Abstract
Background
Treatment of recent tuberculosis infection in children <2 years old is essential because of high risk of progression to disease, but diagnosis is hindered by the inaccuracy of the tuberculin skin test (TST). More accurate T cell-based tests for infection could enhance diagnosis by optimizing TST interpretation.
Methods
979 child tuberculosis contacts in Istanbul underwent TST and enzyme-linked immunospot (ELISpot) testing. Using ELISpot results as a reference standard, we assessed the effect of age and BCG-vaccination on sensitivity and specificity of TST, and computed optimal TST cut-off points (OCPs) using receiver operator characteristic curves.
Results
Using a ≥10mm TST cut-off point, sensitivity of TST was 66% in children <2y, lower than in older children (p=0.006). Specificity was 75% in BCG-vaccinated children, compared with 92% in unvaccinated children (p=0.001). OCPs improved TST specificity in children with 1 BCG scar with little loss of sensitivity. Despite use of OCPs, sensitivity of TST remained <70% in children <2y, specificity remained <87% in BCG-vaccinated children >2y and overall accuracy was low in children with >1 BCG scar.
Conclusions
Negative TST results cannot exclude tuberculosis infection in child tuberculosis contacts <2 years old, supporting use of preventive therapy regardless of TST results in this age group. In children >2 years old, accuracy of TST can be improved by adjustment of cut-off points in BCG-vaccinated children but remains poor in children with >1 BCG scar. This methodology can define optimal TST cut-off points for diagnosis of tuberculosis infection tailored to target populations.
Keywords: ELISpot, tuberculin skin test, BCG, tuberculosis, diagnosis
Introduction
Children with Mycobacterium tuberculosis infection are at high risk of developing active tuberculosis [1, 2]. Infected children <2 years of age have a ≥20% risk of progression to active disease, which rises to 50% in infants [2]. The risk falls in children >2 years and is approximately 5% in the 2-5 year old age group [2]. Given that isoniazid therapy prevents progression to tuberculosis [3], rapid, accurate diagnosis of childhood M tuberculosis infection is a global public health priority.
However, routine diagnosis of M. tuberculosis infection is based on the tuberculin skin test (TST), which handicaps management of childhood tuberculosis infection. Prior BCG vaccination may affect specificity of the TST [4], and some countries adjust TST cut-off points in vaccinated children. However, there is no consensus on whether, or how much, the TST cut-off point should be increased [5, 6].
Sensitivity of the TST in young children is unknown and guidelines for the management of child tuberculosis contacts therefore vary widely. Some national tuberculosis control programmes recommend isoniazid preventive therapy for young children on the basis of TST results [5, 7], while others recommend it for all child tuberculosis contacts under a certain age, regardless of TST results [6, 8-11].
T cell-based interferon-gamma release assays (TIGRA) represent a significant advance on the TST [12-15], and in this study, the interferon-gamma enzyme-linked immunospot (ELISpot) assay was utilized. Although further research is required, much published evidence indicates that in adults the ELISpot is more specific, and more sensitive than the TST [16-28]. Although there are fewer studies in children, the available evidence shows that the assay is more sensitive than TST in children with active tuberculosis [29-31], and although there is no gold-standard test for latent tuberculosis infection (LTBI), ELISpot appears more sensitive than TST in contact investigations in children and infants [17, 30, 32-34]. Thus, available evidence suggests that this ELISpot assay is more specific and more sensitive than TST for detection of LTBI in children.
However, TIGRAs are not yet suitable for all populations because they are expensive and require a blood sample and laboratory equipment. Therefore, most children may fail to benefit from this important medical advance. We reasoned that TIGRAs might be used to inform and improve the interpretation and diagnostic accuracy of TST. In the absence of a gold-standard test, we used ELISpot as a surrogate reference standard for LTBI to optimize use of TST in children with recent tuberculosis exposure. ELISpot was applied in parallel with TST in 979 child household contacts in Istanbul, Turkey, which has an intermediate prevalence of tuberculosis (40/100,000) [35], a very low prevalence of HIV-infection in children [36], and a policy of universal childhood BCG vaccination. We classified ELISpot-positive children as infected and ELISpot-negative children as uninfected and then assessed the effect of young age and BCG-vaccination on sensitivity and specificity of TST.
Methods and Materials
Study participants
All adults diagnosed with sputum smear-positive pulmonary tuberculosis at the 7 government-funded tuberculosis clinics in east Istanbul between October 2002 and May 2004 and who had children living in the household were invited to participate as previously described [30].
Ethical approval was granted by the Institutional Review Board of Marmara University School of Medicine, Istanbul, The Turkish Ministry of Health, Ankara and the World Health Organization Steering Committee on Research Involving Human Subjects, Geneva.
The Turkish Ministry of Health guidelines for BCG vaccination are as follows: all children are vaccinated intradermally with BCG Pasteur 1173-P2 (Serum Institute of India Ltd., Pune, India) between 2 and 3 months of age and a booster vaccination is administered in the first year of primary school, at 6 to 7 years of age. BCG vaccination coverage in Turkish children was 79% in 2004 [37].
Clinical evaluation
1,024 child contacts of the 443 index patients with sputum smear-positive pulmonary tuberculosis were enrolled at the Paediatric Infectious Diseases Clinic, Marmara University School of Medicine where medical histories were taken, physical examination and investigations performed and demographic information recorded, as previously described [30]. Out of a total of 1024 children enrolled, complete demographic, clinical, ELISpot and TST data were available for 979 as previously described [30].
TST
TST was administered by the Mantoux method as previously described [30]. For interpretation and analysis of TST induration, three different universal cut-off points of induration were used to define a positive TST result: ≥5mm; ≥10mm; ≥15mm.
Ex-vivo interferon-gamma ELISpot assays
ELISpot assays were performed as previously described [30, 32]. This assay has subsequently been developed into the regulatory approved commercially available T-SPOT.TB® assay (Oxford Immunotec, Abingdon, UK), which uses the same ESAT-6 and CFP-10 peptides. Our predefined cut-off point is the standard used in all previous studies based on our assay, amounting to 9 studies in 1916 participants [16, 17, 19, 20, 22, 29, 32, 38, 39]. Specificity and sensitivity of this assay are described in the corresponding studies [16, 17, 19, 20, 22, 29, 32, 38, 39] and recent reviews [13, 14, 34, 40].
Statistical methods
Determinants of disease prevalence, test sensitivity and specificity were identified using logistic regression modeling, the significance of each factor assessed using likelihood ratio and Wald tests. Observed receiver operator characteristic (ROC) curves were plotted for each subgroup, the areas under curves estimated using the trapezoidal rule, and the significance of differences in areas under the curve were tested.
Smoothed ROC curves were constructed to minimize random error and remove digit preference, before identifying optimal cut-off points (OCPs). Curves were fitted using the Dorfman and Alf maximum likelihood latent scale binormal model [41], and fitted values of sensitivity and specificity obtained at each observed cut-off point. Estimates of the probability of false-negative (Prob(FN)) and false-positive (Prob(FP)) diagnoses accounting for disease prevalence were computed for each cut-off point as:
OCPs were computed using prevalence of infection estimates obtained from the logistic regression model for each subgroup.
The first optimal cut-off point criteria minimized total diagnostic error, identifying the cut-off point at which the sum of the probabilities of false positive and false negative diagnoses of latent tuberculosis infection was lowest, which allowed prevalence of infection to be taken into account. We also report the range of cut-off points within which the probability of diagnostic error was within 2% of this minimum value to describe the sensitivity of overall performance to cut-off point selection. A second optimal cut-off point was defined as the cut-off point at which the consequences of diagnostic error were minimized. Whilst it is clear that false negative diagnoses (missing a case of LTBI) have greater consequences than false positive diagnoses (incorrectly diagnosing LTBI and giving unnecessary treatment), the relative disutility of these consequences is not known. We created a disutility score as:
where k was given values of 2, 5 and 10 corresponding to assumptions of consequences of false negative results being 2, 5 and 10 times more catastrophic than consequences of false positives and identified the cut-off points at which the score was minimized. Results are presented for a value of k=2. We report ranges of cut-off points with disutility values within 2% (relative to the maximum theoretical score) of the optimal cut-off point score.
Analyses were undertaken in Stata V9·0 (Stata Corporation, College Station, TX, USA) using roccomp and rocfit commands, and in Microsoft® Office Excel (Microsoft Corporation, Seattle, WA, USA).
Results
Demographic and clinical characteristics of study participants
Median age of the 979 child contacts of the 414 sputum-smear positive TB cases was 7 years [IQR 3, 11], 50·2% were male, and the average number of contacts per household was 2·5. 770 contacts (78·7%) were BCG-vaccinated, based on presence or absence of a BCG scar, including 115 contacts that had 2 scars and 3 contacts with 3 BCG scars.
TST and ELISpot results
Based on positive ELISpot results, 416 (42·5%) children were deemed infected with M tuberculosis. Proportions of TST-positive children were 60·5%, 50·8%, and 40·1% using cut-off points of ≥5mm, ≥10mm, and ≥15mm, respectively.
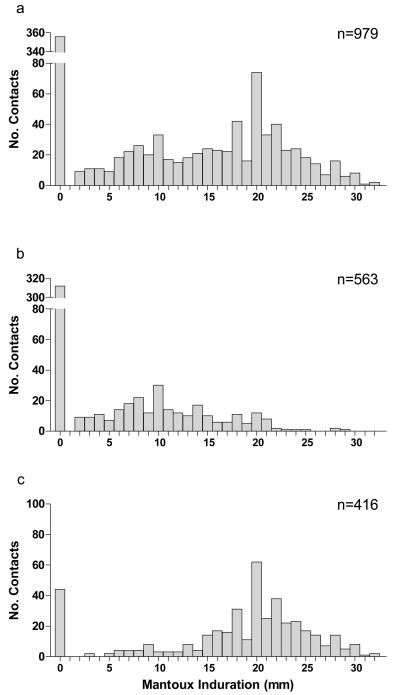
TST indurations for ELISpot-negative children (figure 1b) were lower than for ELISpot-positive children (figure 1c) (median [IQR]: 0 [0,10] mm and 20 [16,23] mm respectively, P<0·0001, Mann Whitney U-test).
Figure 1. Distribution of TST indurations stratified by ELISpot responses.
a illustrates TST indurations for the whole group n=979, b shows TST indurations from ELISpot-negative contacts, and c illustrates TST indurations from ELISpot-positive contacts. Stratification by ELISpot results revealed that the bimodal nature of the overall distribution of TST measurements arises through combining two distinct distributions with different modal values, reflecting the infected and uninfected subgroups.
Sensitivity and specificity of TST relative to ELISpot across a range of TST cut-off points
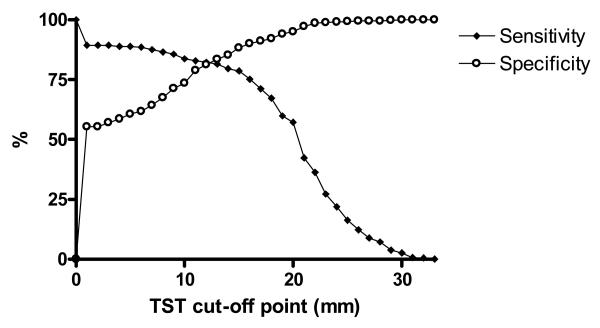
With a cut-off point of ≥1mm, the relative specificity remains above 50%, reaching a plateau at 25 to 30mm and the relative sensitivity starts at 89·4% gradually declining to 78·6% at 15mm and more steeply thereafter (figure 2).
Figure 2. Sensitivity and Specificity of the TST by cut-off point, using ELISpot as a reference standard for LTBI.
The filled diamond line represents sensitivity, and the hollow circle line represents specificity. Sensitivities were calculated as the cumulative proportion of ELISpot positive contacts with reactions equal to or larger than the TST cut-off point, and specificity as the cumulative proportion of ELISpot negative contacts with indurations smaller than the cut-off point.
Impact of age on sensitivity and specificity of TST relative to ELISpot
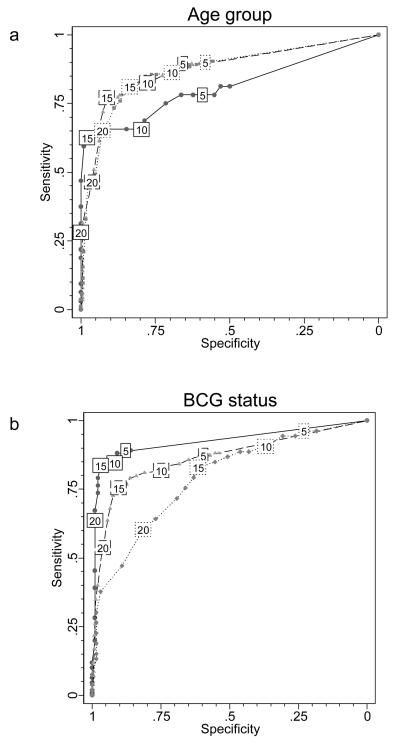
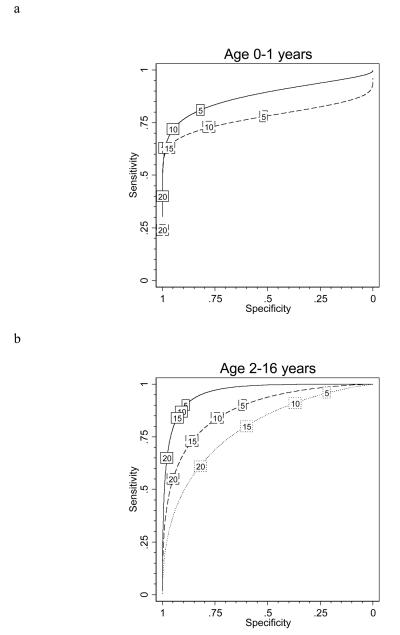
The shape of the ROC curve was different for children aged <2 years compared with older children (figure 3a). Using a ≥15mm cut-off point, relative sensitivity decreased from 80% in children aged 2-16 years to 63% in children aged <2 years (P=0·024) and from 85% to 66% using the ≥10mm cut-off point (P=0·006) (table 1). The ≥5mm cut-off point gave the best relative sensitivity of 78·1% in the under 2 year olds, which was still significantly lower than in older children (P=0·049), at the cost of a very low relative specificity of 59·2% (table 1). Thus at all cut-off points, relative sensitivity of TST was lower in children <2 years old, and even using the ≥5mm cut-off point, 7 infected infants would have been missed because of false-negative TST results.
Figure 3. Observed ROC curves of TST test performance compared with ELISpot results by age and BCG scar status.
a shows the observed ROC curves stratified by age group; the solid line indicates performance in those aged under 2 (n=130); the dashed line in those aged 2-5 (n=275); and the dotted line in those aged 6-16 years old (n=574). b illustrates the observed ROC curves stratified by BCG vaccination scar status; the solid line indicates performance in those with no scar (n=209); the dashed line in those with 1 scar (n=652) and the dotted line in those with 2 or more scars (n=118). Values in boxes indicate TST threshold in millimetres.
Table 1. Sensitivity and specificity of TST relative to ELISpot as a reference standard, stratified by age and BCG status.
| Cutpoint ≥5mm for all | Sensitivity | 95%CI | n | Specificity | 95%CI | n | ||
|---|---|---|---|---|---|---|---|---|
| All participants | 88.9 | 85.5 - 91.8 | 416 | 60.6 | 56.4 - 64.6 | 563 | ||
| Age groups | P-value cf <2yrs | P-value cf <2yrs | ||||||
| <2 | 78.1 | 60.0 - 90.7 | 32 | - | 59.2 | 48.8 - 69.0 | 98 | - |
| 2-5 | 89.5 | 82.3 - 94.4 | 114 | 0.099 | 65.8 | 58.0 - 73.1 | 161 | 0.282 |
| 6-16 | 90.0 | 85.8 - 93.3 | 270 | 0.051 | 58.2 | 52.5 - 63.8 | 304 | 0.867 |
| 2-16 combined | 89.8 | 86.4 - 92.7 | 384 | 0.049 | 60.9 | 56.3 - 65.3 | 465 | 0.758 |
| BCG vaccination status | P-value cf no BCG | P-value cf no BCG | ||||||
| no BCG | 89.1 | 81.7 - 94.2 | 110 | - | 88.9 | 81.0 - 94.3 | 99 | - |
| 1 scar | 87.4 | 82.6 - 91.2 | 253 | 0.641 | 59.7 | 54.7 - 64.5 | 399 | <0.0001 |
| >1 scar | 96.2 | 87.0 - 99.5 | 53 | 0.146 | 23.1 | 13.5 - 35.2 | 65 | <0.0001 |
|
Cutpoint ≥10mm for all | ||||||||
| All participants | 83.7 | 79.7 - 87.1 | 416 | 73.5 | 69.7 - 77.1 | 563 | ||
| Age groups | P-value cf <2yrs | P-value cf <2yrs | ||||||
| <2 | 65.6 | 46.8 - 81.4 | 32 | - | 79.6 | 70.3 - 87.1 | 98 | - |
| 2-5 | 82.5 | 74.2 - 88.9 | 114 | 0.044 | 77.6 | 70.4 - 83.8 | 161 | 0.711 |
| 6-16 | 86.3 | 81.6 - 90.2 | 270 | 0.004 | 69.4 | 63.9 - 74.5 | 304 | 0.053 |
| 2-16 combined | 85.2 | 81.2 - 88.6 | 384 | 0.006 | 72.3 | 67.9 - 76.3 | 465 | 0.137 |
| BCG vaccination status | P-value cf no BCG | P-value cf no BCG | ||||||
| no BCG | 84.5 | 76.4 - 90.7 | 110 | - | 91.9 | 84.7 - 96.4 | 99 | - |
| 1 scar | 81.8 | 76.5 - 86.4 | 253 | 0.529 | 74.9 | 70.4 - 79.1 | 399 | 0.001 |
| >1 scar | 90.6 | 79.3 - 96.9 | 53 | 0.297 | 36.9 | 25.3 - 49.8 | 65 | <0.0001 |
|
Cutpoint ≥15mm for all | ||||||||
| All participants | 78.6 | 74.3 - 82.5 | 416 | 88.3 | 85.3 - 90.8 | 563 | ||
| Age groups | P-value cf <2yrs | P-value cf <2yrs | ||||||
| <2 | 62.5 | 43.7 - 78.9 | 32 | - | 98.0 | 92.8 - 99.8 | 98 | - |
| 2-5 | 77.2 | 68.3 - 84.5 | 114 | 0.098 | 91.3 | 85.8 - 95.2 | 161 | 0.048 |
| 6-16 | 81.1 | 75.9 - 85.6 | 270 | 0.017 | 83.6 | 78.9 - 87.5 | 304 | 0.002 |
| 2-16 combined | 79.9 | 75.6 - 83.8 | 384 | 0.024 | 86.2 | 82.8 - 89.2 | 465 | 0.005 |
| BCG vaccination status | P-value cf no BCG | P-value cf no BCG | ||||||
| no BCG | 83.6 | 75.4 - 90.0 | 110 | - | 97.0 | 91.4 - 99.4 | 99 | - |
| 1 scar | 75.5 | 69.7 - 80.7 | 253 | 0.088 | 90.5 | 87.2 - 93.2 | 399 | 0.047 |
| >1 scar | 83.0 | 70.2 - 91.9 | 53 | 0.921 | 61.5 | 48.6 - 73.3 | 65 | <0.0005 |
Table 1 shows a trend towards lower relative specificity in older children which remained significant after adjustment for BCG vaccination status (test for trend across age-categories at a ≥15mm cut-off point: P=0·013). Despite the strong relationships between relative TST sensitivity and specificity with age, the area under the ROC curve, a measure of overall test accuracy, did not significantly differ between the three age-groups (0·81, 0·87 and 0·87 for 0-1, 2-5, 6-16 years respectively; test for difference P=0·54), as can occur when the relationships act in contrasting directions.
Impact of BCG on sensitivity and specificity of TST relative to ELISpot
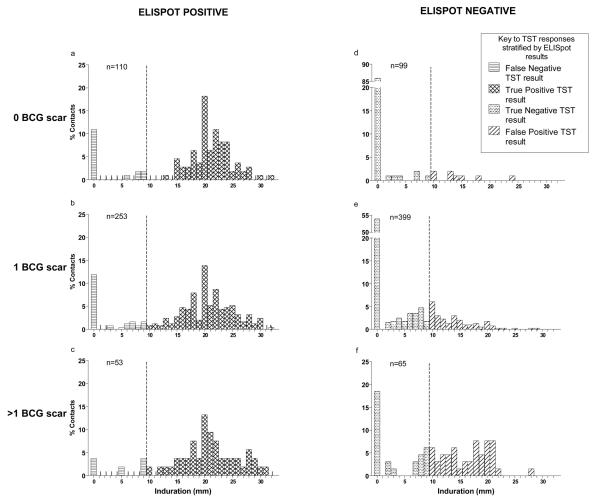
The area under the ROC curve became significantly smaller with increasing number of BCG scars (figure 3b), indicating a reduction in overall test accuracy (0·93, 0·86 and 0·79 for 0, 1, and >1 BCG scars respectively; test for difference P=0·003). The number of BCG scars significantly adversely affected TST specificity at all cut-off points but did not affect sensitivity (table 1). To investigate this further we stratified the distribution of Mantoux indurations by ELISpot results and number of BCG scars (figure 4).
Figure 4. Distribution of TST indurations stratified by RD1 ELISpot responses and BCG status.
Figures a, b, and c show TST indurations in ELISpot-positive children, and figures d, e, and f show the TST indurations for ELISpot-negative children. Figures a and d show TST indurations for children who were unvaccinated, b and e show TST indurations of children who exhibited 1 BCG scar, and c and f show TST indurations for children who had more than one BCG scar. The dashed-line represents a uniform ≥10 mm TST cut-off point defining positive and negative TST indurations. Distributions of TST indurations in ELISpot-positive children (figures 4a, b and c) were similar, indicating that increasing numbers of BCG scars does not affect TST sensitivity. In contrast, results for ELISpot-negative children (figures 4d, e and f) revealed a substantial reduction in the proportion of children with 0mm induration and an increase in the proportion with ≥10mm induration with increasing numbers of BCG vaccination scars, resulting in lower specificity. The percentage of ELISpot-negative contacts with TST results above 0 mm with no scar, 1 scar and >1 scar were 14%, 46% and 82% respectively (X2trend = 73.3, P<0.0001). The percentage of ELISpot-negative contacts with positive TST results, as defined by a cut-off point of ≥10mm, were 8%, 25% and 63% for children with no BCG scar, 1 BCG scar and >1 BCG scars respectively (X2trend = 59.3, P<0.0001).
Optimization of TST cut-off points to minimize diagnostic error relative to ELISpot
OCPs that minimized the sum of the probabilities of false-positive and false-negative diagnoses taking into account prevalence of infection were identified from smoothed ROC curves generated for groups defined by age (<2 vs. 2-16) and BCG scar status (0 vs. 1 vs. >1) (figure 5 and table 2). The 2-5 and 6-16 age-groups were combined as there was no significant difference in prevalence of infection or TST performance between the groups (table 1 and figure 3a).
Figure 5. Fitted ROC curves of TST test performance compared with ELISpot results according to age and BCG scar status.
Fitted ROC curves of TST test performance compared with ELISpot results according to age and BCG scar status for a, under 2 year olds, and b 2-16 year olds. Solid lines indicate performance in those with no scar; dashed lines in those with 1 scar; dotted lines in those with 2 or more scars. Values in boxes indicate TST threshold in mm.
Table 2. Optimal cut-off points for the study population stratified by age and BCG status.
Optimal cut points were computed for the prevalence identified in the study population. OCP = Optimal Cut-off Point, CP = Cut-off Point, PPV = Positive Predictive Values, NPV = Negative Predictive Values. The range of cut-off points on either side of the OCP which only increased the error rate by a maximum of 2% was also identified for each group. In our study population, TST performance was most accurate in unvaccinated children aged 2-16 in whom approximately 9 out of every 10 diagnoses will be correct. Performance is slightly lower in BCG naïve children aged <2 years, where 7 out of 8 diagnoses will be correct. In vaccinated children aged 2-16, TST was erroneous once in every 5 diagnoses for those with 1 BCG scar, and once in every 3 diagnoses for those with more than 1 BCG scar.
| Minimise total error rate |
Minimise disutility score with cost FN twice cost of FP |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BCG scars | Sample size | Prevalence* (%) | OCP | CP with error rates within 2% of OCP |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Error rate of CP (%) | OCP | CP with scores within 2% of OCP |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Error rate of CP (%) |
| <2 | 0 | 33 | 33 | 13 | (10–15) | 69 | 97 | 91 | 86† | 13 | 10 | (7-13) | 72 | 95 | 87 | 87 | 13 |
| <2 | 1 | 97 | 22 | 16 | (14–17) | 62 | 98 | 91 | 90† | 10 | 16 | (13-16) | 62 | 98 | 73 | 91 | 10 |
| 2-16 | 0 | 176 | 56 | 2 | (2–15) | 91 | 88 | 90 | 89 | 10 | 2 | (2-10) | 91 | 88 | 90 | 89 | 10 |
| 2-16 | 1 | 555 | 42 | 15 | (11–18) | 73 | 86 | 79 | 81 | 20 | 10 | (2-15) | 84 | 74 | 70 | 86 | 22 |
| 2-16 | >1 | 118 | 42 | 20 | (18–21) | 61 | 82 | 72 | 74 | 27 | 16 | (11-19) | 78 | 63 | 61 | 80 | 31 |
The results are based on the observed prevalence of infection in the 5 population subgroups. Optimal cut-off points were also computed for three arbitrary prevalences of infection; 5%, 20% and 40%. At 40% infection prevalence the optimal cut-off points were lower than at lower levels of prevalence. In children aged <2 years, optimal cut-off points appeared relatively unaffected by infection prevalence: a cut-off point of ≥15mm was robust except in unvaccinated children where disease prevalence was 40%, when a lower cut-off point of ≥10mm had the lowest error rate (16%). Cut-off point selection in children aged >2 years was more directly affected by prevalence. For unvaccinated children, cut-off points of ≥20, ≥15 and ≥10mm fell within a 2% margin of error of the optimal computed cut-off points at prevalences of 5%, 20% and 40%, respectively. Cut-off points for children with one scar were around 5mm higher, and for those with 2 or more scars around 10mm higher than in unvaccinated children (data not shown). Despite the use of optimal cut-off points tailored to different levels of infection prevalence, the overall error rate of TST relative to ELISpot increased with increasing prevalence of infection in the target population (data not shown).
Despite the lower sensitivity of TST in children aged under 2 years, the NPV in this age group is similar to that observed in older children because the prevalence of infection in children under 2 years is half that in the older children.
Table 2 shows the OCPs for prevalences of infection estimated from the observed data. Results are presented for two different analytical strategies (see Statistical Methods). Estimated performance of TST relative to ELISpot at these OCPs and the range of cut-off points adjacent to the OCPs with similar overall performance are shown in table 2.
In unvaccinated children >2 years where relative TST specificity and the prevalence of infection are high (table 2), the probability of false-positive results is low. Optimization therefore computed a low cut-off point of ≥2mm to minimize the summed probabilities of false-negative and false-positive results.
Because of the serious adverse clinical consequences of false-negative results in children aged <2 years, we calculated OCPs for disutility multipliers of k=5 and k=10 (see Statistical Methods). In vaccinated children aged <2 years, at the observed population infection prevalence of 22%, OCPs using disutility multipliers of 5 and 10 were, ≥13mm and ≥11mm, respectively. In unvaccinated children aged <2 years, a disutility multiplier of 10 gave an OCP of ≥4mm, which amounted to a cut-off of any observed response (as 4mm was the smallest observed response in this group); even this OCP yielded a relative diagnostic sensitivity of only 81%. If the disutility multiplier (k) exceeded 10.7, treating all patients would be a preferable strategy than even this lowest cut-off point option, given the observed study prevalence.
However, even using computed OCPs, the overall error rate of TST relative to ELISpot, rises from 10% in unvaccinated children >2 years old to 20% or more in children with ≥1 BCG vaccination scars (table 2).
OCPs were also calculated for the 5 groups using arbitrary prevalences of 5%, 20% and 40%. Cut-off point selection for children <2 years old was unaffected by prevalence of infection, while OCP selection for children aged >2 years was related to prevalence (Table 2, footnote).
Discussion
The lack of a gold standard for LTBI greatly complicates interpretation of TST results which in turn represents a substantial obstacle to improving tuberculosis control. Setting cut-off points has therefore relied upon empirical comparison of TST indurations in presumptively infected and uninfected populations. Such analyses have hitherto been based on population distributions of TST results from patients with active tuberculosis or hypothetical distributions of TST results computed by subtracting the distribution of TST results in unexposed individuals from that in tuberculosis contacts [42, 43]. We used ELISpot results as a surrogate reference standard in child tuberculosis contacts to assess the effect of BCG vaccination and young age on specificity and sensitivity of TST and optimize TST cut-off points.
The lower relative sensitivity of TST in children <2 years old suggests that the delayed type hypersensitivity response to M. tuberculosis infection in infants is weaker than in older children [44]. However, ELISpot can detect very low levels of T cell responses to M. tuberculosis infection. This explains its high diagnostic sensitivity relative to TST in infants [17, 33] and young children [29] with immature cellular immune systems, and in HIV-infected individuals [12, 22, 29].
Our analysis to minimize diagnostic error assumed that the consequences of false-positive and false-negative results were equal, but in practice the clinical consequences of not treating infected children aged <2 years are more severe than treating uninfected children, because of the high risk of progression to tuberculosis and its attendant high morbidity and mortality [1, 45, 46]. In order to account for how different clinical outcomes affect clinicians' interpretation of diagnostic test results, we computed an alternative set of OCPs which minimized a disutility score. This weighted the cut-off point selection process in favor of minimizing false-negative results and generated cut-off points that were equal to or lower than cut-off points for minimizing the diagnostic error score, as relative TST sensitivity was maximized at the cost of relative specificity. Given the imperative for early treatment of tuberculosis infection in very young children, our results suggest that TST lacks sufficient sensitivity to reliably rule out a diagnosis of tuberculosis infection amongst household contacts in this age group. Some national guidelines recommend that child contacts <2 years old should receive isoniazid preventive therapy on the basis of positive TST results [5]. Our results lend greater support to a policy of giving universal preventative therapy to all child tuberculosis contacts <2 years, regardless of TST results.
In children aged 2-5 years, sensitivity of TST relative to ELISpot was not significantly different to its performance amongst children aged over 6 years. Therefore, given that children >2 years are at substantially lower risk of primary progression to active disease than younger children [2], and given that the relative sensitivity of TST did not increase further with increasing age, 2 years may be a suitable age threshold above which TST results can be used to guide targeting of isoniazid preventive therapy to child contacts.
In unvaccinated children, TST specificity was high but declined progressively as the number of previous BCG vaccinations increased. In ELISpot-negative children with >1 scar, indurations of ≥20mm were not uncommon. The recommended cut-off points in Turkey, while slightly different to our OCPs, nonetheless performed within a 2% margin of error compared with the OCPs that were computed to minimize disutility where k=2.
The OCPs represent the best possible performance for the TST in this population, given ELISpot as the reference standard; however, even these gave error rates between 10 and 27%. The problem was most pronounced in children with >1 BCG scar. Several countries perform a second BCG vaccination in children (e.g. Turkey and Russia) and use TST to diagnose LTBI [10]. Previous studies reached contradictory conclusions about the impact of repeat BCG vaccination on TST [47-49]. Our results indicate that repeat vaccination has a substantial impact on TST induration which renders interpretation of TST unreliable, and even use of OCPs results in a high proportion of false-positive results amongst child contacts.
In contrast, TST specificity in children under 2 years was high, despite the close proximity in time to BCG vaccination (table 1). Thus, notwithstanding the poor sensitivity of TST in children under 2 years, its high specificity, using a ≥15mm cut-off point, makes a positive TST result a reliable marker of tuberculosis infection in very young children.
Specificity of TST varies across different populations and regions of the world and depends, in part, on the level of environmental mycobacterial exposure, as well as BCG vaccination status [4, 50]. Thus, while our OCPs are of direct relevance to clinical practice in Turkey, they cannot be extrapolated to other populations. Our approach for deriving OCPs, however, is generalizable. Where deployment of TIGRAs is not yet possible, testing sentinel populations by TST and TIGRA would enable tuberculosis control programmes to set more accurate TST cut-off points tailored to the whole target population.
In contrast to the consistent evidence from low and medium prevalence countries for correlation of TIGRA results with tuberculosis exposure, a recent Gambian study using a variation of our assay with different thresholds for scoring results, sensitivity and specificity [51, 52] found poor correlation and further work is required in high-burden settings. Although the ELISpot assay used in our study represents an improvement on TST [12, 16-19, 21, 22, 25, 32, 38] and is already recommend by several national guidelines, it is not a perfect test of LTBI and our OCPs will have been based on imperfect diagnoses in a proportion of our population,
Our findings could inform tuberculosis control policy. The low sensitivity of TST in children <2 years old supports the use of isoniazid preventive therapy in these household contacts regardless of TST results. In children with two BCG scars, TST specificity was very poor even after adjusting the cut-off point. Given that a second BCG vaccination confers no additional protection against tuberculosis disease [53] or infection [30] yet renders the TST almost uninterpretable, it may be impeding tuberculosis control efforts. Finally, our study provides a mechanism through which the scientific advance of T cell-based testing could be used to improve management of childhood tuberculosis infection in resource-limited settings.
Acknowledgements
We dedicate this article to Prof. Mujdat Basaran, who died 6 years ago. He was the founder of the Department of Paediatrics, Marmara University School of Medicine, and contributed to tuberculosis immunology research with many publications. We thank all the children who took part in the study, and their parents. We thank Zeliha Arslan and Filiz Ozturk for referring contacts from the tuberculosis clinics in Istanbul and Imogen Staveley for contributing to the reading and analyzing of ELISpot assays. We acknowledge the crucial support of the Istanbul Association for the Fight Against Tuberculosis, and the physicians and nurses of the seven government-run tuberculosis clinics in the Asian side of Istanbul. We also thank The Department of Tuberculosis of The Ministry of Health of Turkey for their support. We are very grateful to Ben Marais, Luca Richeldi, Frank Cobelens, Hans Rieder, Beate Kampman, Susan Liebeshuetz, Paul Newton, Paul Heath, Paul Fine and John Innes for critical appraisal of the manuscript and helpful discussions. This work was funded by the Wellcome Trust and the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). DPSD's PhD studentship was supported by the Sir Halley Stewart Trust. JJD was funded by a Department of Health Senior Research Scientist in Evidence Synthesis Award. KAM was supported by a Wellcome Trust Prize studentship. AL is a Wellcome Senior Research Fellow in Clinical Science.
Footnotes
Conflict of Interest Statement Professor Lalvani is lead inventor for several patents underpinning T cell-based diagnosis. The Lalvani ELISpot was commercialised by an Oxford University spin-out company (T-SPOT.TB®, Oxford Immunotec Ltd, Abingdon, UK) in which Oxford University and Professor Lalvani have minority shares of equity. Professor Lalvani acted as non-executive director to Oxford Immunotec from 2003-07. Dr Dosanjh and Dr Millington are named inventors on patents relating to T cell-based diagnosis. No other authors have a potential conflict of interest.
References
- 1.Gedde-Dahl T. Tuberculous infection in the light of tuberculin matriculation. Am J Hyg. 1952 Sep;56(2):139–214. doi: 10.1093/oxfordjournals.aje.a119547. [DOI] [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med. 2006 May 15;173(10):1078–90. doi: 10.1164/rccm.200511-1809SO. [DOI] [PubMed] [Google Scholar]
- 3.Hsu KH. Thirty years after isoniazid. Its impact on tuberculosis in children and adolescents. JAMA. 1984 Mar 9;251(10):1283–5. doi: 10.1001/jama.251.10.1283. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax. 2002 Sep;57(9):804–9. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Chronic Conditions . Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians; London: 2006. Tuberculosis. [PubMed] [Google Scholar]
- 6.American Thoracic Society Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000 Apr;161(4 Pt 1):1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 7.Fundaçao Nacional de Saude Brasilia Tuberculose - Guia de Vigilancia Epidemiologica Ministerio de Saude. 2002:1–99. [Google Scholar]
- 8.South African Department of Health The South African Tuberculosis Control Programme - Practical Guidelines. 2000 [Google Scholar]
- 9.The World Health Organization Treatment of Tuberculosis: Guidelines for National Programmes. 2003 [Google Scholar]
- 10.Türkiye Cumhuriyeti Sağlık Bakanlığı A. Türkiye'de tüberkülozun kontrolü için başvuru kitabı. 2003. http://wwwistanbulsaglikgovtr/w/sb/bh/belge/tuberkitabipdf.
- 11.Chauhan LS, Arora VK. Management of pediatric tuberculosis under the revised national tuberculosis control programme. Indian J Pediatr. 2004 Apr;71(4):341–3. doi: 10.1007/BF02724102. [DOI] [PubMed] [Google Scholar]
- 12.Richeldi L. An Update on the Diagnosis of Tuberculosis Infection. Am J Respir Crit Care Med. 2006 Jun 23; doi: 10.1164/rccm.200509-1516PP. [DOI] [PubMed] [Google Scholar]
- 13.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007 Mar 6;146(5):340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007 Jun;131(6):1898–906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 15.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004 Dec;4(12):761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001 Jun 23;357(9273):2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 17.Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004 Aug 1;170(3):288–95. doi: 10.1164/rccm.200403-307OC. [DOI] [PubMed] [Google Scholar]
- 18.Shams H, Weis SE, Klucar P, et al. Enzyme-linked Immunospot and Tuberculin Skin Testing to Detect Latent Tuberculosis Infection. Am J Respir Crit Care Med. 2005 Nov 1;172(9):1161–8. doi: 10.1164/rccm.200505-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalvani A, Pathan AA, McShane H, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001 Mar;163(4):824–8. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 20.Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001 Nov 1;167(9):5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 21.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005 Aug;24(8):529–36. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 22.Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. Aids. 2002 Nov 22;16(17):2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 23.Zellweger JP, Zellweger A, Ansermet S, de Senarclens B, Wrighton-Smith P. Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis. 2005 Nov;9(11):1242–7. [PubMed] [Google Scholar]
- 24.Arend SM, Thijsen SF, Leyten EM, et al. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med. 2007 Mar 15;175(6):618–27. doi: 10.1164/rccm.200608-1099OC. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara G, Losi M, D'Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006 Apr 22;367(9519):1328–34. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 26.Porsa E, Cheng L, Graviss EA. Comparison of an ESAT-6/CFP-10 peptide-based enzyme-linked immunospot assay to a tuberculin skin test for screening of a population at moderate risk of contracting tuberculosis. Clin Vaccine Immunol. 2007 Jun;14(6):714–9. doi: 10.1128/CVI.00073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang YA, Lee HW, Hwang SS, et al. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007 Sep;132(3):959–65. doi: 10.1378/chest.06-2805. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Choi HJ, Park IN, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006 Jul;28(1):24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 29.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004 Dec 18;364(9452):2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 30.Soysal A, Millington KA, Bakir M, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005 Oct 22-28;366(9495):1443–51. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 31.Detjen AK, Keil T, Roll S, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007 Aug 1;45(3):322–8. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 32.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003 Apr 5;361(9364):1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L, Ewer K, Losi M, et al. T-cell-based diagnosis of neonatal multidrug-resistant latent tuberculosis infection. Pediatrics. 2007 Jan;119(1):e1–5. doi: 10.1542/peds.2006-1057. [DOI] [PubMed] [Google Scholar]
- 34.Lalvani A, Millington KA. T cell-based diagnosis of childhood tuberculosis infection. Curr Opin Infect Dis. 2007 Jun;20(3):264–71. doi: 10.1097/QCO.0b013e32813e3fd8. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization World Health Organization Global TB database. 2005. http://wwwwhoint/tb/country/global_tb_database/en/indexhtml.
- 36.Turkish Ministry of Health Reported cases and carriers of AIDS distribution by age and gender. 2005. http://www.saglik.gov.tr/extras/istatistikler/2005geribildirim/Tablo%2021htm.
- 37.World Health Organization Immunization profile - Turkey. 2005. http://www.who.int/immunization_monitoring/en/globalsummary/countryprofileselectcfm.
- 38.Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001 Feb 1;183(3):469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 39.Dosanjh DP, Hinks TS, Innes JA, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med. 2008 Mar 4;148(5):325–36. doi: 10.7326/0003-4819-148-5-200803040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008 Aug 5;149(3):177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorfman DD, Alf E. Maximum liklihood estimation of parameters of signal detection theory-a direct solution. [Binormal ROC curve-dichotomous diagnostic test]. Psychometrika. 1968;33:117–24. doi: 10.1007/BF02289677. [DOI] [PubMed] [Google Scholar]
- 42.Berkel GM, Cobelens FGJ, de Vries G, Draayer-Jansen IWE, Borgdorff MW. Tuberculin skin test: estimation of positive and negative predictive values from routine data. Int J Tuberc Lung Dis. 2005;9(3):310–6. [PubMed] [Google Scholar]
- 43.Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969 Apr;99((4):Suppl):1–132. [PubMed] [Google Scholar]
- 44.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004 May;8(5):658–74. [PubMed] [Google Scholar]
- 45.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974 Feb;99(2):131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 46.Starke JR. Childhood tuberculosis during the 1990s. Pediatr Rev. 1992 Sep;13(9):343–53. doi: 10.1542/pir.13-9-343. [DOI] [PubMed] [Google Scholar]
- 47.Murray DL, Brewer TF. Multiple bacille Calmette-Guerin vaccinations and interpretation of the Mantoux test in children. Clin Infect Dis. 1995 Dec;21(6):1533–4. doi: 10.1093/clinids/21.6.1533. [DOI] [PubMed] [Google Scholar]
- 48.Shaw LW. Field studies on immunization against tuberculosis. I. Tuberculin allergy following BCG vaccination of school children in Muscogee County, Georgia. Public Health Rep. 1951 Nov 2;66(44):1415–6. [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus JH, Khassis Y. The tuberculin sensitivity in BCG vaccinated infants and children in Israel. Acta Tuberc Pneumol Scand. 1965;46(2):113–22. [PubMed] [Google Scholar]
- 50.Black GF, Weir RE, Floyd S, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002 Apr 20;359(9315):1393–401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 51.Hill PC, Brookes RH, Fox A, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004 Apr 1;38(7):966–73. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- 52.Hill PC, Brookes RH, Adetifa IM, et al. Comparison of enzyme-linked immunospot assay and tuberculin skin test in healthy children exposed to Mycobacterium tuberculosis. Pediatrics. 2006 May;117(5):1542–8. doi: 10.1542/peds.2005-2095. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues LC, Pereira SM, Cunha SS, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005 Oct 8;366(9493):1290–5. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]