Abstract
Self-fertilization and apomixis have often been seen as alternative evolutionary strategies of flowering plants that are advantageous for colonization scenarios and in bottleneck situations. Both traits have multiple origins, but different genetic control mechanisms; possible connections between the two phenomena have long been overlooked. Most apomictic plants, however, need a fertilization of polar nuclei for normal seed development (pseudogamy). If self-pollen is used for this purpose, self-compatibility is a requirement for successful pollen tube growth. Apomictic lineages usually evolve from sexual self-incompatible outcrossing plants, but pseudogamous apomicts frequently show a breakdown of self-incompatibility. Two possible pathways may explain the evolution of SC: (1) Polyploidy not only may trigger gametophytic apomixis, but also may result in a partial breakdown of SI systems. (2) Alternatively, frequent pseudo self-compatibility (PSC) via aborted pollen may induce selfing of pseudogamous apomicts (mentor effects). Self-fertile pseudogamous genotypes will be selected for within mixed sexual–apomictic populations because of avoidance of interploidal crosses; in founder situations, SC provides reproductive assurance independent from pollinators and mating partners. SI pseudogamous genotypes will be selected against in mixed populations because of minority cytotype problems and high pollen discounting; in founder populations, SI reactions among clone mates will reduce seed set. Selection for SC genotypes will eliminate SI unless the apomict maintains a high genotypic diversity and thus a diversity of S-alleles within a population, or shifts to pollen-independent autonomous apomixis. The implications of a breakdown of SI in apomictic plants for evolutionary questions and for agricultural sciences are being discussed.
Keywords: Apomixis, Pseudogamy, Endosperm, Self-incompatibility, Selfing, Polyploidy
Introduction
Self-fertilization (selfing) and apomixis are the two major pathways of reproduction in angiosperms besides the normal sexual outcrossing system. Both modes of reproduction are derived features, and both of them have multiple origins in various lineages of angiosperms (Charlesworth et al. 2005; Van Dijk and Vijverberg 2005). In many respects, both selfing and apomixis have similar evolutionary and ecological implications (Table 1). Both breeding systems allow for the reproduction via a single parental individual and potentially the founding of a population from a single seed. Both breeding systems potentially provide reproductive assurance independently from pollinators or pollen vectors. Therefore, both apomixis and selfing have been thought to be major advantages for colonization scenarios (Baker’s Law; Baker 1955, 1967; Baker and Stebbins 1965) and genetic bottlenecks. Baker’s law more generally invokes the importance of reproductive assurance in electing for self-fertilization. Beyond the colonization phase, uniparental reproduction might alleviate reduced fecundity resulting from pollen limitation in small founder populations and compensate for lack of suitable pollinators in the colonized region (Rambuda and Johnson 2004). Indeed, both selfing and apomixis have been frequently reported for invasive plants (e.g., Carino and Daehler 1999; Rambuda and Johnson 2004; Brock 2004; Fehrer et al. 2007). Selfing is often found in pioneer plants, in weeds and in island endemics (e.g., Barrett 1996; Bernardello et al. 2001), while apomixis is a frequent mode of reproduction of species colonizing previously glaciated areas and high altitudes (Bierzychudek 1985; Hörandl 2006). Both modes of reproduction result in a reduction of genotypic diversity within populations.
Table 1.
Summary of advantages and disadvantages of reproductive systems and polyploidy in angiosperms
| Mode | Advantage | Disadvantage |
|---|---|---|
| Sexual outcrosser | Maintenance of genetic diversity | Mating partner and pollen vector needed |
| Sexual selfer | Uniparental reproduction, frequent (as dominant: c. 20%; as casual: c. 40%)a |
Increased homozygosity, potential inbreeding depression |
| Autogamy, cleistogamy | Pollinator independent | |
| Geitonogamy, facilitated selfing | Pollinator dependent, pollen and seed discounting |
|
| Polyploid sexual | Novel genomic composition, fixed heterozygosity, often self-compatible, reproductive isolation, high frequencies (40–70%)b |
Possible disturbances of meiosis and gene expression, aneuploidy |
| Pseudogamous apomixis | Heterozygosity, heterosis | Reduced genotypic diversity |
| Reproductive isolation | Pollen dependent | |
| Potential minority cytotype disadvantages | ||
| Low frequencies (<1%)c | ||
| Pseudogamous apomixis with SI | Mating partner and pollen vector needed; Pollen-discounting, SI reaction among clone mates |
|
| Pseudogamous apomixis with SC and autogamyd |
Uniparental reproduction, pollinator independent |
|
| Pseudogamous apomixis with SC, geitonogamy or facilitated selfingd |
Uniparental reproduction | Pollinator dependent |
| Autonomous apomixis | Heterozygosity, heterosis | Reduced genotypic diversity |
| Uniparental reproduction, pollinator independent |
Endosperm balance strongly disturbed | |
| Self-fertility not needed | Very low frequenciesc | |
| No minority cytotype disadvantages |
Only the endosperm is fertilized, not the embryo
However, each of these breeding systems has different frequencies, modes of origin and genetic control mechanisms. The great majority of angiosperms (c. 95%) have hermaphroditic individuals and therefore a potential for self-fertilization or selfing. Selfing is a more frequent trait than apomixis and occurs at least occasionally in up to 40% of species of angiosperms (e.g., Richards 1997). Selfing may occur within the same flower (autogamy), within specialized, closed flowers (cleistogamy), between different flowers of the same individual (geitonogamy), or by pollinators that transfer also self-pollen within a flower (facilitated selfing). While autogamy and cleistogamy provide reproductive assurance independent from pollinators, geitonogamy and facilitated selfing are still pollinator dependent (Lloyd and Schoen 1992). Geitonogamy reduces pollinator visits to outcrossing partners and is therefore connected to a high amount of pollen and seed discounting, i.e., the loss of outcrossing pollen or seed (Lloyd 1992). Since self-fertilization results in a loss of heterozygosity in the offspring and potentially leads to inbreeding depression, geitonogamy and facilitated selfing have no advantage for self-compatible plants. These traits are just non-adaptive byproducts of outcrossing. Only autogamy and cleistogamy have actual selective advantage by providing independence from pollinator visits (Lloyd 1992).
Self-fertilization requires the breakdown of self-incompatibility systems that operate before fertilization. SI systems have probably evolved early in angiosperms in the context of the evolution of hermaphroditic flowers out of a self–non-self recognition system (Leach and Mayo 2005). Self-incompatibility (SI) results from the failure of pollen grains to adhere to the stigma, or to failure of pollen tubes to grow down the style. A broad range of mechanisms either prevents self-pollen to attach to the stigma (herkogamy, dichogamy, and heteromorphies) or rejects self-pollen by the pistil based on protein recognition systems of the same genotype. The proteins in pollen and pistil are different and act according to a lock-and-key mechanism (Charlesworth et al. 2005). The proteins are under monogenic or multigenic control of S alleles. The SI reaction can be based either on proteins controlled by the genotype of the pollen (gametophytic SI, GSI); in most taxa with GSI, the pollen S-allele product interacts with stylar RNase in a key-and-lock mechanism. Other systems are based on proteins derived from the anther tapetum (i.e., controlled by the genotype of the pollen donor) and expressed in the exine of the pollen (sporophytic SI, SSI). These proteins accumulate in the pollen coat and interact with those of the stigma. It would extend the scope of this review to report in detail the various forms genetic control mechanisms of SI (see Richards 1997; De Nettancourt 2001; Charlesworth et al. 2005; Leach and Mayo 2005).
The breakdown of SI mechanisms may have a genetic basis and can evolve via mutations of S-alleles, inactivation of proteins, gene silencing effects or via polyploidy. Mutations for self-compatibility (SC) are expected to be selected in bottleneck situations and loss of genetic diversity of S alleles in populations (Busch and Schoen 2008). Mutations in GSI systems that change pollen specificity and render the plants self-fertile are expected to be rapidly fixed (unless selection acts against self-fertilization via inbreeding depression). In SSI systems, loss or malfunctions of stigmatic proteins will render plants self-fertile. Selfing may have also a physiological background and can be induced by immature or aging pollen or pistils, late pollination, high temperatures, irradiation and other processes influencing the activity of the proteins involved in the SI systems (De Nettancourt 2001). A special case of breakdown of SI is the so-called “mentor effect”, which means that a mixture of compatible and incompatible pollen and self-pollen will lead to a breakdown of SI. Mentor effects can be increased by using inactivated or dead pollen (De Nettancourt 2001). These processes are summarized under the term pseudo-self-compatibility (PSC). PSC could be an evolutionary transition to SC by natural selection of PSC plants (Busch and Schoen 2008). Transitions from SI to SC or even between SI, SC and PSC happen frequently within plant families and genera (Stone 2002; Igic et al. 2003; Ferrer and Good-Avila 2006).
Apomixis, a mode of reproduction via asexually formed seed (Asker and Jerling 1992), occurs in c. 40 families and 440 genera of angiosperms (Carman 1997). At the species level, it occurs in less than 1% of the species (Mogie 1992). Apomixis occurs in many widespread and ecologically diverse plant groups (e.g., dandelions, hawkweeds, hawthorns, blackberries, Kentucky bluegrass, Citrus spp). Apomictic development of embryos follows two main pathways: (1) the development of an unreduced embryo sac from a somatic cell of the nucellus (apospory) or from an unreduced megaspore mother cell (diplospory); the unreduced egg cell develops without fertilization into an embryo (gametophytic apomixis). This form of reproduction is taxonomically scattered, but mostly found in three families of angiosperms (Asteraceae, Poaceae, Rosaceae). (2) Alternatively, embryos are formed directly out of somatic tissues within the ovules outside an embryo sac. This form of reproduction is called adventitious embryony and is often observed in tropical plants. In tropical trees, pollen limitation may selectively favor adventitious embryony to increase fecundity (Whitton et al. 2008). Adventitious embryony is taxonomically scattered, with many representatives in orchids, Celastraceae and Rutaceae (e.g., Citrus). Adventitious embryony usually occurs in parallel to normal sexual reproduction, often within multiseeded, fleshy fruits and is often connected to polyembryony (Naumova 1993). However, in both forms of apomixis, the maternal genotype is transmitted to the offspring without a loss of heterozygosity.
Apomixis is heritable and under genetic control, but hardly ever obligatory. Research on the developmental features and control mechanisms has shown that gametophytic apomixis is rather a deregulation of the normal sexual pathway than an independent trait. A temporal and/or spatial deregulation of gene expression may cause apomeiotic formation of the embryo sac and the parthenogenetic development of the embryo (Koltunow and Grossniklaus 2003; Curtis and Grossniklaus 2007). Polyploidy and hybridization may trigger the shifts in control mechanisms resulting in gametophytic apomixis via repatterning of gene expression (e.g., Grimanelli et al. 2001; Carman 1997, 2001, 2007). Apomixis may be controlled by a single dominant Mendelian factor (Nogler 1984) or by a complex of a few, closely linked genes (Ozians-Akins and Van Dijk 2007). Mapping studies of the apomixis locus suggest a clustering of apomixis-specific molecular markers in large non-recombinant regions (Vijverberg and Van Dijk 2007; Ozias-Akins and Van Dijk 2007). Transitions from sexual reproduction to gametophytic apomixis are infrequent and probably not possible via single mutations (Van Dijk and Vijverberg 2005; Hörandl 2009a, b). Three steps, i.e., the formation of an unreduced embryo sac (apomeiosis), the development of the unfertilized egg cell (parthenogenesis) and the endosperm formation, have to be coupled to establish a fully functional apomixis. Mutants of Arabidopsis may develop unreduced embryo sacs, but apomeiosis is not coupled with parthenogenetic development of the egg cell (Ravi et al. 2008). Facultative sexuality can result from an uncoupling of apomeiosis and parthenogenesis, or from sexual and apomictic embryo sac development within the same nucellus. However, reversals from apomixis to full sexuality are extremely rare (Nogler 1984; Chapman et al. 2003). Little is known about the genetic control of adventitious embryony. Segregation studies on Citrus suggest a single-locus dominant control of apomixis (Garcia et al. 1999). The somatic embryogenesis receptor kinase (SERK) may be an important component of somatic embryo initiation (Koltunow and Grossniklaus 2003). Kantama et al. (2006) found rare maternal genotypes of putative adventitious embryony in AtSERK1 transgenic populations of Arabidopsis thaliana; however, these genotypes were not heritable. Adventitious embryony does not show such a strong correlation to polyploidy (Richards 1997).
In the majority of apomictic plants (c. 90%; Mogie 1992), production of viable seed depends on the presence of pollen, i.e., fertilization of the polar nuclei is still required for normal endosperm development (pseudogamy). Only a few apomictic taxa, mainly occurring in Asteraceae, can develop the endosperm autonomously. In many angiosperms, a 2:1 maternal/paternal ratio in the endosperm is optimal for seed development because of genomic imprinting (Vinkenoog et al. 2003). Paternal genes promote growth of the endosperm, whereas maternal genes reduce growth, presumably to allocate resources evenly to the progeny. A lower ratio of the paternal genome results in underdevelopment of seeds, while a higher paternal contribution will result in over-proliferation of seeds (Vinkenoog et al. 2003). In the case of gametophytic apomixis, this ratio is disturbed, because an unreduced embryo sac has proportionally a much larger maternal contribution in the endosperm than the paternal one (e.g., a tetraploid apomict with a Polygonum type embryo sac has an octoploid endosperm nucleus, 4x + 4x, which is fertilized by a 2x pollen nucleus). Plants with gametophytic apomixis have developed various modifications of developmental pathways to maintain the optimal 2 m:1p ratio in the endosperm (Koltunow and Grossniklaus 2003; Savidan 2007). For instance, not only in polyploid apomictic Ranunculus auricomus, but also in many Rosaceae, the majority of seeds are formed by fertilization of polar nuclei with both sperm nuclei, thus preserving the 2 m:1p ratio in the endosperm (Spielmann et al. 2003; Savidan 2007, Talent and Dickinson 2007). Some taxa tolerate deviations from the endosperm balance (e.g., in Rosaceae subtribe Pyrinae, Talent and Dickinson 2007). In mutants of Arabidopsis, double fertilization events can be uncoupled, and both embryo and endosperm can develop autonomously (Koltunow and Grossniklaus 2003). In autonomous apomicts, genomic imprinting can probably be bypassed (Curtis and Grossniklaus 2008). Talent (2009) suggests that actually, the mode of embryo sac formation determines imprinting patterns in the endosperm of polyploid genomes, which would explain the frequent (but incomplete) connection of diplospory to autonomous apomixis and of apospory to pseudogamy. Alternatively, other tissues of the ovule take over the nutritional function for the embryo, so that a retarded endosperm has no negative effects (Vinkenoog et al.2003). This may be the case in Asteraceae, where the endosperm generally is small and may be lost during maturation of the embryo (e.g., Vinkenoog et al. 2003; Krahulcová and Suda 2006). Adventitious embryos usually are nourished by endosperm produced by sexual embryo sacs present in the same ovule; adventitious embryony is therefore not connected to shifts of endosperm balance, but still largely pollen dependent.
Sexual selfing and apomixis have often been seen as alternative evolutionary strategies that have evolved under different conditions: selfing occurs more frequently in annual plants than in perennials; among perennials, herbaceous species self-fertilize more frequently than in woody species (Barrett et al. 1996; Barringer 2007). In annuals, selection for reproductive assurance might be stronger (Stebbins 1950). Moreover, selfing is followed by a loss of heterozygosity and, potentially, by inbreeding depression because of the expression of homozygous deleterious recessive alleles (Charlesworth and Charlesworth 1987). In annual selfers, selection against homozygous deleterious alleles may lead to rapid purging effects by removing the individuals carrying these alleles from the population. This purging effect is followed by a subsequent increase of fitness after inbreeding (e.g., Crnokrak and Barrett 2002; Carr and Dudash 2003). In perennials with a long generation turnover, such a purging is slower, and continued selfed progeny are expected to decrease the fitness of the population. Moreover, large plants with large inflorescences or a large number of flowers have further increased frequencies of geitonogamy, i.e., frequent self-fertilization between different flowers of the same individual. The disadvantages of geitonogamy may further contribute to low frequencies of selfing among woody plants (e.g., Barrett et al. 1996).
Apomixis, in contrast, evolves almost exclusively in perennials, including both herbaceous and woody plants. Apomictic annuals are extremely rare (e.g., some species of Erigeron, Aphanes). Apomixis not only provides reproductive assurance, but also avoids a loss of heterozygosity in the offspring because the egg cell maintains the parental genotype. Apomixis therefore avoids effects of inbreeding depression and may additionally confer some advantages because of heterosis effects. Isoenzyme studies on various genera show that levels of observed heterozygosity of apomicts markedly exceed the values of their sexual relatives in all genera studied so far (Hörandl et al. 2001; Hörandl and Paun 2007). Apomixis maintains heterozygosity of hybrid genotypes by the avoidance of meiotic segregation and therefore harbors intra-individual allelic variation. Selfers, in contrast, maintain recombination and segregation at heterozygous loci during meiosis. In comparison to apomixis, selfing maintains therefore a higher inter-individual genotypic variation, which may confer an important selective advantage (Burt 2000). Selfing is easier to establish than apomixis and may therefore provide a more rapid and more flexible response to changing environments (Burt 2000; Hörandl 2006).
The role of polyploidy
Both apomixis and selfing are frequently, but not necessarily connected to polyploidy. Polyploidy is a frequent trait among flowering plants, and 35–70% of species are estimated to be of polyploid origin (Ramsey and Schemske 1998). Polyploid cytotypes can evolve rapidly either via somatic doubling or, more frequently, via the fusion of unreduced gametes. Non-reduction of gametes in diploid plants can occur during both macrosporogenesis and microsporogenesis because of a disturbed meiosis, but usually non-reduction of pollen and egg cells is not correlated. Most data are available on the formation of unreduced pollen, which can be readily induced not only by such environmental factors as rapid temperature changes, wounding, water and nutritient stress, but also by hybridization (Ramsey and Schemske 1998). The major pathways to polyploid cytotypes are the formation of a triploid (2n + n), which produces partly 2n gametes fusing with another 2n gamete (2n + 2n). High sterility of triploids may limit the success of this pathway, while increased fertility can be gained on the tetraploid or reachieved at the hexaploid level. Polyploidy can also arise within one generation by the fusion of two unreduced gametes (2n + 2n; Ramsey and Schemske 1998). On the tetraploid level, a regular meiosis with bivalent formation can be re-established that substantially increases fertility. Establishment of an outcrossing polyploid cytotype is hampered by a minority cytotype disadvantage (Levin 1975, 2002): the newly arisen polyploid individual represents a minority in the population and will mainly receive haploid pollen from neighboring diploid plants, resulting in infertile aneuploid crosses. For this reason, uniparental reproduction, as provided by apomixis and selfing, is advantageous for establishment of newly arisen polyploid cytotypes.
A correlation of polyploidy and self-compatibility has long been known, but it is less pronounced than the association of gametophytic apomixis and polyploidy. A recent statistical evaluation based on 235 species of flowering plants suggests that indeed polyploids self-fertilize more frequently than diploids (Barringer 2007). Monocotyledons and rosids exhibit higher levels of selfing among polyploids than among diploids, while this association was not confirmed for asterids. In contrast, correlations of SI-systems (including GSI, SSI and heteromorphies) and polyploidy were not confirmed by Mable (2004). Only if mixed mating systems are classified as SC, then the number of SC polyploid species exceeds significantly those of SC diploids according to the dataset of Mable (2004). Occasional self-fertilization due to PSC in otherwise SI taxa and different methodical approaches may explain different outcomes in these two studies. Mable (2004) and Barringer (2007) may have also underestimated actual rates of selfing because studies based on progeny arrays based on genetic markers may be overrepresented, while studies using pollination experiments may have been overlooked. Both authors discuss the possibility that autopolyploids might have lower frequencies of selfing than allopolyploids because the latter might be less sensitive to inbreeding depression. However, the database is insufficient for testing these hypotheses.
A breakdown of SI in polyploids is frequently observed in cases of mono factorial GSI. Self-compatible pollen production occurs in pollen grains heterozygous for two S-alleles (De Nettancourt 2001; Stone 2002). Certain S-alleles are dominant over others, and the pollen grain expresses the S specificity of the dominant allele. Alternatively, interactions between two S-alleles can lead to a loss of expressivity for each of the two alleles (De Nettancourt 2001). These processes may result in different compatibility reactions of different pollen tubes of the same plant. During the formation of polypoids via a triploid bridge, the breakdown of SC in the pollen enhances selfing and formation of tetraploids (Ramsey and Schemske 1998). Autotetraploids arising from diploids with a single-locus GSI can express semi-self-compatibility (Richards 1997). In allotetraploids with a single-locus system, similar mechanisms may result in SC. Duplications of the S-locus may also result in self-compatibility (Stone 2002). In multi-locus systems, polyploidy will not eliminate SI. If the allotetraploid originates from parents with S-alleles controlled by different loci, then a two-locus SI system can be established in the allotetraploid (e.g., Richards 1997). A complex four-locus SI system is e.g., known from Ranunculus repens (Lundqvist 1994, 1998). Self-incompatibility of tetraploids is further known from various families of angiosperms.
To summarize, SC in polyploid plants is a frequent but not at all a necessary phenomenon, but can be established under certain circumstances quite rapidly as a consequence of the formation of diploid pollen from tetraploid plants. In turn, SC helps to form tetraploids via the triploid bridge (Ramsey and Schemske 1998). In the case of a partial breakdown of SI in the pollen, SC genotypes but may be secondarily selected for during establishment, because they overcome the mate limitation of a minority cytotype. Under a condition of mate limitation, natural selection should favor SC genotypes (Busch and Schoen 2008).
Polyploidy and apomixis are also closely linked. Plants with gametophytic apomixis are almost exclusively polyploid; the only well-documented cases of apomixis on the diploid level are Boechera holboellii, which is probably a paleopolyploid (Koltunow and Grossniklaus 2003), and some species of Paspalum (Siena et al. 2008). In both cases, sexuality is also present on the diploid level. Most taxa with gametophytic apomixis have a genetically controlled and heritable formation of unreduced embryo sacs, but maintain the meiotic reduction of pollen. It is still under intense dispute whether polyploidy is a prerequisite or a supporting factor of apomixis. Polyploidy can rapidly change the genome by favoring development of gene silencing, novel functions of duplicated genes and alterations of gene expression via epigenetic changes (e.g., Comai 2005; Chen 2007). Alterations in gene expression occur immediately after polyploidization (e.g., Martelotto et al. 2005). Duplications of the genome could also trigger spatial and temporal alterations of gene expression regulating the normal sexual pathway, resulting in an apomictic phenotype (Grimanelli et al. 2001; Koltunow and Grossniklaus 2003; Curtis and Grossniklaus 2007). Such alterations are expected to be more profound in allopolyploids with a hybrid genome than in autopolyploids with just a duplicated genome (Grimanelli et al. 2001). Carman (1997, 2001, 2007) has proposed an elaborate hypothetical model for the natural origin of apomixis via allopolyploidy: sexual species that have differentiated in the timing of meiosis, embryo sac development and embryogenesis are brought together by range fluctuations caused by environmental changes (e.g., glaciations). In hybrids, rare apomeiotic (unreduced) embryo sacs arise and will cause polyploidization via fertilization and formation of triploid (2n + n) hybrids as a first step. Epigenetic modifications in the polyploids would then lead to a collapse of the timing of developmental pathways, where developmental steps that are normally expressed in sequence now occur simultaneously, thus fixing apomeiotic development of the embryo sac and full expression of apomixis.
Carman (2007) occasionally observed apomeiotic embryo sacs in experimentally produced F1 hybrids of diploid, sexual species of Sorghum (Poaceae) and Antennaria (Asteraceae). The parents are heterochronous in the timing of their developmental pathways. Frequencies of apomeiosis partially increase in segregating F2 generations (J. Carman, personal communication). According to these findings, the formation of F1 hybrids with an altered timing of reproductive pathways would be the first step toward apomixis, and polyploidy would stabilize apomixis in the hybrid genome.
The lethal effect theory by Nogler (1984) based on experimental work on Ranunculus auricomus suggests that the allele controlling aposporous embryo sac development (A−) is dominant for apomixis but has lethal recessive effects in the gametes. Apomixis can be therefore inherited only by diploid gametes heterozygous for A+ A−, while haploid gametes carrying A−would not be viable. Recessive lethal effects in the pollen or in the egg cell have indeed been observed in a couple of other apomictic taxa, mainly in Asteraceae and Poaceae (Ozias-Akins and Van Dijk 2007). Thus, mutations leading to apomixis are more likely established in lineages that had previously become polyploid (Whitton et al. 2008). Van Dijk and Vijverberg (2005) suggest that two individually deleterious mutations, one for apomeiosis (A) and one for parthenogenesis (P), must occur in individuals heterozygous at their respective loci (Aa and Pp). Hybridization combines the individual mutations in a polyploid hybrid (Aaa/Ppp) to establish a primary apomict.
The almost exclusive polyploidy of apomictic plants may be also an important prerequisite for a breakdown of SI systems in heteroallelic diploid pollen that is produced by a tetraploid plant. Thus, polyploidy may be established in parallel with apomixis and SC. The question arises—are these traits simply a parallelism following polyploidy, or are they connected by selective forces?
Apomixis and selfing: is there a correlation?
It has long been known that sexual relatives of apomictic plants are usually self-incompatible and obligate outcrossers (Gustafsson 1953; Asker and Jerling 1992). Gustafsson’s (1953) detailed survey of sexual taxa from c. 48 genera containing apomicts shows that eleven of them are dioecious or monoecious, 25 are self-sterile and 10 more are probably self-sterile as inferred from floral structure, but no genus is entirely self-fertile on the diploid sexual level. Possible exceptions of at least some sexual self-compatible taxa occur in Burmannia (Burmanniaceae), Zephyranthes (Amaryllidaceae), Aphanes (Rosaceae), Taraxacum (Asteraceae; Asker and Jerling 1992), Boechera (Brassicaceae; Roy 1995) and Poa pratensis (Barcaccia et al.1998). A correlation of apomixis and heteromorphic SI systems is missing on family level (De Nettancourt 2001). A monomorphic SI system based on a specific pollen–stigma combination occurs in both sexual and apomictic Limonium (Plumbaginaceae; Palop-Esteban et al. 2007). From this pattern, it can be concluded that the progenitors of apomictic taxa were mostly obligate outcrossers with homomorphic stigmatic or stylar SI systems. The frequent origin of apomixis from hybrids may explain why apomixis originates rather from obligate sexual outcrossers than from diploid sexual selfers. In outcrossing taxa prone to hybridization and polyploidization, a shift to gametophytic apomixis may be easier than in selfers.
However, in apomicts, a breakdown of self-compatibility systems has been observed in couple of taxa from different families (with the exception of those taxa where also apomicts are dioecious; Gustafsson 1953). An update of the available data suggests that SC in apomicts occurs mainly in the case of pseudogamy (Table 2), suggesting a causal association of self-compatibility and pseudogamy. Self-compatibility occurs also in taxa with adventitious embryony, where usually a part of the seed is derived sexually, and maintenance of a pollen function is to be expected. In the case of autonomous apomixis, the association to SC is probably weak, because pollen is not needed for seed set. Some Asteraceae with autonomous apomixis even have precocious development of embryo sacs before pollination (e.g., in Crepis; Noyes 2007), whereas in some taxa, the anthers are completely aborted (e.g., Erigeron; Noyes and Soltis 1996). In fact, there is no strong selection for maintenance of pollen function. For taxa with autonomous apomixis, however, the record remains also uncertain for methodical reasons; the frequently conducted bagging + emasculation tests are only informative about autonomous apomixis, but cannot give evidence for self-compatibility (e.g., Kissling et al. 2006 on Nardus stricta). In contrast to sexual selfing plants, progeny arrays using molecular markers are not useful for the assessment of self-fertilization of apomictic plants, because the offspring is maternal. A useful methodical approach is flow cytometric seed screening for assessment of autonomous versus pseudogamous apomixis (Matzk et al. 2000; Talent and Dickinson 2007; Hörandl et al. 2008) and microscopic studies of pollen tube growth for the assessment of SI reactions. Moreover, pseudogamy and autonomous apomixis are not completely exclusive: some taxa exhibit both modes of endosperm formation (Table 2). The taxonomic distribution of SI systems in the families with pseudogamous taxa suggests that SI may break down in both SSI and GSI systems (Table 2).
Table 2.
Records of pseudogamous taxa with SI in sexual related species and SC (inferred from self-fertility) in apomictic cytotypes
| Taxon | Sexual | Apomictic | Literature source |
|---|---|---|---|
| Asteraceae (SSI) | |||
| Parthenium | SI | SC | Gustafsson (1953) |
| Brassicaceae (SSI) | |||
| Boechera | Mostly SC | SCd | Roy (1995) |
| Clusiaceae | |||
| Hypericum | Probably SI | SC?d |
Gustafsson (1953); Robson (1981); Barcaccia et al. (2006) |
| Myrtaceae | |||
| Eugenia | SI | Partly SC | Gustafsson (1953) |
| Poaceae (GSI) | |||
| Paspalum | SI | SC | Quarin (1992, 1999) |
| Thrasya | SI | SC | Acuna et al. (2005) |
| Ranunculaceae (GSI) | |||
| Ranunculus auricomus | SI | SC | Rutishauser (1954); Hörandl (2008) |
| Ranunculus kuepferi | SI | Partly SCd |
Huber (1988); Cosendai and Hörandl (submitted) |
| Rosaceae (GSI) | |||
| Amelanchier | SI | SC | Dickinson et al. (2007) |
| Crataegus | SI | SC | Dickinson et al. (2007) |
| Malus | ? | SC | Dickinson et al. (2007) |
| Rubus | SI | SC |
Gustafsson (1953); Nybom (1986); Kollmann et al. (2000) |
| Sorbus | SI | SC | Dickinson et al. (2007) |
| Rutaceae | |||
| Citrus | ? | SCd | Gustafsson (1953) |
The predominant SI system of the respective family is given in parentheses (on species and genus level, detailed information on GSI or SSI is largely missing)
Occasionally also autonomous endosperm development
Pseudogamous apomicts usually have the same general type of inflorescence, similar size and structure of flowers, as their sexual relatives and do not produce cleistogamous flowers. Special adaptations to autogamy are rare: in some apomictic taxa of Rubus, self-pollination is facilitated by stamens arching over the stigmas (Nybom 1986). Otherwise, the floral features of outcrossing progenitors are usually maintained. Also, shifts from perennial to annual life forms have so far not been reported. Self-pollination may occur regularly not only via autogamy, but also via geitonogamy and facilitated selfing. Geitonogamy is especially expected in plants with many flowers per individual, such as in grasses or in woody Rosaceae. If the plant is SC, all forms of self-pollination can be used for endosperm fertilization and therefore potentially increase female fecundity. Since there is no negative effect of increased homozygosity in the offspring of an apomict, all forms of self-pollination, including geitonogamy and facilitated selfing have the advantage of uniparental reproduction. This ability to reproduce with a single parent helps to establish single founder individuals and may help to explain why SC pseudogamous plants usually maintain the normal “outcrossing syndrome,” producing large inflorescences, many and normal-sized flowers, and a high pollen production. For instance, among hawthorns (Crataegus spp.), SI diploids have pollen/ovule ratios in the same magnitude of 104, as polyploid SC pseudogamous apomicts (104 and 103); a significant reduction of pollen production, as typical for sexual selfers, has not been observed (Dickinson and Phipps 1986). Mathematical modeling even predicts an increased male investment with self-pollination in pseudogamous apomicts, in contrast to sexual plants (Noirot et al. 1997). On the other hand, continued self-fertilization could be disadvantageous because of a loss of heterozygosity in the endosperm (Friedman et al. 2008). The effects of self-fertilized endosperm on female fitness in apomicts need to be studied.
So far, there is no indication that SI systems and embryo sac development display any linkage between genes controlling apomixis and genes controlling SI systems. The numerous crossing experiments reviewed by Ozias-Akins and Van Dijk (2007) suggest that apomixis is usually controlled by one locus or a cluster of tightly linked loci with one allele dominant for the formation of an unreduced embryo sac (via diplospory or apospory). Parthenogenesis is typically controlled independently or by a locus tightly linked to that for apomeiosis. Inheritance of apomixis is usually characterized by segregation distortion and suppression of recombination (Ozias-Akins and Van Dijk 2007). Apomixis may be also under epigenetic control (Koltunow and Grossniklaus 2003; Curtis and Grossniklaus 2007). SI systems can be monogenic or multigenic, but rely rather on polymorphisms of S alleles. Unfortunately, in the numerous crossing experiments between sexual and apomictic cytotypes for mapping apomixis loci (Ozias-Akins and Van Dijk 2007), a co-segregation of self-compatibility in the offspring has not been scored. However, since apomixis affects the development of the embryo sac, but the breakdown of SI mostly affects the pollen and the stigma, a genetic linkage cannot be readily assumed. Also, the importance of a paternal genomic contribution on endosperm development has been only recently detected and sheds a new light on a possible evolutionary connection of apomixis and self-incompatibility. So far, little attention has been paid to a connection of self-compatibility, self-pollination and pseudogamy. Self-fertility in pseudogamous plants has been documented in Crataegus (Dickinson and Phipps 1986; Dickinson et al. 1996), in Paspalum (Quarin 1992) and in Ranunculus (Hörandl 2008). Based on these model systems, a hypothetical selection model for the evolution of self-fertility in apomictic plants is proposed.
A model of evolution of self-compatibility in apomixis
From observations on sexual polyploids, we can hypothesize that SC genotypes arise in apomicts, at least occasionally, as a consequence of polyploidization. Apomixis is expected to fix the genotypic constitution of S-alleles or other genetic factors controlling SI because they bypass meiosis and segregation. In pseudogamous apomicts, establishment of SC genotypes could be explained either by regular PSC or by a selection model for SC genotypes.
Not only the mode of origin, but also the population dynamics and fitness parameters need to be considered in the establishment and evolution of SC in apomicts. Apomixis is often connected to a lower female fertility, as shown by Michaels and Bazzaz (1986) and Hörandl (2008). Low pollen fertility in apomicts arising from hybridization is widespread (e.g., Asker and Jerling 1992; Izmailow 1996; Hörandl et al. 1997; Meirmans et al. 2006; Voigt et al. 2007; Van Dijk 2007), largely as a consequence of meiotic disturbances during microsporogenesis. Low pollen quality may also reduce female fitness because of partial failure of endosperm fertilization (Hörandl 2008). In the case of reduced male and female fitness, an apomict cannot establish itself within a predominantly sexual, outcrossing population, even if introgression of apomixis into sexual forms is taken into account (Hörandl and Temsch 2009). The apomict has a better chance to become established at some distance to the source population (Hörandl 2008).
Inside a sexual parental population, the SI pseudogamous apomictic plant represents a minority cytotype and will mainly receive haploid pollen from neighboring diploid plants. For endosperm fertilization, this is disadvantageous because the optimal endosperm balance is disrupted (e.g., a tetraploid apomict pollinated by haploid pollen would have a ratio of 8 m:1p in the endosperm, resulting in potential underdevelopment of the endosperm). The apomict consequently suffers from low fertility and fails to become established in the population. If another SI apomict of the same ploidy level is present within the population, it can act as a pollen donor, but this individual will allocate pollen only to endosperm formation; its genome is not transferred to the apomictic embryo. Therefore, the apomictic pollen donor does not allocate pollen to the production of its own offspring. Because of high pollen discounting, the genotype of the SI pollen donor will therefore also disappear from the population. The SI apomicts could produce offspring only if different cross-compatible genotypes are present in the population and if they would use their pollen exclusively for cross-pollination of another apomictic individual. This is unlikely in a mixed sexual–apomictic population. In fact, mathematical modeling predicts that SC is necessary for maintenance of pseudogamous apomictic populations (Noirot et al. 1997).
Outside the sexual population, a SI pseudogamous apomictic plant can only reproduce successfully if it grows within the pollinator radius and can be pollinated occasionally by another genotype. If seed set is successful, the apomictic mother plant will surround itself with its own maternal offspring. Most diaspores are just gravity dispersed and fall near the mother plant, resulting in local clones. Seed set in such clones would fail because of predominant pollination by neighboring plants and a SI reaction of genetically identical clone mates. Thus, the clone will go extinct after the first generation. Without SC, the apomictic clone comes into a dead end, because it will be mostly pollinated by SI pollen of the neighboring clone mates, which will result in seed abortion (Fig. 1).
Fig. 1.
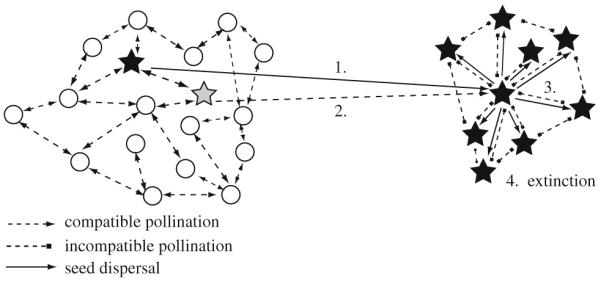
Model for the evolution of self-compatibility in pseudogamous apomicts. The apomicts (stars) are assumed to arise within a SI diploid sexual population (circles). A SI apomict (black star) largely fails to establish within the sexual populations because of a minority cytotype disadvantage and lower fertility than sexuals. SI pollen donors suffer from high pollen discounting and will disappear from the population (Noirot et al. 1997). If pollinated by another apomictic genotype (gray star), seeds may establish in some spatial distance to the source population (1), but this SI plant must be still pollinated by another genotype (2). The SI apomict surrounds itself with SI offspring (3) and goes extinct after the first generation (4) because of mother–offspring and sibling incompatibility. Any self-fertile genotype will be selected for
In this scenario, any SC genotype, although initially rare, will be rapidly selected for, because it can establish itself outside the sexual population within the pollinator’s radius, or even after long distance dispersal via single seeds. Within a sexual population, the SC genotype may not be able to increase frequencies of apomixis if fitness is lower than in sexual plants (Hörandl and Temsch 2009). Nevertheless, such SC plants may at least maintain their own genotype because they avoid a minority cytotype disadvantage (Levin 1975, 2002), which is effective because of endosperm fertilization. Moreover, apomictic reproduction bypasses recombination and segregation during meiosis and consequently also segregation of S-alleles; the allelic composition of the SC genotype will be fixed. The SC genotype will surround itself with its own maternal offspring, therefore providing sufficient pollen for endosperm fertilization. According to this model, even low initial frequencies of SC genotypes might come soon to fixation of SC in apomictic lineages.
For the origin of an SC apomictic genotype, two possibilities have to be considered: (1) SC is inherited from a polyploid, partly SC sexual ancestor, or has arisen via mutations or (2) PSC is not heritable but triggered continuously via mentor effects. Since frequencies of mentor effects are in general very low (Tas and Van Dijk 1999; Hörandl and Temsch 2009), a genetical model of heritable SC may better explain the observed predominance of SC.
SI pseudogamous apomictic lineages can reproduce successfully only if a diversity of S-alleles is maintained within a population. This factor may explain the fact that apomictic populations hardly ever display a strictly clonal population structure. Numerous population genetic studies have shown that apomictic lineages do indeed harbor genetic variation and usually show a few deviating genotypes even within predominantly clonal populations (reviewed by Gornall 1999; Hörandl et al. 2001; Hörandl and Paun 2007). Thus, different ploidy levels of sexuals and apomicts, a clonal population structure and fitness parameters may actually influence selection for SC.
To summarize, the evolution of SC in pseudogamous apomicts may involve the following steps:
SI and outcrossing of sexual progenitors promote interspecific hybridization and/or polypoidization.
Hybridization and polyploidization result at least partially in SC genotypes and trigger independently the shift to apomixis. Apomixis initially fixes both SI and SC genotypes. Alternatively (or additionally), large amounts of aborted pollen resulting from meiotic disturbances during microsporogenesis may induce a breakdown of SI via mentor effects (PSC).
Among the newly arisen apomictic plants, SC genotypes will be selected for because all forms of self-pollination contribute to female fecundity. Self-fertile genotypes can establish within and outside the parental population via self-pollination. Within a predominantly sexual population, apomictic SI genotypes will be limited because they represent a minority cytotype as recipients of pollen for endosperm formation. Moreover, apomictic SI genotypes will allocate pollen to other genotypes but not to the production of their own offspring. Outside the parental population, lack of pollen will limit seed set of SI genotypes, and occasional cross-pollination will just result in a local SI clone. This clone will suffer from low fertility because of SI reactions among clone mates. Selection will eliminate SI genotypes among the apomicts.
SC genotypes will be easier fixed in the population if apomixis is highly obligate and connected to a clonal population structure, but SI genotypes may be maintained in highly facultative apomicts with a high genotypic diversity within populations and consequently a high diversity of S-alleles.
Accordingly, the model applies mainly to pseudogamous apomicts with (1) at least a partial SC, (2) a polyploid cytotype that has a different ploidy level from that of the sexual progenitor and (3) a highly clonal population structure where SC allows for pollination among clone mates. This model, however, does not apply to apomicts with autonomous endosperm formation. Here, the SI system of sexual progenitors may be simply maintained because of the lack of selection against it (Van Dijk 2007). A higher female fertility of apomicts compared to sexual plants and reproductive assurance may in fact be the major evolutionary advantage of autonomous apomicts, such as dandelions (e.g., Van Dijk 2007).
Case studies
So far, no studies are available to explicitly test this hypothetical selection model for SC in pseudogamous apomicts. Nevertheless, information from three families indirectly supports the model.
Ranunculaceae–Ranunculus
Recent studies on the apomictic Ranunculus auricomus complex have given insights into possible evolutionary pathways causing self-compatibility to arise via polyploidization. Most polyploid taxa are aposporous, with a single-locus control of apospory, and have pseudogamous endosperm development (Rutishauser 1954; Izmailow 1966; Nogler 1971, 1984; Hörandl 2008). Molecular marker studies, flow cytometric and morphometric analyses strongly suggest that hybridization between diploid sexual cytotypes of R. carpaticola and autotetraploid sexual R. cassubicifolius has resulted in the origin of a hexaploid apomictic cytotype (R. carpaticola × R. cassubicifolius; Hörandl and Greilhuber 2002; Paun et al. 2006b; Hörandl et al. 2009). Polyploidization, however, is only partially connected to a change of SI systems. Pollinator exclusion tests via bagging of flowers revealed that the diploid sexual taxa of the R. auricomus complex (R. notabilis, R. cassubicifolius and R. carpaticola) are strictly self-incompatible with complete seed abortion after bagging. Autopolyploid sexual cytotypes of R. cassubicifolius randomly collected in a wild population were also self-incompatible; only individuals that have been produced by manual crossings of the genetically most distant parental individuals of the populations showed a partial breakdown of SI (Hörandl 2008). The crosses may have produced genotypes heterozygous for S-alleles that have the earlier described heteroallelic effects in the pollen. Such rare SC genotypes may have been involved in the parentage of the hexaploid apomictic hybrid cytotypes, resulting in at least partly SC apomicts. The hexaploid pseudogamous hybrid derivative (R. carpaticola × R. cassubicifolius) showed a breakdown of SI and normal seed set in all individuals examined after bagging experiments (Hörandl 2008). In accordance with the expectations of the model, the population structure of these hexaploid apomicts is highly clonal, and sexual and apomictic bio-types have different ploidy levels (Paun et al. 2006a). Self-fertility and their ability to establish founding populations may explain the distributional success of these hexaploid pseudogamous cytotypes (Hörandl et al. 2009).
In this experimental setup, PSC via high temperatures or aging was rather unlikely, but mentor effects cannot be ruled out. Apomictic plants usually produce high amounts of aborted pollen (e.g., Izmailow 1996;Hörandl et al. 1997; Voigt et al. 2007), resulting in mixtures of dead and viable self-pollen on the stigma. Mentor effects have been proved to occur in Taraxacum (Tas and Van Dijk 1999; Brock 2004), Hieracium (Mraz 2003) and in sexual taxa of Ranunculus (Hörandl and Temsch 2009), but occur in general at very low frequencies. Whether high proportions of aborted pollen can induce selfing in apomictic plants as well remains to be studied. If aborted pollen grains consistently trigger induced selfing, then the pseudogamous apomict could reproduce successfully, as long as a minor proportion of fertile self-pollen is being produced.
Poaceae–Paspalum
Among grasses, the genus Paspalum offers an interesting model system for studying the connection of self-fertilization and apomixis. Around 75% of the diploid sexual species are self-sterile, whereas in tetraploid sexual species, self-fertility is predominant. Several species have both SI diploid sexual and self-fertile apomictic pseudogamous polyploid cytotypes (3x, 4x, 5x, 6x; Quarin 1992). In contrast, diploid SC species of the genus do not have apomictic polyploid cytotypes. Quarin (1992) hypothesizes that selective forces for polyploidy and apomixis were missing in SC diploid sexual species. One of the best studied sexual/apomictic model systems is Paspalum notatum, a species with diploid sexual cytotypes with a genetical SI system and tetraploid apomictic SC cytotypes (Quarin 1992, 1999). Autotetraploid sexual cytotypes have been produced artificially by colchicine treating self-fertile plants after controlled self-pollination (Martínez et al. 2001). Here, the breakdown of the GSI system is probably caused by polyploidization as described earlier. Since shifts to apomixis must have occurred after polyploidization (Quarin 1992), SC in the apomicts could have been inherited from sexual autopolyploid progenitors. Experimental cross-pollinations of 4x apomictic plants of Paspalum notatum using various cytotypes as pollen donors confirmed that fertilization of endosperm nuclei and seed set arose independent from ploidy level of the pollen donor, albeit with a lower seed set after interploidal crosses (Quarin 1999). Chromosome counts of the endosperm confirmed that the two unreduced polar nuclei were fertilized by one sperm nucleus, suggesting a low sensitivity to endosperm imbalance (Quarin 1999). However, it cannot be ruled out that embryo sacs with only one polar nucleus can be also formed, as in other Panicoideae, and also in other species of Paspalum, which would maintain the optimal 2 m:1p genomic ratio in the endosperm (Matzk et al. 2000; Savidan 2007). Therefore, minority cytotype effects need to be studied. In accordance with a selection model for SC, genetic variation in isolated tetraploid apomictic populations of P. notatum is partly clonal (Daurelio et al. 2004).
Rosaceae
Similar to Ranunculus, the apomictic genera of Rosaceae are pseudogamous and show SI in sexual species and a breakdown of SI in polyploid apomicts in at least four genera (Table 2). Among hawthorns (Crataegus), the black-fruited species of sect. Douglasii are a well-studied model system with SI diploid sexual and SC polyploid apomictic taxa (Dickinson et al. 1996). Triploids likely originated via allopolyploidy, and backcrossing of allotriploids with the diploid parents might have resulted in allotetraploids (Lo et al. 2009). Crosses between different cytotypes and seeds with unbalanced endosperm are frequent. Thus, the endosperm balance requirement might be relaxed, probably by imprinting mechanisms acting before fertilization (Talent and Dickinson 2007; Talent 2009). Minority cytotype disadvantages because of endosperm imbalance might consequently select only weakly against SI in mixed populations. Crataegus suksdorfii comprises diploid sexual SI cytotypes with a restricted distribution in the Pacific Northwest, and triploid and tetraploid SC apomictic cytotypes with a broad distribution in western North America. Molecular population genetic studies suggest isolation by distance in the sexual SI populations, whereas gene flow in apomictic SC polyploid populations is not limited by distance, but rather by apomixis and SC. Genetic structure of polyploid apomicts suggest rapid colonization and successful establishment outside the diploid area, which is probably enhanced by bird dispersal. Genetic variation in the polyploid apomicts is at least partly clonal and mainly distributed among populations, whereas sexual plants have individual genotypes and genetic diversity distributed within populations (Lo et al.2009). In this model system, clonal population genetic structure and rapid establishment after long distance dispersal are probably the strongest selective forces for SC pseudogamous apomicts.
Pollination experiments using bagged flowers in naturally polyploid apomictic Rubus populations confirm pseudogamy, SC and the ability for autogamy (Kollmann et al. 2000); however, effects of crosses between ploidy levels and endosperm imbalance were not studied. Genetic variation in seedling populations was assessed using isoenzymes and AFLP markers and show a predominantly clonal population structure with only a low percentage of deviating genotypes (Kollmann et al. 2000). These preliminary results are also accordance with the expectations of a model that SC is selected for in clonal pseudogamous populations.
Suggestions and implications for future research
Many details in this hypothetical framework need to be analyzed further. First, we need to establish a broader database on the co-occurrence of pseudogamy, autonomous apomixis and self-compatibility in various apomictic taxa. A linkage of genetic control mechanisms has so far not been explored. The role of fertilization in seed development, endosperm development and the maintenance of endosperm balance need to be studied further to understand the relevance of SI systems for the functionality of apomixis. Here, it will be interesting to examine taxa that have both autonomous and pseudogamous endosperm formation (Table 2). Taxa with a low sensitivity of endosperm imbalance may allow for an evolutionary transition from pseudogamy to autonomous apomixis. In such cases, it is likely that SI will be maintained because of the lack of selection against it. If a balanced endosperm and pseudogamy is obligatory, then selection would rather favor a breakdown of SI.
The comparison of diploid sexual, polyploid sexual and polyploid apomicts, and the reconstruction of the evolutionary origin and parentage of polyploid apomicts will give insights into the evolution of genetic SI-systems via natural selection. Population genetic studies will provide information whether SI systems can be maintained through maintenance of sufficient genetic diversity within a population, or whether clonal population structure will favor a breakdown of SI via selection of SC genotypes. Further, it needs to be clarified whether a genetic SC system or PSC is actually the mechanism of a breakdown of SI. The functional background of the earlier mentioned mentor effect is barely understood, and it remains unclear whether aborted pollen is sufficient to cause PSC. Both experimental and physiological studies with aborted pollen and/or pollen of different ploidy levels would be useful to understand the functionality and frequencies of mentor effects.
These studies are relevant in answering further evolutionary questions. Without SC, pseudogamous apomixis loses the evolutionary advantage of reproduction via single individuals. The need of mating partners is seen as a major disadvantage of sexual reproduction (e.g., Bell 1982). The breakdown of SI, however, is an additional functional constraint for successful shifts from sexuality to apomixis. The rarity and difficulty of establishment of successful apomictic reproduction appear to be a major constraint in seed plants (Hörandl 2009a, b). Similar to animals (Engelstädter 2008), such constraints may actually limit frequencies of asexuality in natural populations and contribute to maintenance of sex in advanced eukaryotes (Hörandl 2009a, b). The ability of uniparental reproduction is probably also of major importance for understanding the wide distributions of apomictic plants (Baker 1967; Hörandl 2006, 2009a, b; Hörandl et al. 2008).
The study of SI systems, however, is also important for a major target of applied apomixis research, namely the production of apomictic crops. The fixation of heterozygous, highly vigorous genotypes with a favorable combination of alleles is seen as a major promise for increasing productivity in plant breeding (e.g., Spillane et al. 2001; Ozias-Akins and Van Dijk 2007; Curtis and Grossniklaus 2008; Ravi et al. 2008; Meadows 2009). For the large-scale production of apomictic crops, either SC or the shift to autonomous endosperm development is a prerequisite for seed set. The pollination dependence of endosperm development and sensitivity to endosperm imbalance in many grasses have turned out to be a major functional problem for the development of apomictic crops (Curtis and Grossniklaus 2008). If a single clone is growing on a crop field, the apomictic plants would mainly receive pollen from neighboring plants with the same genotype. To avoid infertility because of SI reactions among clone mates on a crop field, either SC or autonomous apomixis must be achieved.
Acknowledgments
This work was funded by the Austrian Research Foundation (FWF), P 19006-B03, and the Austrian Academy of Sciences, Commission for Interdisciplinary Ecological Studies (KIÖS), P 2007-03. I thank Timothy A. Dickinson, Scott D. Russell and one anonymous referee for valuable suggestions on the manuscript.
References
- Acuna CA, Martinez EJ, Quarin CL. Sexual diploid and apomictic tetraploid races in Thrasya petrosa (Gramineae) Austr J Bot. 2005;53:479–484. [Google Scholar]
- Asker SE, Jerling L. Apomixis in plants. CRC Press; Boca Raton, USA: 1992. [Google Scholar]
- Baker HG. Self compatibility and establishment after long distance dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- Baker HG. Support for Baker’s law—as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Baker HG, Stebbins GL. The genetics of colonizing species. Academic Press; New York: 1965. [Google Scholar]
- Barcaccia G, Mazzucato A, Albertini E, Zethof J, Gerats A, Pezzotti M, Falcinelli M. Inheritance of parthenogenesis in Poa pratensis L.: auxin test and AFLP linkage analyses support monogenic control. Theor Appl Genetics. 1998;97:74–82. [Google Scholar]
- Barcaccia G, Arzenton F, Sharbel TF, Varotto S, Parrini P, Lucchin M. Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity. 2006;96:322–334. doi: 10.1038/sj.hdy.6800808. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. The reproductive biology and genetics of island plants. Phil Trans Roy Soc London B Biol Sci. 1996;351:723–733. [Google Scholar]
- Barrett SCH, Harder LD, Worley AC. The comparative biology of pollination and mating in flowering plants. Phil Trans Roy Soc London B Biol Sci. 1996;351:1271–1280. [Google Scholar]
- Barringer BC. Polyploidy and self-fertilization in flowering plants. Amer J Bot. 2007;94:1527–1533. doi: 10.3732/ajb.94.9.1527. [DOI] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. California Press; Berkeley: 1982. [Google Scholar]
- Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile) Bot Rev. 2001;67:255–308. [Google Scholar]
- Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- Brock MT. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Amer J Bot. 2004;91:656–663. doi: 10.3732/ajb.91.5.656. [DOI] [PubMed] [Google Scholar]
- Burt A. Sex, recombination and the efficacy of selection-was Weismann right? Evolution. 2000;54:337–351. doi: 10.1111/j.0014-3820.2000.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Busch JW, Schoen DJ. The evolution of self-incompatibility when mates are limiting. Trends Pl Sci. 2008;13:128–136. doi: 10.1016/j.tplants.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Carino DA, Daehler CC. Genetic variation in an apomictic grass, Heteropogon contortus, in the Hawaiian Islands. Molec Ecol. 1999;8:2127–2132. doi: 10.1046/j.1365-294x.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc. 1997;61:51–94. [Google Scholar]
- Carman JG. The gene effect: genome collisions and apomixis. In: Savidan Y, Carman JG, Dresselhaus T, editors. The flowering of apomixis: from mechanisms to genetic engineering. CIMMYT; Mexico DF: 2001. pp. 95–110. [Google Scholar]
- Carman JG. Do duplicate genes cause apomixis? In: Hörandl E, Grossniklaus U, van Dijk P, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Gantner; Ruggell: 2007. pp. 169–194. [Google Scholar]
- Carr DE, Dudash MR. Recent approaches into the genetic basis of inbreeding depression in plants. Phil Trans Royal Soc London B. 2003;358:1071–1084. doi: 10.1098/rstb.2003.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Houliston GJ, Robson B, Iline I. A case of reversal: the evolution and maintenance of sexuals from parthenogenetic clones in Hieracium pilosella. Int J Pl Sci. 2003;164:719–728. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:237–268. [Google Scholar]
- Charlesworth D, Vekemans X, Castric V, Glemin S. Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytol. 2005;168:61–69. doi: 10.1111/j.1469-8137.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol. 2007;58:377–406. doi: 10.1146/annurev.arplant.58.032806.103835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genetics. 2005;6:836–846. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- Crnokrak P, Barrett SCH. Purging the genetic load: a review of experimental evidence. Evolution. 2002;56:2347–2358. doi: 10.1111/j.0014-3820.2002.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. Amphimixis and apomixis: two sides of the same coin. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner Ruggell; Liechtenstein: 2007. pp. 37–62. [Google Scholar]
- Curtis MD, Grossniklaus U. Molecular control of autonomous embryo and endosperm development. Sex Plant Reprod. 2008;21:79–88. [Google Scholar]
- Daurelio LD, Espinoza F, Quarin CL, Pessino SC. Genetic diversity in sexual diploid and apomictic tetraploid populations of Paspalum notatum situated in sympatry or allopatry. Pl Syst Evol. 2004;244:189–199. [Google Scholar]
- de Nettancourt D. Incompatibility and incongruity in wild and cultivated plants. 2nd edn. Springer; Berlin: 2001. [Google Scholar]
- Dickinson TA, Phipps JB. Studies in Crataegus L. (Rosaceae: Maloideae) XIV. The breeding system of Crataegus crus-galli sensu lato in Ontario. Amer J Bot. 1986;73:116–130. doi: 10.1002/j.1537-2197.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
- Dickinson TA, Belaoussof S, Love RM, Muniyamma M. North American black-fruited hawthorns. I. Variation in floral construction, breeding system correlates, and their possible evolutionary significance in Crataegus sect. Douglasii Loudon. Folia Geobot Phytotax. 1996;31:355–371. [Google Scholar]
- Dickinson TA, Lo E, Talent N. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Pl Syst Evol. 2007;266:59–78. [Google Scholar]
- Engelstädter J. Constraints on the evolution of asexual reproduction. BioEssays. 2008;30:1138–1150. doi: 10.1002/bies.20833. [DOI] [PubMed] [Google Scholar]
- Fehrer J, Krahulcová A, Krahulec F, Chrtek J, Rosenbaumová R, Bräutigam S. Evolutionary aspects in Hieracium subgenus Pilosella. In: Hörandl E, Grossniklaus U, van Dijk P, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Gantner Ruggell; Liechtenstein: 2007. pp. 359–390. [Google Scholar]
- Ferrer MM, Good-Avila SV. Macrophylogenetic analyses of the gain and loss of self-compatibility in the Asteraceae. New Phytol. 2006;173:401–414. doi: 10.1111/j.1469-8137.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- Friedman WE, Madrid EN, Williams JH. Origin of the fittest: relating female gametophyte development to endosperm genetics. Int J Pl Sci. 2008;169:79–92. [Google Scholar]
- Garcia R, Asins MJ, Forner J, Carbonell EA. Genetic analysis in Citrus and Poncirus by genetic markers. Theor Appl Genetics. 1999;99:511–518. doi: 10.1007/s001220051264. [DOI] [PubMed] [Google Scholar]
- Gornall RJ. Population genetic structure in agamospermous plants. In: Hollingsworth PM, Bateman RM, Gornall RJ, editors. Molecular systematics and plant evolution. Taylor and Francis; London: 1999. pp. 118–138. [Google Scholar]
- Grimanelli D, Leblanc O, Perotti E, Grossniklaus U. Developmental genetics of gametophytic apomixis. Trends Genet. 2001;17:597–604. doi: 10.1016/s0168-9525(01)02454-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson A. Apomixis in higher plants. Part II. The causal aspects of apomixis. Lunds Univ Arsskr. 1953;43:71–178. [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytol. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. Evolutionary implications of self-compatibility and reproductive fitness in the apomictic Ranunculus auricomus polyploid complex (Ranunculaceae) Int J Plant Sci. 2008;169:1219–1228. doi: 10.1086/591980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. Geographical parthenogenesis: opportunities for asexuality. In: Schön I, Martens K, Van Dijk P, editors. Lost sex. Springer; Heidelberg: 2009a. pp. 161–186. [Google Scholar]
- Hörandl E. A combinational theory for maintenance of sex. Heredity. 2009b doi: 10.1038/hdy.2009.85. (in press) (published online doi:10.1038/hdy.2009.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Greilhuber J. Diploid and autotetraploid sexuals and their relationships to apomicts in the Ranunculus cassubicus group:insights from DNA content and isozyme variation. Plant Syst Evol. 2002;234:85–100. [Google Scholar]
- Hörandl E, Paun O. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials and ecology. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner Ruggell; Liechtenstein: 2007. pp. 169–174. [Google Scholar]
- Hörandl E, Temsch E. Introgression of apomixis into sexual species is in the Ranunculus auricomus complex inhibited by mentor effects and ploidy barriers. Ann Bot. 2009;104:81–89. doi: 10.1093/aob/mcp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Dobeš C, Lambrou M. Chromosomenund Pollenuntersuchungen an österreichischen Sippen des Ranunculus auricomus-Komplexes. Bot Helv. 1997;107:195–209. [Google Scholar]
- Hörandl E, Jakubowsky G, Dobeš C. Isozyme and morphological diversity within apomictic and sexual taxa of the Ranunculus auricomus complex. Pl Syst Evol. 2001;226:165–185. [Google Scholar]
- Hörandl E, Cosendai A-C, Temsch E. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecol Div. 2008;1:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Greilhuber J, Klimova K, Paun O, Temsch E, Emadzade K, Hodálová I. Reticulate evolution and taxonomic concepts in the Ranunculus auricomus complex (Ranunculaceae): insights from morphological, karyological and molecular data. Taxon. 2009;58:1194–1215. [PMC free article] [PubMed] [Google Scholar]
- Huber W. Natürliche Bastardierungen zwischen weißblühenden Ranunculus-Arten in den Alpen (Natural hybridizations between white-flowered species of Ranunculus in the Alps) [German with English abstract] Veröff Geobot Inst ETH Zürich. 1988;100:1–160. [Google Scholar]
- Igic B, Bohs L, Kohn JR. Historical inferences from the self-incompatibility locus. New Phytol. 2003;161:97–105. [Google Scholar]
- Izmaiłow R. Macrosporogenesis in the apomictic species Ranunculus cassubicus. Acta Biol Cracov. 1966;8:183–195. [Google Scholar]
- Izmaiłow R. Reproductive strategy in the Ranunculus auricomus complex (Ranunculaceae) Acta Soc Bot Polon. 1996;65:167–170. [Google Scholar]
- Kantama L, Lambert Y, Hu HF, de Jong H, de Vries S, Russinova E. Use of the SSLP-based method for detection of rare apomictic events in a sexual AtSERK1 transgenic Arabidopsis population. Sex Plant Reprod. 2006;19:73–82. [Google Scholar]
- Kissling WD, Lord JM, Schnittler M. Agamospermous seed production of the invasive tussock grass Nardus stricta L. (Poaceae) in New Zealand–evidence from pollination experiments. Flora. 2006;201:144–151. [Google Scholar]
- Kollmann J, Steinger T, Roy BR. Evidence of sexuality in European Rubus (Rosaceae) species based on AFLP and allozyme analysis. Amer J Bot. 2000;87:1592–1598. [PubMed] [Google Scholar]
- Koltunow A, Grossniklaus U. Apomixis, a developmental perspective. Annu Rev Plant Biol. 2003;54:547–574. doi: 10.1146/annurev.arplant.54.110901.160842. [DOI] [PubMed] [Google Scholar]
- Krahulcová A, Suda J. A modified method of flow cytometric seed screen simplifies the quantification of progeny classes with different ploidy levels. Biol Plant. 2006;50:457–460. [Google Scholar]
- Leach C, Mayo O. Outbreeding mechanisms in flowering plants. J Cramer; Stuttgart: 2005. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. Oxford University Press; Oxford: 2002. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int J Pl Sci. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. Self- and cross-fertilization in plants. I. Functional dimensions. Int J Pl Sci. 1992;153:358–369. [Google Scholar]
- Lo EYY, Stefanovic S, Dickinson TA. Population genetic structure of diploid sexual and polyploidy apomictic hawthorns (Crataegus; Rosaceae) in the Pacific Northwest. Molec Ecol. 2009;18:1145–1160. doi: 10.1111/j.1365-294X.2009.04091.x. [DOI] [PubMed] [Google Scholar]
- Lundqvist A. The self-incompatibility system in Ranunculus repens (Ranunculaceae) Hereditas. 1994;120:151–157. [Google Scholar]
- Lundqvist A. Disomic control of self-compatibility in the tetraploid Ranunculus repens (Ranunculaceae) Hereditas. 1998;128:181–183. [Google Scholar]
- Mable BK. Polyploidy and self-compatibility: is there an association? New Phytol. 2004;162:803–811. doi: 10.1111/j.1469-8137.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- Martelotto LG, Ortiz JPA, Stein J, Espinoza F, Quarin CL, Pessino SC. A comprehensive analysis of gene expression alterations in a newly synthesized Paspalum notatum autotetraploid. Plant Sci. 2005;169:211–220. [Google Scholar]
- Martínez EJ, Urbani MH, Quarin CL, Ortiz JPA. Inheritance of apospory in bahiagrass, Paspalum notatum. Hereditas. 2001;135:19–25. doi: 10.1111/j.1601-5223.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant J. 2000;21:97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Meadows R. Engineering sexless seeds as a path to high-yield crops. PLos Biol. 2009;7(6):e1000118. doi: 10.1371/journal.pbio.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirmans PG, den Nijs HCM, Van Tienderen PH. Male sterility in triploid dandelions: asexual females vs. asexual hermaphrodites. Heredity. 2006;96:45–62. doi: 10.1038/sj.hdy.6800750. [DOI] [PubMed] [Google Scholar]
- Michaels HJ, Bazzaz FA. Resource allocation and demography of sexual and apomictic Antennaria parlinii. Ecology. 1986;67:27–36. [Google Scholar]
- Mogie M. The evolution of asexual reproduction in plants London. Chapman and Hall; UK: 1992. [Google Scholar]
- Mraz P. Mentor effects in the genus Hieracium s.str. (Compositae, Lactuceae) Folia Geobot. 2003;38:345–350. [Google Scholar]
- Naumova TN. Apomixis in angiosperms. Nucellar and integumentary embryony. CRC press; Boca Raton: 1993. [Google Scholar]
- Nogler GA. Genetik der Aposporie bei Ranunculus auricomus s.l. W. Koch. I. Embryologie. Ber Schweiz Bot Gesell. 1971;81:139–179. [Google Scholar]
- Nogler GA. Genetics of apospory in apomictic Ranunculus auricomus: 5 conclusion. Bot Helv. 1984;94:411–423. [Google Scholar]
- Noirot M, Couvet D, Hamon S. Main role of self-pollination rate on reproductive allocations in pseudogamous apomicts. Theor Appl Genet. 1997;95:479–483. [Google Scholar]
- Noyes RD. Apomixis in the Asteraceae: diamonds in the rough. Funct Pl Sci Biotechnol. 2007;1:207–222. [Google Scholar]
- Noyes RD, Soltis DE. Genotypic variation in agamospermous Erigeron compositus (Asteraceae) Amer J Bot. 1996;83:1292–1303. [Google Scholar]
- Nybom H. Active self-pollination in blackberries (Rubus subgen. Rubus, Rosaceae) Nordic J Bot. 1986;5:521–525. [Google Scholar]
- Ozias-Akins P, Van Dijk PJ. Mendelian genetics of apomixis in plants. Annu Rev Genetics. 2007;41:509–537. doi: 10.1146/annurev.genet.40.110405.090511. [DOI] [PubMed] [Google Scholar]
- Palop-Esteban M, Segarra-Moragues JG, Gonzalez-Candelas F. Historical and biological determinants of genetic diversity in the highly endemic triploid sea lavender Limonium dufourii (Plumbaginaceae) Mol Ecol. 2007;16:3814–3827. doi: 10.1111/j.1365-294X.2007.03449.x. [DOI] [PubMed] [Google Scholar]
- Paun O, Greilhuber J, Temsch E, Hörandl E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae) Molec Ecol. 2006a;15:897–910. doi: 10.1111/j.1365-294X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- Paun O, Stuessy TF, Hörandl E. The role of hybridization, polyploidization and glaciation in the origin and evolution of the apomictic Ranunculus cassubicus complex. New Phytol. 2006b;171:223–236. doi: 10.1111/j.1469-8137.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- Quarin CL. The nature of apomixis and its origin in panicoid grasses. Apomixis Newsl. 1992;5:8–15. [Google Scholar]
- Quarin CL. Effect of pollen source and pollen ploidy on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex Pl Repr. 1999;11:331–335. [Google Scholar]
- Rambuda TD, Johnson SD. Breeding systems of invasive alien plants in South Africa: does Baker’s rule apply? Diversity Distrib. 2004;10:409–416. [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst. 1998;29:467–501. [Google Scholar]
- Ravi M, Marimuthu MPA, Siddiqi I. Gamete formation without apomixis in meiosis. Nature. 2008;451:1121–1124. doi: 10.1038/nature06557. [DOI] [PubMed] [Google Scholar]
- Richards AJ. Plant breeding. Chapman and Hall; London, UK: 1997. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae) 2. Characters of the genus. Bull Brit Mus Nat Hist. 1981;8:55–226. [Google Scholar]
- Roy BA. The breeding system of six species of Arabis (Brassicaceae) Amer J Bot. 1995;92:1797–1810. [Google Scholar]
- Rutishauser A. Die Entwicklungserregung des Endosperms bei pseudogamen. Ranunculusarten Mitt Naturforsch Gesell Schaffhausen. 1954;25:1–45. [Google Scholar]
- Savidan Y. Apomixis in higher plants. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner; Ruggell: 2007. pp. 15–22. [Google Scholar]
- Siena LA, Sartor ME, Espinoza F, Quarin CL, Ortiz JPA. Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sex Plant Reprod. 2008;21:205–215. [Google Scholar]
- Spielmann M, Vinkenoog R, Scott RJ. Genetic mechanisms of apomixis. Phil Trans Roy Soc London B. 2003;358:1095–1103. doi: 10.1098/rstb.2003.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane C, Steimer A, Grossniklaus U. Apomixis in agriculture: the quest for clonal seeds. Sex Plant Reprod. 2001;14:179–187. doi: 10.1007/s00497-001-0117-1. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Variation and evolution in plants. Columbia University Press; New York: 1950. [Google Scholar]
- Stone JL. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. Quart Rev Biol. 2002;77:17–32. doi: 10.1086/339200. [DOI] [PubMed] [Google Scholar]
- Talent N. Evolution of gametophytic apomixis in flowering plants: an alternative model from Maloid Rosaceae. Theory Biosci. 2009;128:121–138. doi: 10.1007/s12064-009-0061-4. [DOI] [PubMed] [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytol. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Tas ICQ, Van Dijk PJ. Crosses between sexual and apomictic dandelions (Taraxacum) I. The inheritance of apomixis. Heredity. 1999;83:707–714. doi: 10.1046/j.1365-2540.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk PJ. Potential and realized costs of sex in dandelions, Taraxacum officinale s.l. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner; Ruggell: 2007. pp. 215–233. [Google Scholar]
- Van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms: a reappraisal. In: Bakker F, Chatrou L, Gravendeel B, Pelser PB, editors. Plant species-level systematics: new perspectives on pattern and process. Gantner; Ruggell: 2005. pp. 101–116. [Google Scholar]
- Vijverberg K, Van Dijk PJ. Genetic linkage mapping of apomixis loci. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner; Ruggell: 2007. pp. 137–158. [Google Scholar]
- Vinkenoog R, Bushell C, Spielman M, Adams S, Dickinson HG, Scott RJ. Genomic imprinting and endosperm development in flowering plants. Molec Biotechnol. 2003;25:149–184. doi: 10.1385/MB:25:2:149. [DOI] [PubMed] [Google Scholar]
- Voigt ML, Melzer M, Rutten T, Mitchell-Olds T, Sharbel TF. Gametogenesis in the apomictic Boechera holboellii complex: the male perspective. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. ARG-Gantner; Ruggell: 2007. pp. 235–258. [Google Scholar]
- Whitton J, Sears C, Baack EJ, Otto SP. The dynamic nature of apomixis in the angiosperms. Int J Pl Sci. 2008;169:169–182. [Google Scholar]


