tests in Taiwan. His family denied any history of drug use, and he did not drink alcohol. He was diagnosed with moderate-severe AD.6
New-onset sleeptalking had begun > 8 years before presentation (shortly before the dementia was first recognized), but there was no associated dreaming, parasomnia activity, or sleep bruxism.
Therapy of AD with rivastigmine 1.5 mg twice a day was started in November 2008, and after 1 month the dose was increased to 1.5 mg in the morning and 3 mg after dinner. The patient's family very soon became aware of recurrent nocturnal episodes after his going to sleep, of leaving the bedroom, opening the back door, and going outdoors at 03:00–04:00; he fell down several times and sustained multiple abrasion injuries. These events caused great concern in the family. The 3 mg evening dose of rivastigmine was discontinued; the parasomnia promptly remitted, with disappearance of sleep-shouting and peculiar behaviors, but with occasional benign sleeptalking. His cognitive function then progressively worsened. An evening dose of rivastigmine 1.5 mg was prescribed in January 2009, along with the initiation of clonazepam 0.5 mg bedtime therapy (for presumed RBD). His sleep remained uneventful. The evening rivastigmine dose was increased to 3 mg in February 2009, while maintaining 1.5 mg in the morning and clonazepam 0.5 mg at bedtime. The next month, in preparation for polysomnography (PSG), he stopped the clonazepam; on the first night off clonazepam, while continuing to take 3 mg rivastigmine at bedtime, injurious behaviors during sleep reappeared, with the patient going outdoors, falling down hard and sustaining multiple abrasion wounds. He had no recall for any part of these events, nor was there any associated dreaming.
There was no past history of sleepwalking (SW) or any other positive sleep history. His usual sleep time was from 21:00 to 07:00. There was no psychiatric history, and medical history was positive for osteoarthritis, chronic obstructive pulmonary disease, and hypertension (with the latter 2 conditions under effective therapy). There was no positive family sleep history.
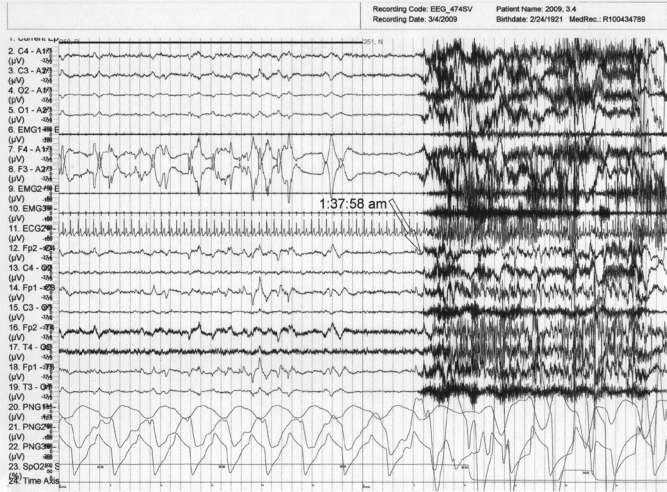
Overnight PSG monitoring, utilizing standard recording and scoring methods, was then performed after discontinuation of clonazepam for 2 days, and the patient continued taking 3 mg rivastigmine in the evening (total daily dose, 4.5 mg). An expanded EEG (seizure montage) with fast paper speed was used. During the PSG study, the patient exhibited 3 episodes of bilateral arm waving with moaning and shouting; each episode arose exclusively from REM sleep, and lasted 8–25 seconds; the 3 episodes were unaccompanied by EEG seizure-like activity. The patient awoke spontaneously after each of the three episodes, and had no recall of dreaming or of his behaviors. The mean duration of the shouting was 18.3 seconds, and the mean interval between the three RBD episodes was 148.6 minutes. The patient had 3 REM sleep periods, and RBD episodes occurred during each of the 3 REM sleep periods. Figure 1 illustrates one such episode. Dense REMs immediately preceded the episode of arm-waving and shouting. There is prominent loss of REM-atonia in the submental EMG and excessive phasic twitching across EMG channels.
Figure 1.
Nocturnal PSG (60-sec epoch) during the first REM sleep period and the emergence of a behavioral episode at 1:37:58 a.m.
Prominent bilateral arm waving and shouting are recorded on videotape during the time that the submental EMG (channel 6) demonstrates elevated tone with excessive phasic twitching. Leg EMGs (ant. tibialis; channels 9–10) display twitching during most of this interval. Dense REMs are present leading up to the behavioral episode (channels 7–8). EEG montage (channels 12–19) shows typical REM sleep activation, without any seizure activity prior to the episode onset with generalized muscle artifact. The electrocardiogram (channel 11) does not show an increase in heart rate preceding the behavioral release.
There were no episodes of sleep bruxism, snoring, apneas/hypopneas, oxygen desaturations, periodic breathing, periodic limb movements, or precipitous arousals from NREM sleep with any behaviors. The patient and his son were interviewed. Neurological exam and psychiatric interview were conducted (by S-B Y), which confirmed the previous negative findings, including the lack of parkinsonism. Rigidity, posture instability, and tremor were not detected. Mild bradykinesia was present that was attributed to advanced age and being slowed down by osteoarthritis.
DISCUSSION
This 88-year-old man with AD is the second reported patient with RBD induced by the acetylcholinesterase inhibitor rivastigmine, and is the oldest reported patient with PSG-documented RBD. On challenge and rechallenge, rivastigmine caused acute and reversible RBD in a dose-dependent manner. The mechanism of action is unclear, although the cholinergic system is presumably disturbed in RBD, along with other neurotransmitter systems.2,7 This is the second case of RBD induced by cholinergic therapy of AD.3 Therefore, the brain substrate (AD vs. no dementia) appears to play a crucial role in whether cholinergic therapy will induce RBD in AD patients or control RBD in idiopathic patients.4,5
The build-up of REMs prior to the RBD episodes in our patient during PSG monitoring is in line with recent reports on RBD in which patients showed a close temporal relation between REMs and RBD behavioral events during a majority of these events.8,9
Clonazepam was effective in controlling RBD when the RBD-triggering dose of rivastigmine (3 mg at bedtime; 4.5 mg total daily dose) was being taken. This serves as an example of the need to exercise clinical judgment in deciding whether to continue an “offending agent” (for triggering RBD) when that agent is considered to be beneficial for another condition (i.e., AD).
Sleeptalking may have been the initial prodromal symptom of future RBD in this patient, since it was a new-onset sleep symptom emerging shortly before the onset of dementia. New-onset sleeptalking, or intensification of prior sleeptalking, was reported to emerge in tandem with another neurodegenerative disorder, i.e., multiple system atrophy (MSA), in 18 of 21 patients in a consecutive MSA series.10
Sleeptalking in REM sleep, together with “REM without atonia” and increased phasic EMG activity in REM sleep has been documented in 4 patients without neurologic disorder,11 and comprises subclinical RBD that may eventually develop into clinical RBD. Thus this patient may have already developed subclinical RBD or mild RBD prior to rivastigmine therapy of AD, rendering him more vulnerable to RBD exacerbation by cholinergic therapy.
This patient's walking at night with altered consciousness could be explained in one of 4 ways, possibly in combination: (1) walking during RBD; (2) walking during SW, a disorder of arousal from NREM sleep, which would then comprise the parasomnia overlap disorder (POD) consisting of RBD and SW1,12; (3) walking after an awakening, with dementia-related confusion; and (4) rivastigmine-induced nocturnal wandering with confusion and/or hallucinations and paranoia. Walking with RBD is uncommon and leaving the bedroom is very uncommon. A few cases have been reported, but in one of these cases the walking was probably SW in a patient also with RBD (i.e., POD),13 and in 3 other cases, insufficient information was provided to determine whether the walking was from RBD, or from SW as part of POD.14 With RBD, the eyes are typically closed (as the person is attending to the dream environment), and so interacting with the actual environment by opening doors and leaving the house is quite unlikely. New-onset SW (together with RBD in 4 of the 6 cases, thus comprising the POD) has been reported in Parkinson disease.15
A case of AD with RBD was documented at autopsy to be the “Lewy body variant of AD,”16 indicating mixed parkinsonian and AD histopathology. This may be the correct diagnosis for our case, since RBD is very rare in pure AD.2,4 Nevertheless, the longitudinal clinical features of our case strongly support a diagnosis of AD rather than dementia with Lewy bodies. A definitive diagnosis can only be established at autopsy. The absence of dream-enactment in this case of RBD does not exclude the diagnosis of RBD, since 7% and 13% of RBD patients in two large series did not have dream-enactment, and in the ICSD-2 dream-enactment is not a required diagnostic criterion for RBD.1
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 2.Mahowald MW, Schenck CH. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia: Elsevier Saunders; 2009. in press. [Google Scholar]
- 3.Carlander B, Touchon J, Ondze B, Billiard M. REM sleep behavior disorder induced by cholinergic treatment in Alzheimer's disease. J Sleep Res. 1996;5(Suppl 1):28. [Google Scholar]
- 4.Ringman JM, Simmons JH. Treatment of REM sleep behavior disorder with donepezil: a report of three cases. Neurology. 2000;55:870–1. doi: 10.1212/wnl.55.6.870. [DOI] [PubMed] [Google Scholar]
- 5.Simmons J. Treatment of REM sleep behavior disorder with acetylcholinesterase inhibitors. Sleep. 2009;32:A292. [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR) Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 7.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 8.Manni R, Terzaghi M, Glorioso M. Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep. Sleep. 2009;32:241–5. doi: 10.1093/sleep/32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Hogl B. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Med. 2009;10:174–81. doi: 10.1016/j.sleep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana N, Kimura K, Kitajima K, Shinde A, Kimura J, Shibasaki H. REM sleep motor dysfunction in multiple system atrophy: with special emphasis on sleep talk as its early clinical manifestation. J Neurol Neurosurg Psychiatry. 1997;63:678–681. doi: 10.1136/jnnp.63.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi M, Sugita Y, Tachibana N, et al. Sleep talking in REM sleep could be the prodromal symptom of REM sleep behavior disorder. Sleep Res. 1995;24A:242. [Google Scholar]
- 12.Schenck CH, Boyd JL, Mahowald MW. A parasomnia overlap disorder involving sleepwalking, sleep terrors, and REM sleep behavior disorder in 33 polysomnographically confirmed cases. Sleep. 1997;20:972–81. doi: 10.1093/sleep/20.11.972. [DOI] [PubMed] [Google Scholar]
- 13.Ishigooka J, Westendorp F, Oguchi T, Takahashi A, Sumiyoshi A, Inami M. Somnambulistic behavior associated with abnormal REM sleep in an elderly woman. Biol Psychiatry. 1985;20:1003. doi: 10.1016/0006-3223(85)90198-2. [DOI] [PubMed] [Google Scholar]
- 14.Tachibana N, Sugita Y, Terashima K, Teshima Y, Shimizu T, Hishikawa Y. Polysomnographic characteristics of healthy elderly subjects with somnambulism-like behaviors. Biol Psychiatry. 1991;30:4–14. doi: 10.1016/0006-3223(91)90065-t. [DOI] [PubMed] [Google Scholar]
- 15.Poryazova R, Waldvogel D, Bassetti CL. Sleepwalking in patients with Parkinson disease. Arch Neurol. 2007;64:1524–7. doi: 10.1001/archneur.64.10.1524. [DOI] [PubMed] [Google Scholar]
- 16.Schenck CH, Mahowald MW, Anderson ML, Silber MH, Boeve BF, Parisi JE. Lewy body variant of Alzheimer's disease (AD) identified by postmortem ubiquitin staining in a previously reported case of AD associated with REM sleep behavior disorder. Biol Psychiatry. 1997;42:527–8. doi: 10.1016/S0006-3223(97)00228-X. [DOI] [PubMed] [Google Scholar]