Abstract
Introduction
Ambient particulate pollution and traffic have been linked to myocardial infarction and cardiac death risk. Possible mechanisms include autonomic cardiac dysfunction.
Methods
In a repeated-measures study of 46 patients 43–75 years of age, we investigated associations of central-site ambient particulate pollution, including black carbon (BC) (a marker for regional and local traffic), and report of traffic exposure with changes in half-hourly averaged heart rate variability (HRV), a marker of autonomic function measured by 24-hr Holter electrocardiogram monitoring. Each patient was observed up to four times within 1 year after a percutaneous intervention for myocardial infarction, acute coronary syndrome without infarction, or stable coronary artery disease (4,955 half-hour observations). For each half-hour period, diary data defined whether the patient was home or not home, or in traffic.
Results
A decrease in high frequency (HF; an HRV marker of vagal tone) of −16.4% [95% confidence interval (CI), −20.7 to −11.8%] was associated with an interquartile range of 0.3-μg/m3 increase in prior 5-day averaged ambient BC. Decreases in HF were independently associated both with the previous 2-hr averaged BC (−10.4%; 95% CI, −15.4 to −5.2%) and with being in traffic in the previous 2 hr (−38.5%; 95% CI, −57.4 to −11.1%). We also observed independent responses for particulate air matter with aerodynamic diameter ≤ 2.5 μm and for gases (ozone or nitrogen dioxide).
Conclusion
After hospitalization for coronary artery disease, both particulate pollution and being in traffic, a marker of stress and pollution, were associated with decreased HRV.
Keywords: air pollution, cardiac event, heart rate variability, myocardial infarction, traffic
Over the last 10 years epidemiologic studies have increasingly found higher daily morbidity and mortality associated with acute changes in ambient particulate matter (PM) exposures [Pope and Dockery 1999; U.S. Environmental Protection Agency (EPA) 2004]. These changes have been reported at concentrations at or near current ambient levels and have raised concerns about the adequacy of the National Air Quality Standards for PM in protecting human populations with an adequate margin of safety. Many of the early studies were criticized because mechanisms underlying the observed effects had not been elucidated at the time, particularly those related to cardiovascular morbidity and mortality. Recent toxicologic and human clinical studies have suggested a variety of PM-mediated mechanisms, including autonomic nervous system responses, particularly among patients with preexisting cardiovascular diseases (Chuang et al. 2007; Zanobetti and Schwartz 2007).
Heart rate variability (HRV), which is under the control of the autonomic nervous system, has been demonstrated to vary with air pollution (Delfino et al. 2005; Gold et al. 2000). Reduced HRV has been associated with increased risk of myocardial infarction in population studies (Tsuji et al. 1996) and has been considered a predictor of increased risk of mortality in patients with heart failure (Anonymous 1996). Although these studies did not assess the impact of acute changes in HRV, a recent study reported that ischemic events are preceded by decreases in high-frequency (HF) HRV in the hour before the event (Kop et al. 2001), and a decrease in HRV also has been reported to precede paroxysmal atrial fibrillation (Bettoni and Zimmermann 2002). Hence, short-term changes in HRV also may be risk factors for more serious consequences.
Local and regional traffic has been proposed as an important source of PM pollution that may increase cardiovascular risk, perhaps through triggering short-term autonomic changes. A recent German study demonstrated that exposure to local traffic within the previous hour was a trigger of myocardial infarction, but the mechanism through which this occurred was not determined (Peters et al. 2004). Traffic can be a source not only of PM pollution but also of stress, a known risk factor for adverse cardiac outcomes, and psychologic stress has been known to influence HRV and autonomic function (Anderson et al. 2007). In a study of traffic patrol troopers, traffic pollution inside the vehicle was demonstrated to increase HRV, systemic inflammation, and cardiac arrhythmias (Riediker et al. 2004). Most other studies have found reduction in HRV with pollution (Brook et al. 2004), but the directionality and clinical implications of the pollution-related cardiac autonomic changes likely vary with patient vulnerability.
With a specific focus on traffic pollution effects, we undertook a study of potentially susceptible patients with recent hospitalization for coronary artery disease to determine whether HRV was affected by ambient changes in PM pollution and a report of being in traffic.
Materials and Methods
Patient recruitment
The study design has been previously described (Chuang et al. 2008). Briefly, before they were discharged, we recruited a panel of patients with documented coronary artery disease from the greater Boston, Massachusetts, area (within Route 495, a 40-km radius of our central monitoring site) who had undergone percutaneous coronary intervention for an acute coronary syndrome (acute myocardial infarction or unstable angina pectoris) or for stable coronary artery disease with worsening symptoms, between October 1999 and January 2003. The presence of an acute myocardial infarction was defined as an elevation in creatine kinase (CK), CK-MB, or troponin I levels greater than three times the upper limit of normal in the setting of typical chest pain and electrocardiogram (ECG) changes. The diagnosis of prior myocardial infarction was extracted from the patients’ hospital record. We excluded those with atrial fibrillation and left bundle branch block because of the intent to evaluate HRV as well as ST-segment depression as outcomes. Coronary artery bypass graft surgery within the preceding 3 months was an exclusion criterion because the nonspecific ST and T-wave changes postoperatively would preclude accurate interpretation of new ST and T-wave changes. Patients with psychiatric illness or drug abuse problems were excluded because of compliance and reliability issues. Active cigarette smoking was an exclusion criterion at entry to the study, and no participant reported current smoking at the time of recruitment. Although four subjects reported recidivist smoking, each reported smoking at only one visit, and only one reported > 0–1 cigarettes per day at that visit (Table 1).
Table 1.
Participant characteristics (n = 46) followed up after an acute coronary event.
| Characteristic | Median (range) | n (%) |
|---|---|---|
| Age (years) | 57 (43–75) | |
| r-MSSD (msec) | 25.3 (6.8–83.5) | |
| SDNN (msec) | 66.8 (11.7–147.7) | |
| HF (msec2) | 90.5 (0.2–1,235) | |
| TP (msec2) | 4,381 (125.1–21,650) | |
| Sex | ||
| Male | 37 (80.4) | |
| Female | 9 (19.6) | |
| No. of visits | ||
| 1 | 45 (36.0) | |
| 2 | 34 (27.2) | |
| 3 | 24 (19.2) | |
| 4 | 22 (17.6) | |
| Total | 125 (100) | |
| Diagnosis | ||
| Myocardial infarction current | 18 (39.1) | |
| Coronary artery disease, not myocardial infarction | 28 (60.9) | |
| Ever used medication | ||
| Statin | 42 (91.3) | |
| Aspirin | 41 (89.1) | |
| Beta blocker | 42 (91.3) | |
| Calcium channel blocker | 5 (10.9) | |
| Angiotensin-converting enzyme inhibitors | 24 (52.2) | |
| Asthma: theophylline and beta antagonist | 2 (4.3) | |
| Digoxin | 2 (4.3) | |
| Diabetes | ||
| No | 35 (76.1) | |
| Yes | 11 (23.9) | |
| Cigarette smoking | ||
| Never | 15 (32.6) | |
| Former | 27 (58.7) | |
| Current | 4 (8.7) | |
| Ethnic group | ||
| White | 43 (93.5) | |
| Black | 1 (2.2) | |
| Asian and other | 2 (4.3) | |
Study protocol
The protocol included a home visit within 2–4 weeks after hospital discharge, followed by three additional follow-up visits at approximately 3-month intervals. At the first visit, a baseline screening questionnaire was administered regarding medications, pulmonary and cardiac symptoms, and smoking history.
Three-lead 24-hr Holter ECG monitoring (Marquette Seer Digital Recorder; Marquette Inc., Milwaukee, WI) was also performed with electrodes in modified V5 and aVF positions. During this monitoring, participants completed a standardized diary defining their activities and location for each half-hour period. For subsequent visits, participants were administered a brief questionnaire regarding cardiac and respiratory symptoms and medication use, and then received 24-hr Holter monitoring. We obtained written informed consent from the study subjects. The study design was reviewed and approved by the human subjects committees of the Brigham and Women’s Hospital and the Harvard School of Public Health.
Digitized Holter monitor recordings were analyzed using a Marquette MARS Workstation (GE Medical Systems, Milwaukee, WI). Tracings were initially reviewed for artifact by a trained technician. HRV was assessed using conventional time domain and frequency domain variables, measured using validated software (Anonymous 1996). Two patients on continuous positive airway pressure for sleep apnea were excluded from data analysis because of the potential influence of positive pressure on HRV outcomes. Time domain variables included the standard deviation of normal-to-normal heart beat intervals (SDNN) and the square root of the mean of the squared differences between adjacent normal RR intervals (r-MSSD). These values were averaged over 30-min clock time intervals to correspond with the resolution of the aerometric measures, which were collected over 30-min intervals. Frequency domain variables included HF measures and total power (TP). Both r-MSSD and HF were used to provide a measure of vagal activity, whereas SDNN and TP were used to provide a measure of sympathovagal activity. To our knowledge there are no validated criteria for half-hour HRV measures to define outliers. We therefore chose to use three times the median of the absolute differences as the cutoff for outliers. This resulted in a loss of approximately 13% of each of the HRV measures used in the analysis.
Information about diabetes diagnosis was abstracted from the medical records.
From diary data, the location of each participant was determined for each half-hour period, with location noted as home or not home, and in traffic defined as driving or riding in a car or riding a bus, subway, or train. For each 30-min interval, we classified each subject as in traffic in the previous 2 hr, not in traffic in the previous 2 hr, or in traffic part of the previous 2 hr. We similarly classified the subjects in relation to being home.
Analyses examined the association between HRV measures and both lagged and accumulated averages of ambient exposures to fine PM [≤ 2.5 μm in aerodynamic diameter (PM2.5)] and black carbon (BC) for periods ranging between 30 min to a few hours and up to 5 days preceding the HRV measures.
Aerometrics
Ambient PM concentrations were measured at a central monitoring site located on the roof of Countway Library, Harvard Medical School, in downtown Boston for the years 1999 to 2003. The median distance of participant homes from the central site monitoring station was 17.6 km. Ambient measures included continuous PM2.5 concentrations measured using a tapered element oscillation microbalance (TEOM) (model 1400A; Rupprecht and Pastashnick, East Greenbush, NY) with a 2.5-μm (PM2.5) cut point. Ambient BC was measured using an aethalometer (model 8021; McGee Scientific, Berkeley, CA).
Hourly measurements of carbon monoxide (CO), ozone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were obtained by taking the mean of site values for each gas from state monitoring sites in Boston: five sites for NO2 and SO2, four sites for CO, and three sites for O3. PM2.5 and BC and the gases concentrations were summarized in half-hour intervals and as running averages from 0.5 hr up to and including 120 hr (5-day moving averages).
Indoor PM2.5 was measured inside the homes of participants continuously using a TEOM with each measurement integrated over periods of half-hour, whereas indoor BC was measured using the aethalometer. BC was measured also outside the participants’ homes (called outdoor BC from now on). Hourly temperature was obtained from the National Weather Service first-order station at Logan Airport.
Statistical analysis
We analyzed the association of HRV with air pollution using generalized additive fixed-effect regression models—that is, we included an indicator variable for each subject. This takes into account the effect of the within-subject similarities due to repeated measurements within each subject and removes the potential for confounding of subject-specific (time-invariant) factors (Hastie and Tibshirani 1990). In addition to controlling for each subject, the models also controlled for day of the week with indicator variables; for traffic; and for average heart rate, hour of the day, date, and mean temperature with penalized splines with 4, 6, 12, and 4 degrees of freedom (df), respectively. Results were not sensitive to changing the number of degrees of freedom for temperature and seasonality. Relative humidity was not a significant predictor.
Traffic was defined as a categorical variable as being in traffic in the previous 2 hr, not in traffic in the previous 2 hr, or in traffic part of the previous 2 hr. To eliminate concurvity between the penalized splines of date and temperature, we first regressed temperature against a penalized spline of date. We used the residuals from this model to control for temperature in our model for HRV. The outcome variables were log-transformed to meet normality assumptions. We assessed the relationship between ambient pollutant and HRV overall and for selected repeated time periods after acute hospitalization. The HRV measurements were analyzed with and without heart rate adjustment. To take into account correlation between adjacent half-hours, we used 6 df in the smoothing function of hour of the day. We checked the assumption of linear dose–response relationships used in the analyses. The analyses were performed using the statistical package R (R Development Core Team 2009).
In secondary analyses we assessed the effect modification by including an interaction term, with discharge diagnoses and history of diabetes. We also examined whether the previous 2-hr associations varied according to whether a subject was home for the previous 2 hr, not home during this time, or home only part of the 2 hr.
Results are presented as percent change scaling the PM2.5 effects and BC effects to the 25th to 75th interquartile range (IQR) for each pollutant at each averaging time period.
Results
We enrolled 46 subjects, whom we studied in their homes on up to four occasions during the course of 1 year after their index event (Table 1). The subjects, all of whom had documented coronary artery disease, ranged in age from 43 to 75 years, approximately 80% were males, and all but three were Caucasian. Eighteen (39%) of the subjects experienced an acute myocardial infarction as part of the index hospitalization. Of the remaining 28 without an acute myocardial infarction, 18 (64%) had a history of previous myocardial infarction. Almost all participants were on statins, aspirin, and beta blockers. Approximately half of the patients (24) also were treated with angiotensin-converting enzyme inhibitors, with all other drugs being used by < 15% of the subjects. Participants with more than one follow-up visit were on average younger than participants with only one visit (57 vs. 62 years old) and were less likely to have ever had a myocardial infarction, although this difference was not statistically significant (Table 1). For the 46 subjects, we obtained 125 separate 24-hr ECG Holter recording sessions, with 4,955 half-hour observations. We had 3,179 observations for indoor PM2.5, 3,365 for indoor BC, and 2,508 for outdoor BC.
Ambient pollution levels were relatively low, with PM2.5 and gases never exceeded the current or proposed National Air Quality Standards (U.S. EPA 2004). However, the distribution of both ambient PM2.5 and BC concentrations during the study periods exhibited substantial variability, as evidenced by IQRs corresponding to an approximately 2.5-fold difference in concentrations. Pollutant variability was greatest for the shorter exposure periods (up to 4 hr). For the longer 3–5 day average concentrations, IQRs differed by 1- to 2-fold (Table 2). When assessing indoor pollution, indoor PM2.5 concentrations were slightly lower compared with the ambient, whereas the indoor BC concentrations were half those observed for the ambient BC. Ten subjects for a total of 17 of 125 visits reported very occasional smoking by other adults in the home “since we last saw you.” The mean levels for either BC or PM were slightly but not significantly higher for observations when smoking was reported versus when it was not (14 vs. 11 μg/m3 for PM half-hourly average).
Table 2.
Distribution of air pollution and weather variables for all subjects (n = 46).
| Percentile |
||||||
|---|---|---|---|---|---|---|
| Variable | 5th | 25th | 50th | 75th | 95th | IQR |
| Mean temperature (°C) | −6.1 | 3.3 | 10 | 18.9 | 27.8 | |
| Ambient pollution | ||||||
| PM2.5 (μg/m3) | ||||||
| 30 min | 1.96 | 5.44 | 9.06 | 13.66 | 25.54 | 8.34 |
| 2-hr mean | 2.54 | 5.65 | 8.92 | 13.58 | 25.07 | 7.93 |
| 72-hr mean | 4.72 | 7.16 | 9.93 | 12.65 | 19.31 | 5.50 |
| 120-hr mean | 5.48 | 7.6 | 9.54 | 12.34 | 15.95 | 4.83 |
| BC (μg/m3) | ||||||
| 30 min | 0.2 | 0.41 | 0.69 | 1.04 | 1.9 | 0.62 |
| 2-hr mean | 0.22 | 0.42 | 0.70 | 1.03 | 1.83 | 0.61 |
| 72-hr mean | 0.38 | 0.59 | 0.80 | 1.07 | 1.41 | 0.48 |
| 120-hr mean | 0.43 | 0.59 | 0.74 | 0.93 | 1.16 | 0.34 |
| NO2 (ppm) | ||||||
| 30 min | 0.011 | 0.017 | 0.021 | 0.027 | 0.036 | 0.011 |
| 2-hr mean | 0.011 | 0.017 | 0.021 | 0.027 | 0.036 | 0.010 |
| 72-hr mean | 0.015 | 0.018 | 0.021 | 0.025 | 0.031 | 0.007 |
| 120-hr mean | 0.015 | 0.018 | 0.021 | 0.024 | 0.029 | 0.006 |
| O3 (ppb) | ||||||
| 30 min | 2.04 | 11.36 | 20.73 | 30.33 | 53.96 | 18.97 |
| 2-hr mean | 2.29 | 11.34 | 20.47 | 30.08 | 54.29 | 18.74 |
| 72-hr mean | 7.95 | 15.71 | 21.87 | 28.33 | 42.33 | 12.62 |
| 120-hr mean | 9.66 | 16.72 | 22.75 | 29.28 | 38.72 | 12.56 |
| Indoor pollution | ||||||
| PM2.5 (μg/m3) | ||||||
| 30 min | 1.39 | 4.46 | 7.88 | 12.41 | 31.17 | 7.95 |
| 2-hr mean | 1.84 | 4.68 | 8.16 | 12.56 | 31.98 | 7.88 |
| 4-hr mean | 2.01 | 4.88 | 8.43 | 12.79 | 30.3 | 7.91 |
| BC (μg/m3) | ||||||
| 30 min | 0.1 | 0.21 | 0.38 | 0.62 | 1.17 | 0.41 |
| 2-hr mean | 0.11 | 0.22 | 0.39 | 0.62 | 1.14 | 0.4 |
| 4-hr mean | 0.11 | 0.22 | 0.4 | 0.63 | 1.12 | 0.41 |
| Outdoor “at home” BC (μg/m3) | ||||||
| 30 min | 0.11 | 0.25 | 0.47 | 0.78 | 1.42 | 0.52 |
| 2-hr mean | 0.11 | 0.26 | 0.49 | 0.77 | 1.38 | 0.51 |
| 4-hr mean | 0.12 | 0.28 | 0.49 | 0.78 | 1.29 | 0.5 |
Ambient pollution and HRV
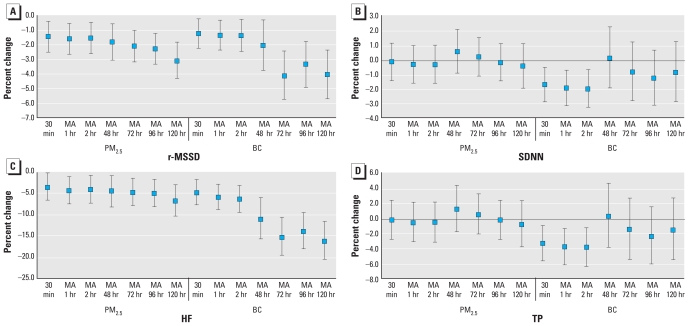
Using ambient concentrations as the exposure measure, we found significant negative associations between both r-MSSD and HF and both PM2.5 and BC for all averaging periods. Overall, we observed a tendency for decreases to be larger with longer averaging times (Figure 1). For example, for PM2.5, at 1 hr for an IQR of 8.2 μg/m3 compared with the 5-day IQR average of 4.8 μg/m3, the decrease in r-MSSD was −1.47% [95% confidence interval (CI), −2.53 to −0.4%] versus −3.1% (95% CI, −4.35 to −1.85%). Effects of PM pollutants on percent change for HF were greater in magnitude than for r-MSSD across all the time periods examined (Figure 1), with a decrease in HF of −4.4% (95% CI, −7.6 to −1.15%) for an IQR increase in PM2.5 the previous hour and a decrease of −6.9% (95% CI, −10.5 to −3.1%) for an IQR increase in the 5-day moving average of PM2.5. The effects were even larger in association with BC with a decrease in HF of −16.4% (95% CI, −20.7 to −11.8%) for an IQR of 0.3-μg/m3 increase in BC for the 5-day moving average. In contrast, for all subjects taken together, the associations noted over the same time periods for SDNN and TP were smaller and were significant only in association with BC at short lags (Figure 1).
Figure 1.
Percent change (95% CI) in r-MSSD (msec; A), SDNN (msec; B), HF (msec2; C), and TP (msec2; D) associated with different averaging times of PM2.5 and BC exposure. MA, moving average. PM2.5 and BC effects are scaled to their IQR.
Previously we reported that those with diabetes had greater ST-segment depression with air pollution exposures (Chuang et al. 2008). Similarly, although we saw effects of pollution on HRV for people with and without diabetes by means of an interaction term, those with diabetes exhibited the largest response to pollution. For example, for a 3-day moving average of PM2.5, the decrease in r-MSSD was −0.9% (95% CI, −2.1 to 0.3%) in those without diabetes versus −9.5% (95% CI, −12.5 to −6.5%) in subjects with diabetes. For a 3-day moving average of BC, the decrease in r-MSSD was −3.2% (95% CI, −5.0 to −1.3%) in those without diabetes and −9.1% (95% CI, −13.2 to −4.9%) in those with diabetes. However, in contrast to our report on the outcome of ST-segment depression (Chuang et al. 2008), those without myocardial infarction but with unstable angina pectoris or worsening stable coronary artery disease showed a stronger effect of PM on HRV than did those with a discharge diagnosis of myocardial infarction. For a 3-day moving average of PM2.5, the decrease in r-MSSD was −2.9% (95% CI, −4.3 to −1.6%) for those without myocardial infarction but with unstable angina pectoris or worsening stable coronary artery disease versus 0.3% (95% CI, −1.8 to 2.4%) for those with myocardial infarction. For a 3-day moving average of BC, the decrease in r-MSSD was −5.9% (95% CI, −7.9 to −3.9%) for those without myocardial infarction but with unstable angina pectoris or worsening stable coronary artery disease, and −0.4% (95% CI, −3.1 to 2.4%) for those with a myocardial infarction. The interaction for diagnosis of diabetes and myocardial infarction was always significant, with a p-value < 0.001. Because > 90% of our patients were consistently taking statins and beta blockers throughout the study, we could not evaluate whether these medications were protective against pollution effects.
Indoor pollution and HRV
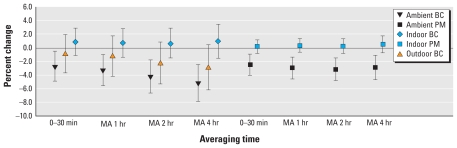
Table 3 and Figure 2 present the effects of indoor compared with outdoor pollution measures on HRV, and the effects of ambient pollution when a subject was at home versus away from home. Because there were more ambient than indoor observations, for comparability of results in Figure 2, we present the indoor, outdoor, and ambient results from the data set with the same number of observations for both indoor and ambient. Table 3 focuses on the more immediate effects of pollution, because we wanted to evaluate cumulative pollution exposures during a period comparable with time spent in traffic (2 hr), or to time spent at home, given that most participants left the house for portions of the day.
Table 3.
Percent change in r-MSSD and HF for ambient and indoor PM2.5 and BC: effects of control for traffic exposure on the association with ambient PM and effect modification.
| Percent change (95% CI) |
|||
|---|---|---|---|
| Exposure | r-MSSD | HF | |
| Models including ambient PM2.5 | |||
| Model 1 | 2-hr mean ambient PM2.5 | −2.0 (−3.3 to −0.6) | −5.2 (−9.2 to −1.1) |
| In traffic, previous 2 hr | −15.2 (−24.8 to −4.4) | −39.2 (−58.0 to −12.0) | |
| In traffic, part of the previous 2 hr | −2.8 (−5.4 to −0.2) | −4.8 (−12.4 to 3.4) | |
| Model 2 | 2-hr mean ambient PM2.5 | −2.2 (−3.6 to −0.9) | −5.9 (−9.8 to −1.8) |
| Model 3 | 2-hr mean ambient PM2.5, not home | −7.9 (−10.3 to −5.3) | −14.8 (−21.6 to −7.4) |
| 2-hr mean ambient PM2.5, home | 0.4 (−1.3 to 2.1) | −1.6 (−6.6 to 3.6) | |
| 2-hr mean ambient PM2.5, home part of time | −4.0 (−7.0 to −0.9) | −9.3 (−17.8 to 0.0) | |
| Models including indoor PM2.5 at home | |||
| Model 4 | 30-min mean indoor PM2.5 | 0.2 (−0.8 to 1.3) | −0.8 (−4.0 to 2.5) |
| Model 5 | 2-hr mean indoor PM2.5 | 0.0 (−1.1 to 1.2) | −1.3 (−4.9 to 2.5) |
| Models including ambient BC | |||
| Model 1 | 2-hr mean ambient BC | −2.2 (−4.0 to −0.4) | −10.4 (−15.4 to −5.2) |
| In traffic, previous 2 hr | −15.7 (−25.2 to −5.0) | −38.5 (−57.4 to −11.1) | |
| In traffic, part of the previous 2 hr | −2.9 (−5.5 to −0.3) | −4.9 (−12.5 to 3.3) | |
| Model 2 | 2-hr mean ambient BC | −2.5 (−4.3 to −0.7) | −11.1 (−15.9 to −5.9) |
| Model 3 | 2-hr mean ambient BC, not home | −6.9 (−10.1 to −3.6) | −17.4 (−25.9 to −8.0) |
| 2-hr mean ambient BC, home | 0.4 (−1.8 to 2.8) | −5.2 (−11.7 to 1.7) | |
| 2-hr mean ambient BC, home part of time | −6.5 (−10.2 to −2.6) | −22.2 (−31.5 to −11.6) | |
| Models including indoor BC at home | |||
| Model 4 | 30-min mean indoor BC | 2.1 (0.0 to 4.2) | 2.1 (−4.3 to 8.8) |
| Model 5 | 2-hr mean indoor BC | 2.0 (−0.2 to 4.4) | 2.3 (−4.7 to 9.8) |
Each model evaluates air pollution using different metrics of exposure as listed in the table. All models adjust for subject and day of the week, average heart rate, hour of the day, date, and mean temperature as described in the text. For example, model 2 has a term for 2-hr mean ambient BC but does not include the three-level traffic variable. PM2.5 and BC effects are scaled to 10 and 1 μg/m3, respectively.
Figure 2.
Percent change (95% CI) in r-MSSD (msec) associated with different averaging times of indoor PM2.5 and BC exposure. MA, moving average. PM2.5 and BC effects are scaled to 10 and 1 μg/m3, respectively.
The percent changes in Figure 2 and Table 3 are scaled to 10 μg/m3 for PM2.5 and to 1 μg/m3 for BC. We found no significant effects of pollution on HRV when the subjects were home, either for ambient or for indoor measured pollution. When subjects were home part of the time, the magnitude of the association of ambient pollution with reduced HRV was larger than when they were home all of the time, but smaller than when they were away from home all of the time. For example, when the patients were away from home during the previous 2 hr, we found a 7.9% (95% CI, −10.3 to −5.3%) decrease in r-MSSD for a 10-μg/m3 increase in PM2.5 and a 6.9% (95% CI, −10.1 to −3.6%) decrease for a 1-μg/m3 increase in BC, compared with a 0.4% (95% CI, −1.3 to 2.1%) decrease and a 0.45% (95% CI, −1.8 to 2.8%) decrease, respectively, when the patients were home. We also found a 14.8% (95% CI, −21.6 to −7.4%) decrease in HF for a 10-μg/m3 increase in PM2.5 and a 17.4% (95% CI, −25.9 to −8%) decrease for a 1-μg/m3 increase in BC when the patients were away from home during the previous 2 hr, compared with a 1.6% (95% CI, −6.6 to 3.6%) decrease and a 5.2% (95% CI, −11.7 to 1.7%) decrease, respectively, when the patients were home.
Traffic and HRV
We defined traffic as driving or riding in a car or riding a bus, subway, or train; cars were the mode of transport for 90% of the observations for time in traffic. Traffic exposure in the previous 2 hr had the largest effect on reduction of HRV, which was independent of the effects of ambient central site–measured BC (Table 3). Traffic was associated with a 15% decrease in r-MSSD and a 39% decrease in HF. We found a dose–response relationship: When the patients were in traffic only part of the previous 2 hr, traffic exposure was associated with a 3% decrease in r-MSSD and a 5% decrease in HF.
Ambient gases and HRV
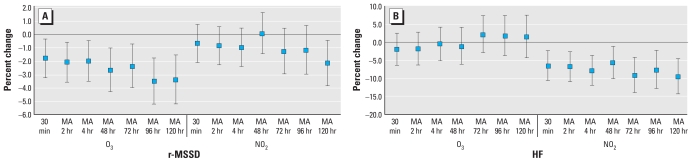
We found decreases in r-MSSD for all averaging time of O3 with a 2.1% (95% CI, −3.5 to −0.6%) decrease for an IQR increase of 19 ppb mean O3 in the previous 2 hr and a 3.4% (95% CI, −5.2 to −1.5%) decrease for an IQR increase of 13 ppb in the 5-day moving average of O3 (Figure 3). HF was not associated with O3 but was associated with NO2 for all averaging times. The association between NO2 and HF was relatively large and consistent across all averaging times, with a decrease of 6.7% (95% CI, −10.8 to −2.5%) for an IQR increase of 0.011 ppm in the 2-hr average NO2, and a decrease of 9.4% (95% CI, −14.1 to −4.4%) for an IQR increase of 0.006 ppm in the 5-day average of NO2.
Figure 3.
Percent change (95% CI) in r-MSSD (msec; A) and HF (msec2; B) associated with different averaging times of O3 and NO2 exposure. MA, moving average. O3 and NO2 effects are scaled to their IQR.
The correlation between the gases and PM2.5 and BC was only modest, with a correlation for the 72-hr average PM2.5 of 0.46 with NO2 and 0.29 with O3; the correlation of BC for the 72-hr average was 0.46 with NO2 and −0.08 with O3.
We tested two-pollutant models using each of the gas and PM measures together (Table 4). For both r-MSSD and HF and for both PM2.5 and BC, the addition of O3 or NO2 to the model did not significantly alter the effects seen in the single-pollutant models. Both O3 and PM2.5 had independent effects on reduced HF.
Table 4.
Percent change in r-MSSD and in HF for the 72-hr mean in two-pollutant models.
| Percent change (95% CI) |
||
|---|---|---|
| Variable (72-hr mean) | PM2.5 | BC |
| r-MSSD | ||
| NO2 | 1.16 (−0.97 to 3.34) | 2.27 (0.00 to 4.59) |
| PM2.5 or BC | −2.32 (−3.41 to −1.21) | −5.53 (−7.71 to −3.29) |
| O3 | −1.13 (−2.92 to 0.69) | −2.50 (−4.11 to −0.86) |
| PM2.5 or BC | −1.71 (−2.71 to −0.70) | −4.06 (−5.81 to −2.28) |
| HF | ||
| NO2 | −7.63 (−13.44 to −1.44) | 1.06 (−5.61 to 8.21) |
| PM2.5 or BC | −2.38 (−6.49 to 1.91) | −15.36 (−20.99 to −9.32) |
| O3 | 6.89 (0.99 to 13.15) | 1.81 (−3.33 to 7.23) |
| PM2.5 or BC | −7.09 (−10.65 to −3.39) | −14.72 (−19.22 to −9.97) |
Pollutant effects are scaled to IQR.
Discussion
These results suggest that short-term traffic exposure and ambient exposure to air pollution are associated with significant reductions in HRV in free-living patients with underlying heart disease, and that recent traffic exposure can cause reductions in HRV as great as 39% in patients 2–6 weeks after hospitalization for coronary artery disease. As in previous studies (Adar et al. 2007b; Gold et al. 2000; Schwartz et al. 2005), we found stronger autonomic dysfunction for HRV measures that represent vagal tone (r-MSSD and HF) than for HRV measures representing overall autonomic responses or sympathetic tone (SDNN and TP). We found associations for both at shorter (2 hr) as well as at longer averaging times (up to 5 days). We observed the greatest effects of PM and BC for longer cumulative averages.
We may not have found an influence of indoor air pollution on HRV because the indoor levels were lower, with few indoor sources of indoor pollution. However, in this same cohort, we did find an effect of both outdoor and indoor BC on increases in T-wave alternans (Zanobetti et al. 2009), an electrophysiologic measure of alternating repolarization waveform indicative of instabilities in cardiac membrane voltage and disruptions in intracellular calcium cycling dynamics. In our cohort, the T-wave alternans was more influenced by short-term cumulative averages of pollution and may be linked to pollution exposure through pathways different from those of HRV. Pollution may influence HRV through direct stimulation of pulmonary reflexes, leading to cardiac or systemic autonomic dysfunction, or through pulmonary/systemic inflammation or oxidative stress, which may lead to autonomic dysfunction (Adamkiewicz et al. 2004; Adar et al. 2007a; Chuang et al. 2007; Park et al. 2005; Peters et al. 2006).
The associations of ambient (central site–measured) BC with our outcomes were uniformly stronger than the associations of BC outside the home with our outcomes (Figure 2). It is possible that ambient central site measures of BC had stronger and larger associations with reduced HRV than did outdoor “outside the home” measures because our ambient measures provide a better measure of regional average exposure during the times when our participants were away from home. The association of reduced HRV in single-pollutant models with only NO2, which can be considered another marker of traffic in addition to BC, provides further support for the effects of pollution of traffic origins. The data also suggest that mixtures of pollutions may influence autonomic cardiac tone, in that both PM pollution and O3 had independent associations with reduced HRV in two-pollutant models.
Our results are consistent with the previous literature on potentially high-risk patients, defined as elderly subjects (with or without documented coronary heart disease) (Creason et al. 2001; Gold et al. 2000; Holguin et al. 2003; Liao et al. 2004; Pope et al. 2004). Others have studied pollution responses in patients with stable coronary heart disease (Lipsett et al. 2006; Timonen et al. 2006), whereas we studied our subjects 2–6 weeks after hospitalization for myocardial infarction, unstable angina pectoris, or worsening stable coronary artery disease. Autonomic dysfunction can precede more serious cardiac electrophysiologic disturbances in vulnerable patients (Lombardi et al. 2000; Monmeneu et al. 2001; Shusterman et al. 2000). HRV is a predictor of long-term cardiac mortality (de Bruyne et al. 1999), although the debate continues as to whether it is a marker or cause of increased cardiac risk. Previously we have described pollution-associated ST-segment depression (Chuang et al. 2008) and increases in T-wave alternans (Zanobetti et al. 2009) in the subjects participating in our study; the finding of traffic- and pollution-associated autonomic dysfunction adds to the evidence that pollution is increasing cardiac risk in this vulnerable population.
Within this population that was vulnerable on the basis of coronary artery disease, people with diabetes were even more vulnerable. This adds to cumulative evidence that type 2 diabetes, which is accompanied by a chronic inflammatory state and by chronic autonomic dysfunction, is a source of vulnerability to pollution (Dubowsky et al. 2006; O’Neill et al. 2005, 2007; Zanobetti and Schwartz 2001, 2002).
The major limitation of this study, besides the relatively small number of study participants, was that we did not study all subjects on all occasions. In addition, although the strongest associations of HRV related to the previous 3 days’ ambient PM exposures, 72-hr integrated indoor or personal exposure measurements were not available for comparison hours. More minor limitations relate to the self-report of medication use, without substantial documentation of compliance. The finding that increasing length of averaging times was associated with increasing effects could relate either to increasing biologic cumulative effects over time or to less measurement error over longer averaging periods. However, in this study we had better estimates of exposure than in most air pollution health effects studies using administrative data only. Although it is possible that our results could reflect time spent indoors at home immediately after hospitalization or time spent sleeping, we did not find that either of these factors modified the effects of indoor pollution on HRV. For the few subjects with environmental tobacco smoke (ETS) exposure in the home “since we last saw you,” it is possible that the indoor PM levels were, in part, due to ETS rather than ambient pollution penetrating indoors, cooking, or cleaning. However we found no association of indoor PM levels with reduced HRV with or without the observations where there was ETS in the home “since we last saw you.”
Although it is possible that being in traffic represents a combination of exposures, we have confidence that the effects of traffic are not a result of chance or the specific vulnerability of a few subjects. Being in traffic for either part or all of the 2 hr before and including HRV measurement was associated with reduced HRV. Although we have only 27 observations for 6 subjects who were in traffic for a full 2 hr, 40 subjects contributed 315 observations when they were in traffic for part of the previous 2 hr (at least one half-hour). It is, however, possible that being in traffic is a measure of a combination of factors with adverse effects. Studies have suggested that stress can trigger adverse cardiac events, including myocardial infarction or arrhythmias (Albert et al. 2007). A limitation of our study is that we did not measure stress while in traffic and therefore cannot disentangle the combination of pollution and stress exposures that “being in traffic” represents. Nevertheless, in the models including “being in traffic” and in the models stratified by being indoors and not in traffic, the independent associations of measured pollution with reduced HRV are not likely to be confounded by the stress of being in traffic. We did not have details on pollution levels in the cars of our study participants. Ventilation in vehicles can vary widely. It would be useful for future studies to evaluate whether reduction of in-vehicle pollution improves HRV and other indicators of cardiac risk in vulnerable populations.
This study provides further evidence that exposure to ambient pollution to both PM2.5 and BC alters autonomic function, particularly among subjects with a history of previous myocardial injury. Traffic exposure, which likely represents a complex combination of local pollution exposure and stress, independently adds to risk incurred by the background of regional pollution. Mixtures with gases such as O3 may add to the risk of PM pollution exposure. Susceptible populations such as individuals with coronary artery disease should be considered when setting national policies and regulations to control levels of PM air pollution and traffic.
Footnotes
This work was supported by National Institute of Environmental Health Sciences grant 5 P01 ES09825, U.S. Environmental Protection Agency (EPA)/Harvard Center on Ambient Particle Health Effects, U.S. EPA PM Center grant R832416, and in part by the U.S. EPA through STAR grant RD832416 to Harvard University.
This research has not been subjected to the Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
References
- Adamkiewicz G, Ebelt S, Syring M, Slater J, Speizer FE, Schwartz J, et al. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204–209. doi: 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ Health Perspect. 2007a;115:507–512. doi: 10.1289/ehp.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007b;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- Albert CM, Rosenthal L, Calkins H, Steinberg JS, Ruskin JN, Wang P, et al. Driving and implantable cardioverter-defibrillator shocks for ventricular arrhythmias: results from the TOVA study. J Am Coll Cardiol. 2007;50:2233–2240. doi: 10.1016/j.jacc.2007.06.059. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- Anonymous Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Critic Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Coull BA, Zanobetti A, Suh H, Schwartz J, Stone PH, et al. Particulate air pollution as a risk factor for ST-segment depression in patients with coronary artery disease. Circulation. 2008;118:1314–1320. doi: 10.1161/CIRCULATIONAHA.108.765669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creason J, Neas L, Walsh D, Williams R, Sheldon L, Liao D, et al. Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol. 2001;11:116–122. doi: 10.1038/sj.jea.7500154. [DOI] [PubMed] [Google Scholar]
- de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, et al. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150:1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. London: Chapman and Hall; 1990. [Google Scholar]
- Holguin F, Tellez-Rojo MM, Hernandez M, Cortez M, Chow JC, Watson JG, et al. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology. 2003;14:521–527. doi: 10.1097/01.ede.0000081999.15060.ae. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Verdino RJ, Gottdiener JS, O’Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events. J Am Coll Cardiol. 2001;38:742–749. doi: 10.1016/s0735-1097(01)01451-6. [DOI] [PubMed] [Google Scholar]
- Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, et al. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 2004;159:768–777. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- Lipsett MJ, Tsai FC, Roger L, Woo M, Ostro BD. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ Health Perspect. 2006;114:1215–1220. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F, Porta A, Marzegalli M, Favale S, Santini M, Vincenti A, et al. Heart rate variability patterns before ventricular tachycardia onset in patients with an implantable cardioverter defibrillator. Participating Investigators of ICD-HRV Italian Study Group. Am J Cardiol. 2000;86:959–963. doi: 10.1016/s0002-9149(00)01130-9. [DOI] [PubMed] [Google Scholar]
- Monmeneu JV, Chorro FJ, Bodi V, Sanchis J, Llacer A, Garcia-Civera R, et al. Relationships between heart rate variability, functional capacity, and left ventricular function following myocardial infarction: an evaluation after one week and six months. Clinic Cardiol. 2001;24:313–320. doi: 10.1002/clc.4960240411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, et al. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. J Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Particle Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, et al. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW. Epidemiology of particle effects. In: Holgate ST, Koren HS, Samet JM, Maynard RL, editors. Air Pollution and Health. London: Academic Press; 1999. pp. 673–705. [Google Scholar]
- Pope CA, III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [[accessed 21 April 2009]]. Available: http://www.R-project.org. [Google Scholar]
- Riediker M, Devlin RB, Griggs TR, Herbst MC, Bromberg PA, Williams RW, et al. Cardiovascular effects in patrol officers are associated with fine particulate matter from brake wear and engine emissions. Particle Fibre Toxicol. 2004;1:2. doi: 10.1186/1743-8977-1-2. [Online 9 December 2004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman V, Aysin B, Weiss R, Brode S, Gottipaty V, Schwartzman D, et al. Dynamics of low-frequency R-R interval oscillations preceding spontaneous ventricular tachycardia. Am Heart J. 2000;139:126–133. doi: 10.1016/s0002-8703(00)90319-3. [DOI] [PubMed] [Google Scholar]
- Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef B, Gold DR, et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: the ULTRA study. J Exp Science Environ Epidemiol. 2006;16:332–341. doi: 10.1038/sj.jea.7500460. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Air Quality Criteria for Particulate Matter. Washington, DC: U.S. Environmental Protection Agency; 2004. [Google Scholar]
- Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles? Am J Respir Critic Care Med. 2001;164:831–833. doi: 10.1164/ajrccm.164.5.2012039. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect. 2007;115:769–775. doi: 10.1289/ehp.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Stone PH, Speizer FE, Schwartz J, Coull BA, Nearing BC, et al. T-wave alternans, air pollution and traffic in high-risk subjects. Am J Cardiol. 2009;104(5):665–670. doi: 10.1016/j.amjcard.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]