Abstract
Objective
We conducted a meta-analysis to quantitatively compare the association between occupation as a painter and the incidence or mortality from lung cancer.
Data sources
PubMed and the reference lists of pertinent publications were searched and reviewed. For the meta-analysis, we used data from 47 independent cohort, record linkage, and case–control studies (from a total of 74 reports), including > 11,000 incident cases or deaths from lung cancer among painters.
Data extraction
Three authors independently abstracted data and assessed study quality.
Data synthesis
The summary relative risk (meta-RR, random effects) for lung cancer in painters was 1.35 [95% confidence interval (CI), 1.29–1.41; 47 studies] and 1.35 (95% CI, 1.21–1.51; 27 studies) after controlling for smoking. The relative risk was higher in never-smokers (meta-RR = 2.00; 95% CI, 1.09–3.67; 3 studies) and persisted when restricted to studies that adjusted for other occupational exposures (meta-RR = 1.57; 95% CI, 1.21–2.04; 5 studies). The results remained robust when stratified by study design, sex, and study location and are therefore unlikely due to chance or bias. Furthermore, exposure–response analyses suggested that the risk increased with duration of employment.
Conclusion
These results support the conclusion that occupational exposures in painters are causally associated with the risk of lung cancer.
Keywords: epidemiology, lung cancer, meta-analysis, painter
Lung cancer is the most common cancer diagnosis worldwide and is the major cause of cancer mortality, particularly among men. The International Agency for Research on Cancer (IARC) estimated that there were > 900,000 new cases of lung cancer each year among men and > 330,000 among women (IARC 2001, 2003). Approximately 90% of the lung cancer burden in developed countries is attributed to smoking, which acts either independently or synergistically with other occupational, lifestyle, or hereditary risk factors (Boffetta and Trichopoulos 2002; Peto et al. 1994). Several agents encountered in the occupational setting, such as asbestos, polycyclic aromatic hydrocarbons, arsenic, beryllium, cadmium, chromium(VI), and nickel compounds, are established carcinogens that target the lung (IARC 2008).
An increased incidence and mortality from lung cancer has been observed in painters, an occupation that employs several million people worldwide (IARC 1989). This has led IARC to classify occupational exposure as a painter as “carcinogenic to humans” (Group 1) (IARC 1989, in press; Straif et al. 2007). Painters are exposed to many known and suspected lung carcinogens through inhalation or dermal contact (IARC 1989; Siemiatycki et al. 2004), such as talc containing asbestos fibers, chromium VI compounds, chlorinated solvents, and cadmium compounds (IARC 1987, 1995, 1999, in press; Straif et al. 2009), although the specific causative agents have not yet been identified.
Cohort and record linkage studies demonstrating a relatively consistent increased incidence and mortality from lung cancer among painters [Alexander et al. 1996; Boice et al. 1999; Dubrow and Wegman 1984; Dunn and Weir 1965; Enterline and McKiever 1963; Gubéran et al. 1989; Guralnick 1963; Hrubec et al. 1995; Logan 1982; Menck and Henderson 1976; Office of Population Censuses and Surveys (OPCS) 1958, 1971, 1978, 1986, 1995; Petersen and Milham 1980; Pukkala 2009; van Loon et al. 1997; Whorton et al. 1983] have supported the IARC Group 1 classification, although potential confounding by tobacco smoking could not be ruled out in several of these studies. (Here we refer to record linkage studies as a subset of cohort studies where two databases are linked, such as a cohort of painters derived from census data and national mortality data, with only minimum demographic information available for the cohort.) Case–control studies have also shown that occupational exposure as a painter is a risk factor for lung cancer (Bethwaite et al. 1990; Bouchardy et al. 2002; Breslow et al. 1954; De Stefani et al. 1996; Finkelstein 1995; Milne et al. 1983; Pohlabeln et al. 2000; Wynder and Graham 1951), albeit somewhat less consistently (Baccarelli et al. 2005; Morabia et al. 1992; Muscat et al. 1998; Vineis et al. 1988; Wünsch-Filho et al. 1998), and the increased risk persisted after adjusting for the potential confounding by smoking (Brüske-Hohlfeld et al. 2000; Coggon et al. 1986; Decouflé et al. 1977; Houten et al. 1977; Jahn et al. 1999; Kjuus et al. 1986; Lerchen et al. 1987; Richiardi et al. 2004; Ronco et al. 1988; Viadana et al. 1976; Williams et al. 1977).
To assess the risk of lung cancer associated with occupational exposure as a painter, we conducted a meta-analysis of cohort, record linkage, and case–control studies to quantitatively compare the results of the different study designs and the potential confounding effect of smoking (by restricting to never-smokers), as well as other analyses to support the causal association. A thorough discussion of the individual studies included in the meta-analysis is not presented here but was summarized in the IARC Monographs (IARC 1989, in press). All of the studies reviewed, including the new studies published since the IARC Monographs, are summarized in Supplemental Material, Tables 1–3, available online (doi:10.1289/ehp.0901402.S1 via http://dx.doi.org/).
Materials and Methods
Selection criteria
All epidemiologic studies included in the previous IARC Monographs were considered (IARC 1989, in press). Further, we searched PubMed (National Center for Biotechnology Information 2009) for articles in any language describing lung cancer in painters referenced in or published since the previous IARC Monograph (IARC 1989) through 24 August 2009, using the following search terms [by text word (tw), MeSH heading (mh), or publication type (pt)]: “paint*[tw]” or “varnish*[tw]” or “lacquer*[tw]”; and “cancer” or “neoplasms[mh]”; and “case-control study[mesh]” or “cohort study[mesh]” or “meta-analysis[mh]” or “review[pt]” or “risk factors[mh]” or “neoplasms/epidemiology” or “neoplasms/etiology” or “neoplasms/CI” or “occupational diseases/etiology” or “occupational diseases/epidemiology” or “occupational diseases/CI” or “occupational diseases/MO” or “occupational exposure/adverse effects” or “death certificates[mh]” or “epidemiologic methods[mh]”; and “lung.” We identified 121 publications after restricting results to studies in humans. From the PubMed search, 69 studies were excluded because they were not epidemiologic studies, did not include original data (they were review articles), did not assess occupation as a painter, or lung cancer was not the outcome. The reference lists of pertinent publications were also reviewed to capture relevant data sources that may not have been identified with the search criteria.
The definition of painter varied between studies and often included other occupations exposed to paints such as plasterers, glaziers, wallpaper hangers, artists, decorators, French polishers, and aerographers [see Supplemental Material, Table 4 (doi:10.1289/ehp.0901402.S1) for definitions]. It is likely that paperhangers and other aforementioned occupations work in the same job environment as painters or may also paint; therefore, we considered this category as painters (Carstensen et al. 1988).
To be included in this meta-analysis, studies had to report estimates of the relative risk (RR), odds ratio (OR), standardized incidence ratio (SIR), standardized mortality ratio (SMR), proportionate mortality ratio (PMR), or proportional registration ratio with corresponding 95% confidence intervals (CIs) for ever-versus-never occupation as a painter or have provided enough information that allowed for their computation. For studies that did not report the ever-versus-never painter category, we estimated the risk estimates and 95% CIs for these categories. For studies that reported only point estimates without corresponding CIs, p-values, or standard errors, or did not report the distribution of data to allow for computation of relative risks and CIs (also for nonoverlapping populations), we made conservative assumptions to estimate RRs and 95% CIs from the data provided on a study-by-study basis. These conservative assumptions underestimated the relative risk (toward the null) and overestimated the width of the CI (i.e., by doubling the variance to approximate a 95% CI adjusted for multiple factors).
For example, overlapping lung cancer cases among African-American (black) men was identified by Morabia et al. (1992) and Muscat et al. (1998). We accounted for this population overlap by approximating the proportion of black male participants (cases and controls) based on distributions presented in other publications detailing this population, applying this proportion to the distribution presented by Morabia et al. (1992) (for black and whites combined) to determine the number of overlapping subjects, and subtracting the overlapping subjects from the distribution presented in Muscat et al. (1998).
Studies were excluded if estimation was impossible. In Supplemental Material, Tables 1–3 (doi:10.1289/ehp.0901402.S1), we use brackets to indicate the RRs and 95% CIs we calculated. For studies with overlapping populations, we included only the publication with the most complete study population. Further comments on study quality and any exclusions made are presented in detail in Supplemental Material, Tables 1–3. In total, we included in the meta-analysis 17 cohort and record linkage studies, 29 case–control studies, and 12 proportionate mortality analyses.
Data abstraction
All articles were assessed independently by three reviewers (A.A., F.M., N.K.S.) who extracted data that included authors, publication date, country of origin, characteristics of the study population including sex, and any details on the definition of painters, incidence versus mortality, lung cancer histology, observed and expected cancer cases (for cohort and proportionate mortality studies), number of exposed cases and controls (for case–control studies), yes/no adjustment for smoking or other occupational carcinogens, relative risks with corresponding 95% CIs, and results on exposure–response [see Supplemental Material, Tables 1–3 (doi:10.1289/ehp.0901402.S1)]. If adjusted and unadjusted results were reported, the most valid point estimate (i.e., adjusted for smoking and other variables) was abstracted. Any discrepancies in data collection were resolved by two other reviewers (N.G., K.S.).
Summary statistics calculated for inclusion in the meta-analysis
For cohort and record linkage studies, relative risk estimates (SIR and SMR) were computed by dividing the observed number of cases by the expected number, based on an external reference population. The corresponding 95% CIs were estimated using the PAMCOMP program (Taeger et al. 2000). If only subgroup results (e.g., by sex or duration of exposure) were reported, fixed-effects models were used to combine stratum- specific data into one summary estimate [see Supplemental Material, Tables 1 and 2 (doi:10.1289/ehp.0901402.S1)].
Wherever possible for the proportionate mortality studies, we used proportional cancer mortality ratios (calculating expected proportions of cancer deaths based on the proportion of cancer mortality in the reference population) in the analysis instead of PMRs as a more conservative approach, because proportional cancer mortality ratios provide a better risk estimate for specific cancer sites when the PMR for all cancer is artificially inflated by a deficit in other causes of death (Dalager et al. 1980) [see Supplemental Material, Table 3 (doi:10.1289/ehp.0901402.S1)]. If several cancer sites are associated with a particular occupation, the PMR can underestimate the RR.
Subgroup analyses were conducted by further restriction to studies with stronger methodologies, such as those studies that adjusted for smoking (Baccarelli et al. 2005; Brüske-Hohlfeld et al. 2000; Burns and Swanson 1991; De Stefani et al. 1996, 2005; Dunn and Weir 1965; Hrubec et al. 1995; Jahn et al. 1999; Kjuus et al. 1986; Lerchen et al. 1987; Levin et al. 1988; Matos et al. 2000; Morabia et al. 1992; Muscat et al. 1998; Notani et al. 1993; Pezzotto and Poletto 1999; Pohlabeln et al. 2000; Pronk et al. 2009; Richiardi et al. 2004; Ronco et al. 1988; Siemiatycki 1991; van Loon et al. 1997; Viadana et al. 1976; Vineis et al. 1988; Williams et al. 1977; Wünsch-Filho et al. 1998; Zahm et al. 1989; Zeka et al. 2006), other occupational risk factors (Jahn et al. 1999; Ronco et al. 1988; Stockwell and Matanoski 1985; van Loon et al. 1997), or population-based case–control studies that adjusted for smoking (Brüske-Hohlfeld et al. 2000; Burns and Swanson 1991; Coggon et al. 1986; Jahn et al. 1999; Lerchen et al. 1987; Levin et al. 1988; Pohlabeln et al. 2000; Richiardi et al. 2004; Ronco et al. 1988; Siemiatycki 1991; Vineis et al. 1988; Zahm et al. 1989; Zeka et al. 2006). Only four of the cohort and record linkage studies provided information on smoking status (Dunn and Weir 1965; Hrubec et al. 1995; Pronk et al. 2009; van Loon et al. 1997).
To allow for inclusion in the meta-analysis, we calculated 95% CIs if they were not presented in the original paper. If a 90% CI was presented and if the upper limit (UL) and lower limit (LL) were proportionally symmetric around the risk ratio (for RR and OR; i.e., if UL/RR = RR/LL), an estimate of the standard error (SE) was calculated by SE = (ln UL – ln LL/3.29), where 3.29 = 2 × 1.645 for 90% CIs. If only a p-value for the null hypothesis was presented, then a test-based SE was estimated using SE = (ln RR)/Zp, where Zp is the value of the standard-normal test statistic corresponding to the p-value using a two-tailed test. The UL and LL of the 95% CI were estimated by RR ± 1.96 (SE), where Zp = 1.96 if p = 0.05 using a two-tailed test (Rothman et al. 2008). A 95% CI corresponding to an unadjusted RR was used in the meta-analysis if a paper did not present enough data to allow for estimation of the adjusted CI.
Statistical analysis
Because cancer incidence data are often more accurate than mortality data, we used SIRs in the analyses instead of SMRs whenever both were presented. However, mortality data for lung cancer are a very reasonable proxy for incidence because of the high fatality of lung cancer and the good quality of data from death certificates (Schottenfeld and Fraumeni 2006). We performed a separate meta-analysis for proportionate mortality studies. The PMRs were, however, not included in the overall meta-analyses because of their often lower quality of exposure assessment and their additional potential for bias. Assuming that the different effect estimates (e.g., SMR, SIR, RR, OR) represent the relative risk, the data were combined for all of the cohort, record linkage, and case–control studies. Subanalyses were also performed by stratifying on study design.
Many of the cohort and record linkage studies used an external reference population to calculate the expected cases. The use of an external reference population may result in a healthy worker effect, so that incidence or mortality rates of cancer in the exposed cohort may spuriously appear lower than in the general population. When the external reference rates used to calculate the expected cases are usually assumed to be known without error, an estimate of the exposure coefficient in a regression could be obtained by a weighted linear regression of the natural log of the adjusted SMR on exposure (Sutton et al. 2000). The risk estimates from nested case–control studies were included with the analysis of cohort studies because, essentially, this design can represent a more efficient way to analyze cohort studies and does not suffer from the problems associated with control selection in a case–control study. Summary ORs (meta-ORs) were obtained separately from the meta-analysis of case–control studies. Subgroup analyses were performed stratified by sex, study region, study design, types of adjustment, and duration of employment.
The I2 statistic quantifies the extent of inconsistency among the studies (Higgins and Thompson 2002). I2 values of 25–50% indicate moderate inconsistency, whereas values > 50% reflect large inconsistencies among studies. We present the I2 values instead of the Cochran’s Q-statistic because the Q-statistic informs about the presence or absence of heterogeneity but does not quantify the extent (Huedo-Medina et al. 2006). We used both random- and fixed-effect models, with weights equal to the inverse of the variance, to calculate a summary risk estimate (DerSimonian and Laird 1986). Results from random-effects models, which account for heterogeneity among studies, are presented.
We conducted sensitivity analyses by dropping one study at a time and examining its influence on the summary effect estimates. Forest plots were used to graphically display the data (Lewis and Clarke 2001). Publication bias was visually assessed using Funnel plots (Deeks et al. 2005). We performed all statistical analyses using STATA (version 10.0; StataCorp, College Station, TX, USA), employing the “metan” command for the meta-analyses (Bradburn 2004).
Results
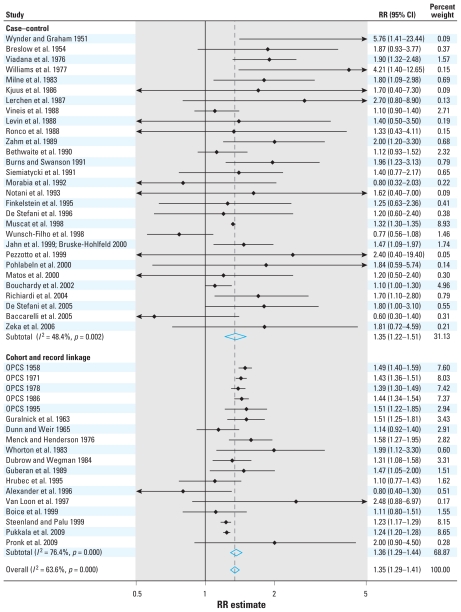
We reviewed 74 reports published since 1951 assessing the relationship between occupation as a painter and the risk of lung cancer [see Supplemental Material, Tables 1–3 (doi:10.1289/ehp.0901402.S1)]. The estimates of the relative risk reported in 47 independent studies ranged from 0.60 to 5.76, with 43 studies reporting an RR > 1.0 (Tables 1 and 2). The combined analysis of 18 cohort and record linkage studies (meta-RR = 1.36; 95% CI, 1.29–1.44; I2 = 76.4%, p = 0) and 29 case–control studies (meta-OR, 1.35; 95% CI, 1.22–1.51; I2 = 48.4%, p = 0.002), including > 11,000 incident cases and/or deaths from lung cancer among painters, demonstrated a significantly increased risk overall in persons who had ever reported occupation as a painter (meta-RR = 1.35; 95% CI, 1.29–1.41; I2 = 63.6%, p = 0) (Figure 1). Although the results of 13 proportionate mortality studies were not included in the combined analysis, they also demonstrated a significantly increased risk of lung cancer in painters (meta-PMR, 1.22; 95% CI, 1.17–1.28). The Forest plot (Figure 1) shows that there was no obvious trend in risk (at least no obvious trend toward a reduction in risk) over time. An influence analysis showed that dropping individual studies did not significantly alter the results (data not shown).
Table 1.
Cohort and record linkage studies assessing lung and respiratory cancer among persons with occupation as a painter by publication date.
| Reference, location, and time period | Cohort description | Exposure assessment | Exposure categories | No. of cases/deaths | HR/RR/SIR/SMR (95% CI) | Adjustment for potential confounders |
|---|---|---|---|---|---|---|
| Pronk et al. 2009, Shanghai, China 1996–2005 | 71,067 never-smoking women who held a job outside the home | Detailed lifetime occupational histories for each job held > 1 year from in-person interview | Painter (construction, automotive industry, and other users) | 6 | HR: 2.0 (0.9–4.5) | Passive smoking, family history of cancer, education |
| Years employmenta | ||||||
| < 10 | 1 | 0.83 (0.12–5.90) | Age, passive smoking (smokers excluded), education level, family history of lung cancer | |||
| ≥ 10 | 5 | 2.75 (1.12–6.73) | ||||
| < 20 | 5 | 2.17 (0.89–5.31) | ||||
| ≥ 20 | 1 | 1.36 (0.19–9.75) | ||||
| Pukkala et al., in press, Denmark 1971–2003, Finland 1971–2005, Iceland 1982–2004, Norway 1961–2003, Sweden 1961–2005 | 15 million people in the 1960, 1970, 1980/1981, and/or 1990 censuses and the 2.8 million incident cancer cases diagnosed in these people in a follow-up until about 2005 were linked to Nordic national registries | Occupation from self-administered census questionnaire | Painters | [3,465] | SIR: [1.24 (1.20–1.28)] | Country, sex, age, period |
| Men | 3,418 | 1.23 (1.19–1.28) | ||||
| Women | 47 | 1.90 (1.40–2.53) | ||||
| Boice et al. 1999, Lockheed Martin Plant, Burbank, Los Angeles County, CA, USA 1960–1996 | 1,216 painters (1,139 men, 77 women) employed ≥ 1 year in the aircraft industry | Detailed job history from work history cards | Painter | 41 | SMR: 1.11 [0.80–1.51] | Age, sex, race, calendar year |
| Steenland and Palu 1999, California, Missouri, New York, Texas, USA, 1975–1994 | 42,170 painters and 14,316 nonpainters with ≥ 1 year union membership | Job titles inferred from union membership records that identified the members’ specialty affiliation and trade of the local union | Painter | 1,746 | SMR: 1.23 (1.17–1.29) | Age, calendar time |
| van Loon et al. 1997, The Netherlands 1986–1990 | 58,729 men, 55–69 years of age, were enrolled from the general Dutch population | Paint exposure from a self-administered questionnaire and case-by-case expert assessment | Paint dust exposure | Age, other occupational exposures, smoking habits, dietary intake of vitamin C, β-carotene, and retinol | ||
| Anyb | 18 | RR: [2.41 (1.07–5.44)] | ||||
| Low | 4 | 2.29 (0.61–8.63) | ||||
| High | 14 | 2.48 (0.88–6.97) | ||||
| p-Value for trend | < 0.01 | |||||
| Alexander et al. 1996, Seattle, WA, USA 1974–1994 | 2,429 chromate-exposed workers employed ≥ 6 months in the aerospace industry | Exposure to chromium (VI) was estimated from industrial hygiene measurements and work-history records | All workers | 15 | SIR: 0.8 (0.4–1.3) | Age, sex, race, calendar year |
| Hrubec et al. 1995, USA 1954–1980 | 1,178 painters assembled from a roster of approximately 300,000 white male veterans of World War I | Occupation and usual industry of employment from mailed questionnaire | Painters, construction, and maintenance | 36 | SMR: 1.1 [0.77–1.43] | Smoking, age, calendar time |
| Bethune et al. 1995; OPCS 1995, England and Wales, United Kingdom 1976–1989 | Men from the 1971 and 1981 census cohorts who died between 1976 and 1989 | Occupation from death certificates | Painters and decorators | NG | SMR: 1.51 (1.22–1.85) | Age, sex, calendar year |
| Gubéran et al. 1989, Switzerland 1971–1984 | 1,916 male painters from the 1970 Geneva census | Occupation from the 1970 census | Painters | 40 | SIR: 1.47 [1.05–2.00] | Age, sex, matrimonial status, calendar year |
| OPCS 1986, Scotland, England, and Wales, United Kingdom 1979–1980, 1982–1983 | Men in Great Britain who died during 1979–1980 and 1982–1983; mortality of men 15–74 years of age in England and Wales in 1981 | Last full-time occupation from death certificate | Painters, decorators, French polishers Men |
779 | SMR: 1.44 [1.34–1.54] | Age, sex |
| Dubrow and Wegman 1984, Massachusetts, USA 1971–1973 | 34,879 white men > 20 years of age | Usual occupation from death certificate | Painters grouped | 110 | SMR : 1.31 [1.08–1.58] | Age |
| Whorton et al. 1983, San Francisco/Oakland SMSA, CA, USA 1976–1978 | 2,200 painting union members (2,197 men, 3 women) | 1976–1977 union membership files | Painter | 15 | SIR: 1.99 [1.12–3.30] | Age, sex, year |
| OPCS 1978, England and Wales, United Kingdom 1970–1972 | Registered deaths of 273,129 men | Last occupation recorded on the death certificate | Painters and decorators | 847 | SMR: 1.39 [1.30–1.49] | Age, sex |
| Menck and Henderson 1976, Los Angeles County, CA, USA 1968–1970 | Pooled mortality and morbidity data of 2,161 deaths from lung cancer and 1,777 incident cases of lung cancer among white males | Last occupation from death certificates and surveillance registry files | Painter | 87 | SMR: 1.58 [1.27–1.95] | Age |
| OPCS 1971, England and Wales, United Kingdom 1959–1963 | Registered deaths of men and women in England and Wales | Last occupation from death certificate | Painters and decorators | Age, sex | ||
| 15–64 years of age | ||||||
| Men and women | 1,506 | SMR: 1.43 [1.36–1.51] | ||||
| Men | 1,502 | 1.43 [1.36–1.50] | ||||
| Single women | 4 | 4.00 [1.09–10.24] | ||||
| Dunn and Weir 1965, California, USA 1954–1962 | Prospective study of > 68,000 men working in “suspicious” occupations (12,512 painters and decorators) | Men were enrolled based on their occupation, identified through unions, and mailed questionnaire | Painters and decorators | 91 | SMR: 1.14 [0.92–1.40] | Age, smoking |
| Enterline and McKiever 1963,Guralnick 1963, USA 1950 | Men who died in the USA in 1950 | Usual occupation and industry recorded from death certificates | Painters and plasterers | 118 | SMR: 1.51 [1.25–1.81] | Age, race |
| OPCS 1958, England and Wales, United Kingdom 1949–1953 | Registered deaths of 221,941 men and women in the broad occupational category of painters and decorators | Occupation at time of death or last occupation from death certificates | Other painters and decorators | Age, sex | ||
| Men and women | 912 | SMR: [1.49 (1.40–1.59)] | ||||
| Men | 909 | [1.49 (1.40–1.59)] | ||||
| Single women | 3 | 3.00 [0.62–8.77] | ||||
Abbreviations: HR, hazard ratio; NG, not given; SMSA, standard metropolitan statistical area. Values in brackets were calculated by us.
Information obtained by contacting authors.
Calculated using a fixed-effects model.
Table 2.
Case–control studies of the association between lung cancer and occupation as a painter by publication date.
| Reference, location, and time period | Characteristics of cases | Characteristics of controls | Exposure assessment | Exposure | No. of exposed cases | OR (95% CI) | Adjustment for potential confounders |
|---|---|---|---|---|---|---|---|
| Zeka et al. 2006, Czech Republic, Hungary, Poland, Romania, Russia, Slovakia, United Kingdom 1998–2002 | 223 never-smoking cases (48 men, 175 women) | 1,039 nonsmoking controls (534 men, 505 women) | Lifetime occupational histories for jobs held ≥ 1 year from in-person interview | Painters | |||
| Men and women | 6 | [1.81 (0.72–4.59)] | None | ||||
| Women | 6 | 1.8 (0.53–6.0) | Sex, age, study center | ||||
| Baccarelli et al. 2005, Leningrad Province (Russia) 1993–1998 | 540 (474 men, 66 women) | 582 (453 men, 129 women) individuals with autopsy-based diagnoses of non–cancer-related and non–tobacco-related conditions, frequency matched by sex, age, area, year of death | Lifetime occupational histories from personal records | Ever painters | 10 | 0.6 (0.3–1.4) | Age, sex, smoking |
| < 10 years | 6 | 0.5 (0.2–1.5) | |||||
| ≥ 10 years | 4 | 0.8 (0.2–3.0) | |||||
| De Stefani et al. 2005, Montevideo, Uruguay 1994–2000 | 338 men | 1,014 males hospitalized for conditions not related to tobacco smoking, matched by age, residence and urban/rural status | Lifetime occupational history from in-person interview | Ever painter | 26 | 1.8 (1.0–3.1) | Age, residence, urban/rural status, education, smoking status and years since quitting and age at start, no. of cigarettes per day |
| Employment (years) | |||||||
| 1–20 | 9.6 (2.6–36.0) | ||||||
| ≥ 21 | 1.2 (0.6–2.2) | ||||||
| p for trend | 0.07 | ||||||
| Richiardi et al. 2004, Turin and Eastern Veneto, Italy, 1990–1992 | 956 men | 1,253 male population-based controls, matched by study area, 5-year age groups | Lifetime occupational history from in-person interview | Ever painters | 62 | 1.7 (1.1–2.8) | Age, study area, smoking (never, ex-, active smokers), no. of job periods, education |
| Bouchardy et al. 2002, cantons of Basel, Geneva, St Gall, Vaud, and Zurich, Switzerland, 1980–1993 | 9,106 men | 49,028 male non–lung cancer registrants | Longest, current, or most recent occupation as recorded at the time of registration (main or best-specified occupation in Zurich Registry) | Plasterers and painters (in the construction industry) | 273 | 1.1 (1.0–1.3) | Age, registry, civil status, period of diagnosis, nationality, urban/rural residence, socioeconomic status, histologic confirmation, information from death certificate only (cases) |
| Matos et al. 2000, Buenos Aires, Argentina, 1994–1996 | 200 men | 397 male controls hospitalized for non–tobacco-related conditions, matched by hospital and age | Full occupational history from in-person interview. Further details requested for occupations held > 1 year. | Ever painters | 16 | 1.2 (0.5–2.4) | Age, hospital, smoking (pack-years), other occupations with significant ORs (p < 0.05) |
| Pohlabeln et al. 2000, 12 centers in Germany, Italy, Portugal, Sweden, United Kingdom, France, and Spain, 1988–1994 | 650 nonsmoking casesa (509 women, 141 men) | 1,542 nonsmoking controls (1,011 females, 531 males) | In-person interview for lifetime occupational history | Ever painters (men) | 6 | 1.84 (0.59–5.74) | Age, center |
| Jahn et al. 1999; Bruske-Hohlfeld et al. 2000, bGermany, 1988–1993, 1990–1996 | 686 women, 3,498 men | 712 female and 3,541 male population controls | Full occupational history and supplementary job-specific modules from in-person interview | Ever painters (women) | 13 | 3.0 (0.73–12.33) | Smoking, asbestos, education, age, region of residence |
| Ever painters/lacquerers | |||||||
| Men | 147 | 1.42 (1.05–1.92) | |||||
| Men and women | [160] | [1.47 (1.09–1.97)]c | |||||
| Pezzotto and Poletto 1999, Rosario City, Argentina, 1992–1998 | 367 men | 586 hospital-based males controls admitted for a non–smoking-related disease at the same hospitals for traumatic conditions, urologic diseases, acute surgical conditions, and other illnesses, matched by age (± 3 years); mean age 60.1 ± 10.2 years | Lifetime occupational history for each job held > 1 year from standardized questionnaire | House painters | 4 | 2.4 (0.4–19.4) | Age, smoking habit, lifelong cigarette consumption |
| Muscat et al. 1998, New York City, Long Island, NY; Philadelphia, PA; Washington, DC; Detroit, MI; Chicago, IL, USA, 1978–1996 | 365 black men and 185 black women | 251 male and 135 female black patients; conditions unrelated to tobacco use, matched by race, sex, 5-year age groups, month of diagnosis | Only “usual” occupation and whether the job entailed regular exposure to an occupational exposure (for a minimum of 8 hr/week) was obtained from interviews with subjects or their next of kin or death certificates | Ever painters | [24] | [1.32 (1.30–1.35)]c | Age, education, smoking |
| Mend | [19] | [0.68 (0.29–1.59)] | |||||
| Women | 5 | 1.8 (0.3–12.3) | |||||
| Wünsch-Filho et al. 1998, São Paulo, Brazil, 1990–1991 | 398 cases (307 men, 91 women) | 860 controls (546 men, 314 women) hospitalized for non–tobacco-related conditions, matched by age, sex, hospital | Full occupational history from in-person interview | Ever painters (men) | 128 | 0.77 (0.56–1.08) | Age, sex, hospital, smoking, cancer in family, migration history, socioeconomic status |
| De Stefani et al. 1996, Montevideo, Uruguay, 1993–1994, South America | 270 men | 383 male hospital-based controls: other cancer sites except oral cavity, pharynx, esophagus, stomach, larynx, and bladder | Lifetime occupational history from in-person interview | Ever painters | 18 | 1.2 (0.6–2.4) | Age, residence, education, tobacco smoking (pack–years), alcohol consumption |
| Employment (years) | |||||||
| 1–20 | 0.9 (0.2–3.0) | ||||||
| ≥ 21 | 1.4 (0.6–3.1) | ||||||
| Finkelstein 1995, Hamilton and Sault Ste-Marie, Ontario, Canada, 1979–1988 | 967 men | 2,821 men who died of any cause other than lung cancer, matched by age, year of death, and city of residence | Occupation (job and industry) from death certificate | Painters and plasterers | 16 | 1.25 (0.63–2.36) | Age, year of death, city of residence |
|
Notani et al. 1993 Bombay, India, 1986–1990 |
246 men | 212 male hospital-based controls diagnosed with cancers of the mouth and oro- or hypopharynx and noncancerous oral disease, frequency matched by age and community | Lifetime occupational history from in-person interview | Ever painters | 6 | 1.62 (0.4–7.0) | Age, community, smoking (two groups) |
| Swanson et al. 1993, Detroit, MI, metropolitan area, USA,1984–1987 | 3,792 males (2,866 white, 926 black) | 1,966 males (1,596 white, 370 black) with colon and rectal cancer | Lifetime occupational and smoking history from telephone interviews with subjects or their surrogates | Painting machine operators, black and white | Age at diagnosis, pack-years of cigarette smoking | ||
| < 10 years | 40 | [1.19 (0.61–2.34)]e | |||||
| ≥ 10 years | 40 | [2.23 (1.05–4.73)]e | |||||
| < 20 years | 53 | [1.15 (0.65–2.04)]e | |||||
| ≥ 20 years | 27 | [4.62 (1.61–13.31)]e | |||||
| Morabia et al. 1992, Detroit, MI; Chicago, IL; Philadelphia, PA; Pittsburgh, PA; New York, NY; Long Island, NY; San Francisco, CA; Birmingham, AL, USA, 1980–1989, American Health Foundation study | 1,793 men | 3,228 controls not hospitalized for lung cancer but including tobacco-related conditions; matched by age, race, hospital, smoking history, admission date | “Usual” occupation and exposure circumstances from in-person interview | Painters | [13] | 0.8 (0.32–2.03) | Age, geographic area, race, smoking, study period |
| Burns and Swanson 1991, Detroit, MI, metropolitan area, USA | 5,935 (3,918 males, 2,017 females) | 3,956 (1,981 males, 1,975 females) with colon and rectal cancer | Lifetime occupational history from telephone interviews to the subjects or to their surrogates | Painters (usual occupation, grouped) | 97 | 1.96 (1.23–3.13) | Age at diagnosis, race, smoking, sex |
| Siemiatycki 1991, Montreal, Canada, 1979–1985 | 857 men | 533 population controls, 1,360 cancer controls | Lifetime occupational history from interview | Construction painter | Age, family income, ethnicity, respondent type, cigarette and alcohol index | ||
| Any exposure | 26 | 1.4 (0.77–2.17) | |||||
| Bethwaite et al. 1990, New Zealand, 1980–1984 | 4,224 men | 15,680 male non–lung cancer registrants | Current/most recent occupation as recorded at the time of registration; smoking history obtained through telephone interview | Painter decorators, steel and other construction painters, car painters, spray painters, signwriters, other unclassified painters | 88 | 1.12 (0.93–1.52) | Age |
| Zahm et al. 1989, Missouri, USA, 1980–1985 | 4,431 white male cases | 11,326 white male non–lung cancer registrants | Occupation at the time of diagnosis abstracted from medical records | Painters, paper hangers, plasterers | 37 | 2.0 (1.2–3.3) | Age, smoking |
| Levin et al. 1988, China, 1984–1985 | 733 men | 760 age-matched population controls | Lifetime occupational history from interview | Ever painter | 15 | 1.4 (0.5–3.5) | Age, smoking |
| Duration (years) | |||||||
| < 10 | 7 | 1.9 (0.36–16.60)f | |||||
| 10–19 | 2 | 2.8 (0.07–62.47)f | |||||
| 20–29 | 5 | 2.2 (0.26–26.67)f | |||||
| ≥ 30 | 1 | 0.3 (0.01–5.81)f | |||||
| > 10 | 8 | [1.34 (0.26–6.92)]e | |||||
| < 20 | 9 | [2.35(0.44–12.47)]e | |||||
| > 20 | 6 | [1.18 (0.18–7.64)]e | |||||
| Ronco et al. 1988, Italy, 1976–1980 | 126 men | 384 men who died from causes other than from smoking-related or chronic lung diseases | Lifetime occupational history from interview with next of kin | Painter | 5 | 1.33 (0.43–4.11) | Age, year of death, smoking, other employment in suspect high-risk occupations |
| Vineis et al. 1988, Analysis of five case–control studies in Louisiana, Florida, Pennsylvania, Virginia, and New Jersey, USA, 1970s and 1980s | 2,973 men | 3,210 men | Lifetime occupational history from interview with subjects or next of kin | Painters | 201 | 1.1 (0.9–1.4) | Age, birth cohort, smoking |
| Lerchen et al. 1987, New Mexico, USA, 1980–1982 | 771 cases (333 men, 173 women) | 771 controls (499 men, 272 women) | Lifetime occupational history from interview | Ever construction painters (men) | 9 | 2.7 (0.8–8.9) | Age, ethnicity, smoking |
| Coggon et al. 1986,g Cleveland, Humberside, Cheshire counties, United Kingdom, 1975–1980 | 738 male bronchial cancer cases | 1,221 other cancers | Occupation from mailed questionnaire | Painters and decorators | 20 | 1.3 (0.62–2.72) | Age, smoking, residence, respondent |
| Kjuus et al. 1986, Norway, 1979–1983 | 176 men | 176 age-matched hospital controls excluding those with physical or mental handicaps, poor general health, or diagnosed with chronic obstructive lung disease | Longest job held from interview and work site records | Painting, paper-hanging (occupation) | 5 | 1.7 (0.4–7.3) | Age, smoking |
| Milne et al. 1983, Alameda County, CA, USA, 1958–1962 | 925 lung cancer deaths (747 men, 178 women) | 4,880 deaths from other cancers (except pancreatic, bladder, nasal, kidney, hematopoietic) that are not known to be strongly associated with occupational risk factors (reported as the “reduced control group”) | Occupation from death certificates | Painters (men) | 24 | 1.80 (1.09–2.98)h | Age |
| Williams et al. 1977, Atlanta, GA; Birmingham, AL; Colorado; Dallas-Ft. Worth, TX; Detroit, MI; Minneapolis-St. Paul, MN; Pittsburgh, PA; San Franciso–Oakland, CA, USA, Third National Cancer Survey | 432 cases | 2,173 patients with cancers other than lung, larynx, oral cavity, esophagus, bladder | Main lifetime employment from survey questionnaire | Painting (men) | 12 | 4.21 (1.40–12.65) (p < 0.01) | Age, race, education, tobacco, alcohol, geographic location |
| Viadana et al. 1976,Decouflé et al. 1977,Houten et al. 1977, Buffalo, NY, USA, 1956–1965 | Lung cancer cases from 11,591 white male cancer cases | Noncancer admissions from the same cancer treatment center | Lifetime occupation from interview before diagnosis | Painter | |||
| Ever | 42 | 1.90 (1.32–2.48) | Smoking, age | ||||
| Breslow et al.1954, California, USA, 1949–1952 | 518 patients | 518 hospital controls matched by hospital, age, sex, race | Interview | Construction and maintenance painters for ≥ 5 years | 22 | [1.87 (0.93–3.77)] | Hospital, age, sex, race |
| Wynder and Graham 1951, St. Louis, MO, USA, NG | 200 cases | 200 controls with a chest disease other than lung cancer | Lifetime occupational history from interview | Painter ≥ 5 years within the last 40 years | 11 | [5.76 (1.41–23.44)] | None |
NG, not given. Values in brackets were calculated by us.
Nonsmokers, subjects who smoked < 400 cigarettes during their lifetime.
BIPS study in Bremen area and Frankfurt/Main area; GSF study in Nordrhein-Westfalen, Rheinland-Pfalz and Bayern, Saarland, Thuringen, and Sachsen.
Fixed-effects model used to calculate a weighted average.
The study partially overlaps with Morabia et al. 1992 and thus some estimations were used to eliminate the overlap in men and the estimated variance was doubled to approximate an adjusted CI.
Calculated using a fixed-effects model.
Variance was doubled to approximate an adjusted 95% CI.
Included in the analysis restricted to case–control studies but excluded from the combined meta-analysis because of possible overlap with OPCS 1986.
The CI was estimated by applying the ratio of reduced/total controls to the observed cell counts reported for the total control group.
Figure 1.
Meta-analysis of all studies assessing lung cancer among persons with occupation as a painter, stratified by study design. Weights are from random-effects analysis. The relative risk estimate for each study is represented by a black diamond, and the horizontal line shows the corresponding 95% CI. The dashed line marks the combined estimate, and the vertical solid line represents no association.
Relative risks were higher in female painters (meta-RR = 2.04; 95% CI, 1.59–2.62) (Jahn et al. 1999; Muscat et al. 1998; OPCS 1958, 1971; Pronk et al. 2009; Pukkala 2009; Zeka et al. 2006) than in males (meta-RR = 1.37; 95% CI, 1.29–1.44). Although there were only seven studies among female painters, the meta-RR was statistically significant. Stratification by study region showed that relative risks were highest in Asia (meta-RR = 1.71; 95% CI, 0.97–3.03; I2 = 0%, p = 0.86), similar in Europe (meta-RR = 1.38 95% CI, 1.28–1.48; I2 = 75.8%, p = 0) and North America (meta-RR = 1.35; 95% CI, 1.26–1.45; I2 = 56.4%, p = 0.001), and lower in South America (meta-RR = 1.17; 95% CI, 0.77–1.76; I2 = 48.8%, p = 0.10). Of the few studies that reported results for specific histologies (De Stefani et al. 1996, 2005; Pezzotto and Poletto 1999; Richiardi et al. 2004; Siemiatycki et al. 1987), relative risks were generally highest among those diagnosed with small-cell cancer, although the CIs were wide because of the small number of cases and because results for the different histologic entities were not reported consistently.
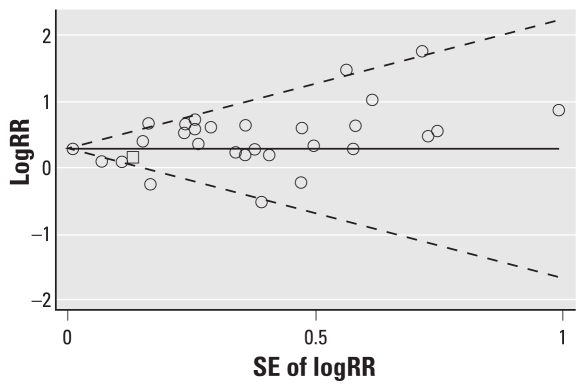
There appeared to be no evidence of publication bias among cohort and record linkage studies (data not shown). However, visual inspection of the funnel plot for 30 independent case–control studies demonstrated some evidence of publication bias: the plot was slightly skewed with a deficit of smaller nonpositive studies (represented by large SEs) (Figure 2). When restricting the analysis to the larger case–control studies that showed both positive and negative results, the meta-OR remained significantly elevated (meta-OR, 1.31; 95% CI, 1.18–1.45; I2 = 51.6%, p = 0.003). There was little difference in the results of case–control studies stratified by hospital-based controls (meta-OR, 1.37; 95% CI, 1.09–1.74; I2 = 59.3%, p = 0.002) or population-based controls (meta-OR, 1.34; 95% CI, 1.18–1.51; I2 = 25.9%, p = 0.16), although the population-based studies were less heterogeneous.
Figure 2.
Begg’s funnel plot with pseudo-95% CIs to assess publication bias in case–control studies of lung cancer among persons reoporting occupation as a painter.
We performed additional analyses to examine the summary estimates when restricted to population-based case–control studies that adjusted for tobacco smoking or other occupational exposures. Restricting to population-based case–control studies that adjusted for smoking demonstrated less heterogeneity between studies and strengthened the results (meta-OR, 1.41; 95% CI, 1.23–1.61; I2 = 0%, p = 0.45). Four cohort studies reported smoking-adjusted results (Dunn and Weir 1965; Hrubec et al. 1995; Pronk et al. 2009; van Loon et al. 1997), with a meta-RR of 1.22 (95% CI, 0.97–1.52; I2 = 23.7%, p = 0.27), slightly lower than the meta-RR for cohort studies that did not adjust for smoking (meta-RR = 1.38; 95% CI, 1.30–1.46; I2 = 80.4%, p = 0). An analysis restricted to never-smokers (meta-RR = 2.00; 95% CI, 1.09–3.67; I2 = 0%, p = 0.97) (Kreuzer et al. 2001; Pronk et al. 2009; Zeka et al. 2006) and never-smokers and nonsmokers (meta-RR = 1.96; 95% CI, 1.15–3.35; I2 = 0%, p = 0.99) (Pohlabeln et al. 2000) demonstrated stronger associations than overall estimates. Regardless of study design, the studies that adjusted for other occupational exposures as well as smoking further strengthened the results (meta-RR = 1.57; 95% CI, 1.21–2.04; I2 = 0%, p = 0.68). Because estimates were relatively consistent between individual studies, regardless of study design, it is reasonable to assume that there is no important confounding by tobacco smoking or other occupational exposures among the studies that were not able to adjust for these factors.
Analysis by duration of exposure (< 10 years vs. ≥ 10 years, < 20 years vs. ≥ 20 years) (Baccarelli et al. 2005; Dalager et al. 1980; Levin et al. 1988; Pronk et al. 2009; Swanson et al. 1993) showed that those exposed ≥ 10 years (meta-RR = 1.95; 95% CI, 1.26–3.02; I2 = 0%, p = 0.63) or ≥ 20 years (meta-RR = 2.00; 95% CI, 1.01–3.92; I2 = 16.4%, p = 0.31) had a higher risk than those exposed < 10 years (meta-RR = 1.13; 95% CI, 0.77–1.65; I2 = 0%, p = 0.46) or < 20 years (meta-RR = 1.37; 95% CI, 0.89–2.13; I2 = 0%, p = 0.54) (reference category, 0 years of exposure), respectively.
Discussion
Previous studies demonstrating an increased risk of lung cancer in painters have allowed IARC to classify occupation as a painter as carcinogenic to humans (Group 1) (IARC 1989, in press). This meta-analysis supports the IARC Group 1 classification by demonstrating a 35% increased risk of lung cancer in painters after adjusting for smoking (meta-RR = 1.35; 95% CI, 1.21–1.51; I2 = 41.2%, p = 0.01). This association was stronger for population-based case–control studies (meta-OR, 1.34; 95% CI, 1.18–1.51; I2 = 25.9%, p = 0.16) or studies that adjusted for other potentially confounding occupational exposures (meta-RR = 1.57; 95% CI, 1.21–2.04; I2 = 0%, p = 0.68). Furthermore, exposure–response analyses suggested that the risk increased with duration of employment. Although paint composition or the painting environment could have differed by major geographic region, the results did not vary much when stratified by region (North America, Europe, Asia, and South America). This is the first meta-analysis that demonstrates a relative increase in incidence/mortality from lung cancer in persons occupationally exposed as painters when restricted to never-smokers (and also nonsmokers), as well as demonstrating a statistically significant, positive duration–response relationship.
It is important to note that the interpretation of a meta-SMR (or meta-SIR) for the cohort and record linkage studies is difficult because different reference populations were used in each study for the calculation of expected cases or deaths (Rothman et al. 2008). Although the cohort studies of painters could assess possibly higher exposures from longer periods of follow-up, exposure assessment in many of the record linkage studies was often crude: Occupation as a painter was usually assessed at a single time point in a census and then linked to death registries. Although there can be relatively poor correspondence between occupation recorded on death certificates and in census records (Dubrow and Wegman 1984; Enterline and McKiever 1963; Guralnick 1963; OPCS 1971, 1978) and there is a chance of false-positive results due to multiple testing of occupations in record linkage studies, the SMRs were remarkably consistent between individual studies, generally ranging between 1.10 and 2.57. This also suggested that the significant results were not likely due to chance. Thus, the approach to combine the cohort and record linkage study SMRs for calculating a meta-SMR seemed to be justified.
In case–control studies, painters may only form a small proportion of the study population, but the full occupational history and additional information on lifestyle factors allowed several studies to adjust for tobacco smoking and some for other occupational carcinogens. An increased lung cancer risk associated with painting was consistently demonstrated in the case–control studies, suggesting that occupation as a painter is a risk factor for lung cancer. Population-based case–control studies may be less subject to selection biases than hospital-based case–control studies (Rothman et al. 2008) because there is generally no concern about the appropriate source population if indeed the general population is represented. However, if response rates are low in population controls, this could result in a lack of comparability with cases and therefore be prone to selection biases. A subanalysis comparing the meta-OR of hospital- based and population-based case–control studies showed similar results.
Estimates of the PMR may be biased if the population under study does not share the same distribution of mortality as the standard population used to compute the proportions for categories other than the ones studied (Rothman et al. 2008). However, the proportionate mortality analyses also showed significantly elevated relative risks for lung cancer in painters within the same range of effect as the analyses overall and in cohort studies, further suggesting that these results remained robust to these biases.
Smoking-adjusted estimates were available for 23 of 29 case–control studies and in only 4 of 18 cohort and record linkage studies. The robustness of the summary estimates after adjusting for tobacco use, and the higher relative risk in never-smokers, suggest that residual confounding by tobacco use is unlikely and that occupation as a painter is independently associated with the risk of lung cancer.
In women, the meta-RR was similar for all studies (meta-RR = 2.04; seven studies) (Jahn et al. 1999; Muscat et al. 1998; OPCS 1958, 1971; Pronk et al. 2009; Pukkala 2009; Zeka et al. 2006) and for studies restricted to never-smokers (meta-RR = 2.00; three studies) (Kreuzer et al. 2001; Pronk et al. 2009; Zeka et al. 2006), further strengthening the evidence that the results are not confounded by smoking. However, female painters (and never-smoking females) may not actually have a higher risk of lung cancer compared with male painters (meta-RR = 1.37; 39 studies). The relative risk in women is higher, which may be due to the fact that women have a lower background lung cancer risk than men (Schottenfeld and Fraumeni 2006).
The robustness of the results is also indicated by the presence of a duration–response relationship, with higher RRs seen for exposure over ≥ 10 years (meta-RR = 1.95) and ≥ 20 years (meta-RR = 2.00) compared with those with < 10 and < 20 years of exposure, respectively (the reference category was no exposure).
Some painters (e.g., in the construction industry) could have been exposed to asbestos. Indeed, a number of studies have shown an increased risk of mesothelioma in painters (Brown et al. 2002; Peto et al. 1995), which is most likely due to occupational asbestos exposure. However, taking into account that the exposure–response relationship for pleural mesothelioma is very different from that for lung cancer, potential asbestos exposure cannot explain all of the increase in lung cancer. Therefore, other suspected carcinogens to which painters are exposed, such as chlorinated solvents, chromium VI compounds, and cadmium compounds (IARC 1987, 1995, 1999, in press; Straif et al. 2009), may also partially explain the increased risk of lung cancer. Very few studies reported results for specific suspected causative agents. van Loon et al. (1997) reported a positive exposure–response relationship with paint dust and Siemiatycki et al. (1987) found a suggestive association with mineral spirits, whereas Alexander et al. (1996) did not find an increased risk of lung cancer in a cohort of painters and other employees in the aerospace industry exposed to chromium VI compounds.
Conclusion
There is great variability and complexity in painting environments, which complicates the interpretation of epidemiologic studies of lung cancer risks in painters. Painters are exposed to a wide variety of chemical mixtures, with compositions that change over time. In more recent decades, a number of hazardous chemicals—including benzene, some other solvents, phthalates (plasticizers), and lead oxides—have been reduced or replaced in paint, although these chemicals are still used in some countries. This trend in reducing exposures to hazardous chemicals in paint has been promoted by the increasing use of water-based paints and powder coatings. New formulations may also contain lower-toxicity solvents, neutralizing agents (e.g., amines), and biocides (IARC 1989, in press). However, this has not yet resulted in lower relative risks for lung cancer in painters, as reported in the more recent observational epidemiologic studies. The elevated risk of lung cancer may also be partly due to the role that other substances may play in increasing the risk of lung cancer among painters.
Although there was not enough information in the studies provided to assess the association of lung cancer with specific chemical agents encountered in painting, the robustness of the estimates in the subgroup analyses (by sex, region, study design, and controlling for smoking and other occupational exposures) and the stronger associations seen in specific subgroups (by duration of exposure) support the conclusion that occupational exposures in painters are causally associated with the risk of lung cancer. Because several million people are employed as painters worldwide and because lung cancer is the most common cancer in painters, even a modest increase in the relative risk is remarkable. It is important for cancer control and prevention to design studies with better exposure assessment to identify the underlying carcinogenic agents encountered in painting.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.0901402.S1 via http://dx.doi.org/).
We thank D. Russell, K. Abdedayem, S. Egraz, and S. Grant for technical assistance.
References
- Alexander BH, Checkoway H, Wechsler L, Heyer NJ, Muhm JM, O’Keeffe TP. Lung cancer in chromate-exposed aerospace workers. J Occup Environ Med. 1996;38:1253–1258. doi: 10.1097/00043764-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Tretiakova M, Gorbanev S, Lomtev A, Klimkina I, Tchibissov V, et al. Occupation and lung cancer risk in Leningrad Province, Russia. Med Lav. 2005;96:142–154. [PubMed] [Google Scholar]
- Bethune A, Harding S, Scott A, Filakati H. Occupational Health Decennial Suplement. Series DS10. London: HMSO; 1995. Mortality of longitudinal study 1971 and 1981 census cohorts; pp. 103–126. [Google Scholar]
- Bethwaite PB, Pearce N, Fraser J. Cancer risks in painters: study based on the New Zealand Cancer Registry. Br J Ind Med. 1990;47:742–746. doi: 10.1136/oem.47.11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Trichopoulos D. Textbook of Cancer Epidemiology. New York: Oxford University Press; 2002. Cancer of the lung, larynx and pleura; pp. 248–280. [Google Scholar]
- Boice JD, Jr, Marano DE, Fryzek JP, Sadler CJ, McLaughlin JK. Mortality among aircraft manufacturing workers. Occup Environ Med. 1999;56:581–597. doi: 10.1136/oem.56.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchardy C, Schuler G, Minder C, Hotz P, Bousquet A, Levi F, et al. Cancer risk by occupation and socioeconomic group among men—a study by the Association of Swiss Cancer Registries. Scand J Work Environ Health. 2002;28:1–88. [PubMed] [Google Scholar]
- Bradburn MJ. Updated and New Commands for Meta-analysis in Stata. 2004. [[accessed 24 August 2009]]. Available: http://www.medepi.net/meta/software/Bradburn_metan_updates.pdf.
- Breslow L, Hoaglin L, Rasmussen G, Abrams HK. Occupations and cigarette smoking as factors in lung cancer. Am J Public Health. 1954;44(2):171–181. doi: 10.2105/ajph.44.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Moradi T, Gridley G, Plato N, Dosemeci M, Fraumeni JF., Jr Exposures in the painting trades and paint manufacturing industry and risk of cancer among men and women in Sweden. J Occup Environ Med. 2002;44:258–264. doi: 10.1097/00043764-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Brüske-Hohlfeld I, Mohner M, Pohlabeln H, Ahrens W, Bolm-Audorff U, Kreienbrock L, et al. Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol. 2000;151:384–395. doi: 10.1093/oxfordjournals.aje.a010218. [DOI] [PubMed] [Google Scholar]
- Burns PB, Swanson GM. The Occupational Cancer Incidence Surveillance Study (OCISS): risk of lung cancer by usual occupation and industry in the Detroit metropolitan area. Am J Ind Med. 1991;19:655–671. doi: 10.1002/ajim.4700190510. [DOI] [PubMed] [Google Scholar]
- Carstensen JM, Pershagen G, Eklund G. Smoking-adjusted incidence of lung cancer among Swedish men in different occupations. Int J Epidemiol. 1988;17:753–758. doi: 10.1093/ije/17.4.753. [DOI] [PubMed] [Google Scholar]
- Coggon D, Pannett B, Osmond C, Acheson ED. A survey of cancer and occupation in young and middle aged men. I. Cancers of the respiratory tract. Br J Ind Med. 1986;43(5):332–338. doi: 10.1136/oem.43.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalager NA, Mason TJ, Fraumeni JF, Jr, Hoover R, Payne WW. Cancer mortality among workers exposed to zinc chromate paints. J Occup Med. 1980;22(1):25–29. [PubMed] [Google Scholar]
- De Stefani E, Boffetta P, Brennan P, Deneo-Pellegrini H, Ronco A, Gutierrez LP. Occupational exposures and risk of adenocarcinoma of the lung in Uruguay. Cancer Causes Control. 2005;16:851–856. doi: 10.1007/s10552-005-2819-4. [DOI] [PubMed] [Google Scholar]
- De Stefani E, Kogevinas M, Boffetta P, Ronco A, Mendilaharsu M. Occupation and the risk of lung cancer in Uruguay. Scand J Work Environ Health. 1996;22:346–352. doi: 10.5271/sjweh.152. [DOI] [PubMed] [Google Scholar]
- Decouflé P, Stanislawczyk K, Houten L, Bross IDJ, Viadana E, editors. A Retrospective Survey of Cancer in Relation to Occupation. DHEW (NIOSH) Publication No. 77-178. Cincinnati, OH: National Institute for Occupational Safety and Health; 1977. [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Systematic Reviews in Health: Care Meta-Analysis Context. London: BMJ Books; 2005. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis; pp. 285–312. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dubrow R, Wegman DH. Cancer and occupation in Massachusetts: a death certificate study. Am J Ind Med. 1984;6:207–230. doi: 10.1002/ajim.4700060305. [DOI] [PubMed] [Google Scholar]
- Dunn JE, Jr, Weir JM. Cancer experience of several occupational groups followed prospectively. Am J Public Health Nations Health. 1965;55:1367–1375. doi: 10.2105/ajph.55.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enterline PE, McKiever MF. Differential mortality from lung cancer by occupation. J Occup Med. 1963;5:283–290. [Google Scholar]
- Finkelstein MM. Occupational associations with lung cancer in two Ontario cities. Am J Ind Med. 1995;27:127–136. doi: 10.1002/ajim.4700270112. [DOI] [PubMed] [Google Scholar]
- Gubéran E, Usel M, Raymond L, Tissot R, Sweetnam PM. Disability, mortality, and incidence of cancer among Geneva painters and electricians: a historical prospective study. Br J Ind Med. 1989;46:16–23. doi: 10.1136/oem.46.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick L, editor. Mortality by Occupation Level and Cause of Death Among Men 20 to 64 Years of Age: USA, 1950. Washington, DC: U.S. Department of Health, Education, and Welfare; 1963. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Houten L, Bross IDJ, Viadana E, Sonnesso G. Occupational cancer in men exposed to metals. Adv Exp Med Biol. 1977;91:93–102. doi: 10.1007/978-1-4684-0796-9_7. [DOI] [PubMed] [Google Scholar]
- Hrubec A, Blair A, Vaught J, editors. Mortality Risks by Occupation among US Veterans of Known Smoking Status 1954–1980. Washington, DC: National Cancer Institute; 1995. [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. IARC Monogr Eval Carcinog Risk Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Occupational exposures in paint manufacture and painting. IARC Monogr Eval Carcinog Risk Hum. 1989;47:329–442. [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Trichloroethylene. IARC Monogr Eval Carcinog Risk Hum. 1995;63:75–158. [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Dichloromethane. IARC Monogr Eval Carcinog Risk Hum. 1999;71:251–315. [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Bray FI, Parkin DM, Pisani P, editors. IARC (International Agency for Research on Cancer) Globocan 2000: Cancer Incidence and Mortality Worldwide, IARC Cancer Bases No. 5. Lyon, France: IARC Press; 2001. [Google Scholar]
- Stewart B, Kleihues P, editors. IARC (International Agency for Research on Cancer) World Cancer Report. Lyon, France: IARC Press; 2003. [[accessed 31 August 2009]]. Available: http://www.iarc.fr/en/publications/pdfs-online/wcr/2003/index.php. [Google Scholar]
- Boyle P, Levin B, editors. IARC (International Agency for Research on Cancer) World Cancer Report. Lyon, France: IARC Press; 2008. [[accessed 31 August 2009]]. pp. 9–510. Available: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.php. [Google Scholar]
- IARC (International Agency for Research on Cancer) Shift-work, painting and fire-fighting. IARC Monogr Eval Carcinog Risks Hum. 98 In press. [Google Scholar]
- Jahn I, Ahrens W, Bruske-Hohlfeld I, Kreuzer M, Mohner M, Pohlabeln H, et al. Occupational risk factors for lung cancer in women: results of a case-control study in Germany. Am J Ind Med. 1999;36:90–100. doi: 10.1002/(sici)1097-0274(199907)36:1<90::aid-ajim13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kjuus H, Skjaerven R, Langard S, Lien JT, Aamodt T. A case-referent study of lung cancer, occupational exposures and smoking. I. Comparison of title-based and exposure-based occupational information. Scand J Work Environ Health. 1986;12(3):193–202. doi: 10.5271/sjweh.2158. [DOI] [PubMed] [Google Scholar]
- Kreuzer M, Gerken M, Kreienbrock L, Wellmann J, Wichmann HE. Lung cancer in lifetime nonsmoking men—results of a case-control study in Germany. Br J Cancer. 2001;84:134–140. doi: 10.1054/bjoc.2000.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchen ML, Wiggins CL, Samet JM. Lung cancer and occupation in New Mexico. J Natl Cancer Inst. 1987;79:639–645. [PubMed] [Google Scholar]
- Levin LI, Zheng W, Blot WJ, Gao YT, Fraumeni JF., Jr Occupation and lung cancer in Shanghai: a case-control study. Br J Ind Med. 1988;45(7):450–458. doi: 10.1136/oem.45.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan WP. Cancer Mortality by Occupation and Social Class 1851–1971. IARC Sci Publ. 1982;36:1–253. [PubMed] [Google Scholar]
- Matos EL, Vilensky M, Mirabelli D, Boffetta P. Occupational exposures and lung cancer in Buenos Aires, Argentina. J Occup Environ Med. 2000;42:653–659. doi: 10.1097/00043764-200006000-00017. [DOI] [PubMed] [Google Scholar]
- Menck HR, Henderson BE. Occupational differences in rates of lung cancer. J Occup Med. 1976;18:797–801. doi: 10.1097/00043764-197612000-00005. [DOI] [PubMed] [Google Scholar]
- Milne KL, Sandler DP, Everson RB, Brown SM. Lung cancer and occupation in Alameda County: a death certificate case-control study. Am J Ind Med. 1983;4:565–575. doi: 10.1002/ajim.4700040410. [DOI] [PubMed] [Google Scholar]
- Morabia A, Markowitz S, Garibaldi K, Wynder EL. Lung cancer and occupation: results of a multicentre case-control study. Br J Ind Med. 1992;49:721–727. doi: 10.1136/oem.49.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Richie JP, Jr, Wynder EL. Lung cancer risk and workplace exposures in black men and women. Environ Res. 1998;76:78–84. doi: 10.1006/enrs.1997.3787. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubMed. 2009. [[accessed 31 August 2009]]. Available: http://www.ncbi.nlm.nih.gov/pubmed/
- Notani PN, Shah P, Jayant K, Balakrishnan V. Occupation and cancers of the lung and bladder: a case-control study in Bombay. Int J Epidemiol. 1993;22:185–191. doi: 10.1093/ije/22.2.185. [DOI] [PubMed] [Google Scholar]
- OPCS (Office of Population Censuses and Surveys) The Registrar General’s Decennial Supplement, England and Wales 1951: Occupational Mortality Tables. Pt II, Vol 2. London: HMSO; 1958. [Google Scholar]
- OPCS (Office of Population Censuses and Surveys) The Registrar General’s Decennial Supplement, England and Wales 1961: Occupational Mortality Tables. London: HMSO; 1971. [Google Scholar]
- OPCS (Office of Population Censuses and Surveys) Occupational Mortality. The Registrar General’s Decennial Supplement, England and Wales 1970–1972: DS No. 1. London: HMSO; 1978. [Google Scholar]
- OPCS (Office of Population Censuses and Surveys) Occupational Mortality 1979–80, 1982–83, Great Britain, Decennial Supplement. DS No. 6. London: HMSO; 1986. [Google Scholar]
- Drever F, editor. OPCS (Office of Population Censuses and Surveys) The Registrar General’s Health and Safety Executive. Occupational Health: Decennial Supplement DS No. 10. London: HMSO; 1995. [Google Scholar]
- Petersen GR, Milham SJ, editors. Occupational Mortality in the State of California 1959–61. Publication No. 80-104. Cincinnati, OH: National Institute for Occupational Safety and Health; 1980. [Google Scholar]
- Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–539. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Heath C, Thun M, editors. Mortality from Tobacco in Developed Countries, 1950–2000. Oxford, UK: Oxford University Press; 1994. [DOI] [PubMed] [Google Scholar]
- Pezzotto SM, Poletto L. Occupation and histopathology of lung cancer: a case-control study in Rosario, Argentina. Am J Ind Med. 1999;36:437–443. doi: 10.1002/(sici)1097-0274(199910)36:4<437::aid-ajim4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pohlabeln H, Boffetta P, Ahrens W, Merletti F, Agudo A, Benhamou E, et al. Occupational risks for lung cancer among nonsmokers. Epidemiology. 2000;11:532–538. doi: 10.1097/00001648-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Pronk A, Coble J, Ji BT, Shu XO, Rothman N, Yang G, et al. Occupational risk of lung cancer among lifetime non-smoking women in Shanghai, China. Occup Environ Med. 2009;66:672–678. doi: 10.1136/oem.2008.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkala E. Occupation and cancer. Follow-up of 15 million people in five Nordic Countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- Richiardi L, Boffetta P, Simonato L, Forastiere F, Zambon P, Fortes C, et al. Occupational risk factors for lung cancer in men and women: a population-based case-control study in Italy. Cancer Causes Control. 2004;15:285–294. doi: 10.1023/B:CACO.0000024223.91059.ed. [DOI] [PubMed] [Google Scholar]
- Ronco G, Ciccone G, Mirabelli D, Troia B, Vineis P. Occupation and lung cancer in two industrialized areas of northern Italy. Int J Cancer. 1988;41(3):354–358. doi: 10.1002/ijc.2910410306. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Philadelphia: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. [Google Scholar]
- Siemiatycki J, editor. Risk Factors for Cancer in the Workplace. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Siemiatycki J, Dewar R, Nadon L, Gerin M, Richardson L, Wacholder S. Associations between several sites of cancer and twelve petroleum-derived liquids. Results from a case-referent study in Montreal. Scand J Work Environ Health. 1987;13:493–504. doi: 10.5271/sjweh.2008. [DOI] [PubMed] [Google Scholar]
- Siemiatycki J, Richardson L, Straif K, Latreille B, Lakhani R, Campbell S, et al. Listing occupational carcinogens. Environ Health Perspect. 2004;112:1447–1459. doi: 10.1289/ehp.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Palu S. Cohort mortality study of 57,000 painters and other union members: a 15 year update. Occup Environ Med. 1999;56(5):315–321. doi: 10.1136/oem.56.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell HG, Matanoski GM. A case-control study of lung cancer in painters. J Occup Med. 1985;27(2):125–126. [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El GF, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F, editors. Methods for Meta-Analysis in Medical Research. New York: Wiley; 2000. [Google Scholar]
- Swanson GM, Lin CS, Burns PB. Diversity in the association between occupation and lung cancer among black and white men. Cancer Epidemiol Biomarkers Prev. 1993;2:313–320. [PubMed] [Google Scholar]
- Taeger D, Sun Y, Keil U, Straif K. A stand-alone Windows applications for computing exact person-years, standardized mortality ratios and confidence intervals in epidemiological studies. Epidemiology. 2000;11:607–608. doi: 10.1097/00001648-200009000-00019. [DOI] [PubMed] [Google Scholar]
- van Loon AJ, Kant IJ, Swaen GM, Goldbohm RA, Kremer AM, van den Brandt PA. Occupational exposure to carcinogens and risk of lung cancer: results from The Netherlands cohort study. Occup Environ Med. 1997;54:817–824. doi: 10.1136/oem.54.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viadana E, Bross IDJ, Houten L. Cancer experience of men exposed to inhalation of chemicals or to combustion products. J Occup Med. 1976;18:787–792. doi: 10.1097/00043764-197612000-00003. [DOI] [PubMed] [Google Scholar]
- Vineis P, Thomas T, Hayes RB, Blot WJ, Mason TJ, Pickle LW, et al. Proportion of lung cancers in males, due to occupation, in different areas of the USA. Int J Cancer. 1988;42:851–856. doi: 10.1002/ijc.2910420610. [DOI] [PubMed] [Google Scholar]
- Whorton MD, Schulman J, Larson SR, Stubbs HA, Austin D. Feasibility of identifying high-risk occupations through tumor registries. J Occup Med. 1983;25:657–660. doi: 10.1097/00043764-198309000-00013. [DOI] [PubMed] [Google Scholar]
- Williams RR, Stegens NL, Goldsmith JR. Associations of cancer site and type with occupation and industry from the Third National Cancer Survey Interview. J Natl Cancer Inst. 1977;59:1147–1185. doi: 10.1093/jnci/59.4.1147. [DOI] [PubMed] [Google Scholar]
- Wünsch-Filho V, Moncau JE, Mirabelli D, Boffetta P. Occupational risk factors of lung cancer in Sao Paulo, Brazil. Scand J Work Environ Health. 1998;24:118–124. doi: 10.5271/sjweh.288. [DOI] [PubMed] [Google Scholar]
- Wynder EL, Graham EA. Etiologic factors in bronchiogenic carcinoma with special reference to industrial exposures. Report of eight hundred fifty-seven proved cases. Arch Ind Hyg Occup Med. 1951;4:221–235. [PubMed] [Google Scholar]
- Zahm SH, Brownson RC, Chang JC, Davis JR. Study of lung cancer histologic types, occupation, and smoking in Missouri. Am J Ind Med. 1989;15:565–578. doi: 10.1002/ajim.4700150509. [DOI] [PubMed] [Google Scholar]
- Zeka A, Mannetje A, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, Lissowska J, et al. Lung cancer and occupation in nonsmokers: a multicenter case-control study in Europe. Epidemiology. 2006;17:615–623. doi: 10.1097/01.ede.0000239582.92495.b5. [DOI] [PubMed] [Google Scholar]