Abstract
Successfully using artificial insemination (AI) is defined as getting cows pregnant when the farmer wants them in-calf and making the best use of appropriate genetic potential. Over the past 30 to 50 years, the percentage of animals in oestrus that stand-to-be-mounted (STBM) has declined from 80% to 50%, and the duration of STBM from 15 h to 5 h; both in parallel with a reduction in first-service-pregnancy-rate from 70% to 40%. Meanwhile, the incidence of lameness and mastitis has not decreased; and it takes more than an extra 40 and 18 days, respectively, to get a lame or mastitic cow in-calf compared to healthy herd-mates. The intensity of oestrus is 50% lower in severely lame cows, and fewer lame cows ovulate. Luteal phase milk progesterone concentrations are also 50% lower in lame cows, and follicular phase oestradiol is also lower in non-ovulating lame cows compared to ovulating animals. Furthermore, lame cows that do not ovulate do not have an LH surge, and the LH pulse frequency in their late follicular phase is lower (0.53 v. 0.76 pulses/h). Thus, we suggest that the stress of lameness reduces LH pulsatility required to drive oestradiol production by the dominant follicle. The consequent low oestradiol results in less-intense oestrus behaviour and failure to initiate an LH surge; hence there is no ovulation. A series of experimental studies substantiate our hypothesis that events activating the hypothalamus–pituitary–adrenal axis interfere at both the hypothalamus and the pituitary level to disrupt LH and oestradiol secretion, and thus the expression of oestrus behaviour. Our inability to keep stress at a minimum by appropriately feeding and housing high-production cows is leading to a failure to meet genetic potential for yield and fertility. We must provide realistic solutions soon, if we want to successfully use AI to maintain a sustainable dairy industry for the future.
Keywords: oestrus, oestradiol, progesterone, LH, stress, lameness, mastitis
Background
The keyword in the title is ‘successfully’ – partially defined as getting cows pregnant when the farmer wants them pregnant, i.e. voluntarily, not because he could not get them pregnant at any other time. The other part of the definition refers to the appropriate use of genetic potential. A bull can successfully inseminate cows but there are few on-farm bulls available with the desired genes, and many adult bulls can be dangerous when running with a herd. Some farmers use a hand-mating bull system by which cows are selected by the herdsman to be introduced into the bull pen but this entails the many disadvantages involved in oestrus detection by humans.
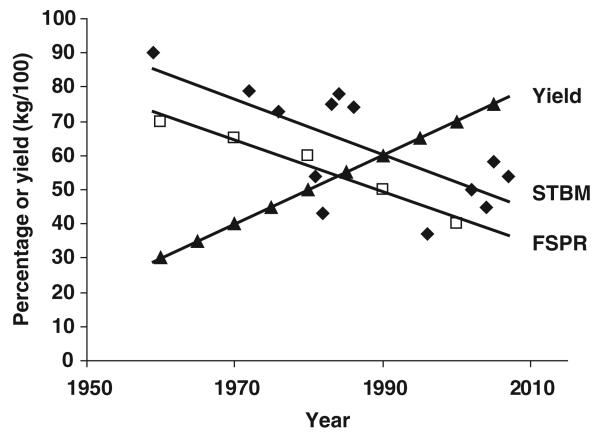
There is substantial evidence that fertility of the modern dairy cow is getting lower with increasing milk yields (Royal et al., 2000; Butler, 2003). Along with this documented decline, the literature over the recent past reveals a parallel decrease in the percentage of cows standing-to-be-mounted (STBM; Figure 1; Hall et al., 1959; Williamson et al., 1972; Esslemont and Bryant, 1976; Glencross et al., 1981; Fonseca et al., 1983; Stevenson et al., 1983; Hackett and McAllister, 1984; Britt et al., 1986; Pennington et al., 1986; Van Vliet and Van Eerdenburg, 1996; LeBlanc et al., 1998; Lyimo et al., 2000; Van Eerdenburg et al., 2002; Roelofs et al., 2004 and 2005a; Walker et al., 2008).
Figure 1.
Percentage of animals standing-to-be-mounted (STBM; ◆), first-service-pregnancy-rate (FSPR; □) and average milk yield (▲) in Holstein Friesian dairy cows reported over the last 50 years (references in text).
Thus, in research studies, although the duration of total primary and secondary signs of oestrus has not changed significantly over the past 30 to 50 years, the percentage of animals STBM (Figure 1) and the duration of STBM have both declined (Table 1). Furthermore, there is evidence that high milk production increases the number of silent heats (averages of 0.7 v. 1.6 silent heats for 28 and 36 kg/day, respectively; Harrison et al., 1990). So, in practical terms, it is not surprising that fewer herdsmen are seeing cows in oestrus. These observations have, of course, been associated with a marked decline in first-service-pregnancy-rate (FSPR; Figure 1). Coupled with the decline in farm labour on dairy units, it is no wonder that it is getting more difficult to successfully artificially inseminate (AI) dairy cows to get them pregnant when required.
Table 1.
Summary of the literature regarding the first and last sign (duration) of behavioural oestrus, or stood-to-be-mounted (STBM) event, within one oestrus period
| Reference | Method | Mean (h) | Range (h) |
|---|---|---|---|
| Wishart, 1972 | Visual | 14.7 ± 1.6 | 10 to 18 |
| Hurnik et al., 1975 | Visual (24 h/day) | 7.5 to 10.1 | |
| Esslemont and Bryant, 1976 | Visual (24 h/day) | 14.9 ± 4.7 (s.d.) | |
| Britt et al., 1986 | Visual (8 h intervals) | 13.8 ± 0.6 (s.e.) | |
| Coe and Allrich, 1989 | Visual (24 h/day) | 14.9 ± 0.7 (s.e.) | 2 to 27 |
| Lyimo et al., 2000 | Visual (30 min every 3 h) | 20.3 ± 10.4 (s.d.) | 6 to 33 |
| Stevenson et al., 1996 | HeatWatch | 14 ± 0.8 (s.e.) | 2.5 to 26 |
| Walker et al., 1996 | HeatWatch | 9.5 ± 6.9 (s.d.) | |
| Dransfield et al., 1998 | HeatWatch | 7.1 ± 5.4 (s.d.) | 0.5 to 36 |
| Xu et al., 1998 | HeatWatch | 8.6 ± 0.46 (s.e.) | 1 to 21 |
| At-Taras and Spahr, 2001 | HeatWatch | 5.83 ± 0.78 (s.e.) | |
| Lopez et al., 2002 | HeatWatch | 3.6 ± 0.8 | 0.2 to 12 |
| Cavalieri et al., 2003 | HeatWatch | 10.9 | 10 to 12 |
| Visual (30 min every 3 h) | 14.4 | 13 to 16 | |
| Roelofs et al., 2005b | Visual (30 min every 3 h) | 11.8 ± 4.4 (s.e.) | |
| STBM only | 5.0 ± 3.0 (s.e.) | ||
| Roelofs et al., 2005a | Pedometers | 10.0 ± 4.2 | |
| Walker et al., 2008 | Visual (30 min every 3 h) | 15.2 ± 1.3 (s.e.) | 3 to 24 |
| STBM only | 10.0 ± 1.1 (s.e.) | 3 to 18 |
HeatWatch refers to an electronic pressure recording device placed on the sacrolumbar region of cows to record STBM.
Factors predisposing to lower fertility and disrupted oestrus
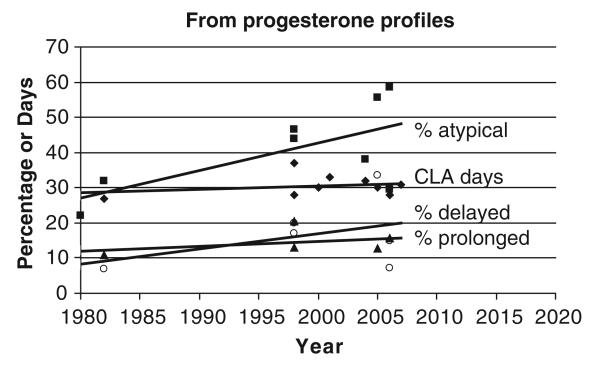
There are several (clinical) ‘production diseases’ associated with lower fertility. We know that low BCS in the early post partum period results in >10 extra days to establish a pregnancy (Lopez-Gatius et al., 2003; Garnsworthy, 2006), whereas cows that have had hypocalcaemia take 13 days longer to get pregnant (Parker, 1992). The calving-to-pregnancy interval is at least 18, 25 and 31 days longer in cows treated for mastitis, retained foetal membranes or endometritis, respectively, compared to healthy herd-mates (Borsberry and Dobson, 1989; Schrick et al., 2001). Lame cows are even less fertile, as it takes them up to an extra 40 days to get pregnant even after treatment (Collick et al., 1989; Melendez et al., 2003; Hernandez et al., 2005; Figure 2). Reviewing studies of milk progesterone profiles, but without detailed acknowledgement of production diseases, the percentage of atypical profiles tends to increase with time (P = 0.08), and also possibly the percentage of cows with delayed onset of luteal activity or with prolonged luteal phases (Figure 3; the observations are too few for rigorous statistical analysis; Bulman and Wood, 1980; Etherington et al., 1991; Opsomer et al., 1998; Royal et al., 2000; Veerkamp et al., 2000; Fulkerson et al., 2001; Horan et al., 2005; Shrestha et al., 2005; McCoy et al., 2006; Petersson et al., 2006a; Patton et al., 2007). A delay in the resumption of ovarian cyclicity after calving certainly contributes to the increased calving–pregnancy interval in diseased animals, for example, an extra 7 days in cows with mastitis and 17 days for lame cows (Huszenicza et al., 2005; Petersson et al., 2006b). However, this does not account for all the delay in getting mastitic or lame cows pregnant again. Once ovarian cyclicity has resumed, the ability to express oestrus is also important.
Figure 2.
Days from calving to pregnancy in cows with different clinical production diseases (RFM retained foetal membranes; BCS body condition score; references in text).
Figure 3.
Days from calving to commencement of luteal activity (CLA; ◆), and percentage of atypical profiles (■), of delayed onset of luteal activity (▲) and prolonged luteal phases (○) reported from milk progesterone studies over the past 30 years (references in text).
Oestrus, follicles and hormones in diseased cows
In view of our early observations that lame cows are less fertile than clinically ‘normal’ cows, we have been assessing the effects of this particular production disease on oestrous behaviour. Increasing severities of lameness (defined in Table 2) have no impact on the incidence of oestrus once ovarian cyclicity has been spontaneously resumed (oestrus observed per period of low progesterone: 20/32, 11/18, 12/17 for not lame, moderately lame and severely lame cows, respectively; Walker et al., 2008). However, the intensity of oestrus was lower in severely lame cows using a weighted scoring system to quantify the intensity of all signs (Van Eerdenburg et al., 2002; scoring system summarised in Table 3; normal n = 18: 583.1 ± 64.9 points; moderately lame n = 9: 657.8 ± 96.8 points; severely lame n = 9: 284.4 ± 42.7; P<0.05).
Table 2.
Lameness scoring scalea
| Lameness score | Description | While standing | While walking | Gait |
|---|---|---|---|---|
| 1 | Non-lame | Level back posture | Level back posture | Normal |
| 2 | Moderately lame | Level back posture | Arched back | Normal to short striding |
| 3 | Severely lame | Arched posture | Arched back | Takes one step at a time; reluctant to bear weight on one or more limbs/feet |
Modified after a previously described 5-point scale (Sprecher et al., 1997) in which the above scores of 1, 2 and 3 are comparable to the scores of 1, 2 and ⩾3 on the Sprecher 5-point scale, respectively.
Table 3.
Point scoring scale for behavioural signs of oestrusa
| Oestrus signs | Points |
|---|---|
| Flehmen | 3 |
| Restlessnessb | 5 |
| Sniffing the vulva of another cow | 10 |
| Mounted but did not stand | 10 |
| Resting chin on the back of another cow | 15 |
| Mounting the rear of another cow | 35 |
| Mounting the head of another cow | 45 |
| Stood-to-be-mounted (STBM) | 100 |
Each time an oestrus sign was observed, the assigned number of points were recorded (Van Eerdenburg et al., 2002).
Can only be recorded once during a single 30-min observation period.
This prompted a more careful evaluation of oestrus behaviour by looking at the frequency of each component of behaviour in groups of eight to 12 cows that had been synchronized with GnRH, followed 7 days later with prostaglandin (GnRH + PG; to increase the number of cows simultaneously in oestrus; adapted from Pursley et al., 1995). Table 4 shows that lame cows (score 1 v. 2 + 3) had a less-intense oestrus than non-lame cows (fewer total points), because the frequencies and duration of certain behaviours were lower in lame cows. Mounting the rear of another cow is an appetitive (courtship) behaviour and chin-resting plus being-mounted-but-not-standing can be construed as ‘testing’ behaviours to determine if cows will STBM. Daily milk progesterone concentrations 4 to 9 days before these oestrus observations were lower in lame cows but surprisingly oestradiol values in the same daily milk samples were not different between lameness groups (Walker et al., 2008). It is well known that prior progesterone exposure in ruminants has a marked effect on the intensity of oestrous behaviour (Fabre-Nys and Martin, 1991).
Table 4.
Mean ± s.e. (range) of the total frequency and duration of behavioural signs of oestrus in non-lame and lame cows (Adapted from SL Walker, personal communication)
| Oestrous signs | Total frequency | Duration (h) | |
|---|---|---|---|
| Sniffing vulva | Non-lame | 20.2 ± 3.1 (0 to 41) | 13.2 ± 1.2 (0 to 18) |
| Lame | 21.9 ± 3.1 (1 to 49) | 12.0 ± 1.2 (3 to 21) | |
| Chin resting | Non-lame | 36.3 ± 5.6 (16 to 78) | 14.4 ± 1.3 (3 to 24) |
| Lame | *24.4 ± 3.6 (0 to 59) | 12.0 ± 1.3 (0 to 21) | |
| Mounting rear of another cow | Non-lame | 14.1 ± 3.4 (1 to 42) | 11.4 ± 1.6 (3 to 24) |
| Lame | **6.1 ± 1.1 (0 to 19) | 9.3 ± 1.3 (0 to 18) | |
| Mounted but did not stand | Non-lame | 2.1 ± 0.7 (0 to 10) | 5.2 ± 1.5 (0 to 15) |
| Lame | 0.9 ± 0.4 (0 to 7) | **1.8 ± 0.7 (0 to 12) | |
| Stood-to-be-mounted (STBM) | Non-lame | 9.3 ± 2.0 (2 to 26) | 10.0 ± 1.2 (3 to 18) |
| Lame | 5.8 ± 1.4 (0 to 18) | 6.8 ± 1.3 (0 to 15) | |
| Total intensity points | Non-lame | 2200 ± 300 | |
| Lame | ** 1400 ± 200 |
Lower values in lame cows (n = 18) compared to non-lame (n = 15) are shown in bold.
P<0.10,
P<0.05
In a subsequent study of ovarian follicular growth and ovulation after GnRH + PG synchronisation, we have recently established that fewer lame cows ovulate compared to non-lame animals (26/37 v. 17/18), although dominant follicles grow to a similar size (15 to 20 mm pre-ovulation). Milk progesterone profiles prior to the follicular phase were lower in lame cows, thus confirming our earlier observations, and oestradiol concentrations in plasma samples every 4 h were lower in non-ovulating lame cows compared to ovulating non-lame cows. In addition, all the lame cows that did not ovulate did not have a surge of luteinising hormone (LH; analysed in 2-hourly blood samples) and the LH pulse frequency in the late follicular phase was lower in non-ovulating lame cows than in ovulating cows (0.53 v. 0.76 pulses/h; P = 0.012). Thus, we suggest that the stress of lameness reduces LH pulsatility to drive oestradiol production by the dominant follicle; the consequent low oestradiol fails to initiate an LH surge and hence there is no oestrus behaviour and no ovulation.
During on-going studies, the ovaries of 52 cows treated by farmers for mastitis were scanned weekly by ultrasound and had dominant ovarian follicles on average 2 mm smaller than in paired healthy herd-mates (G Lloyd, personal communication). Furthermore, cows prone to mastitis, i.e. those with >100 000 somatic cells per ml milk (SCC), appeared to ovulate after GnRH + PG 1 day later than herd-mates with <100 000 SCC (5.5 ± 2.4 v. 4.6 ± 2.2 days, n = 16 and 15, respectively; K Kaneko and S Uppal, personal communication).
Experimental proof
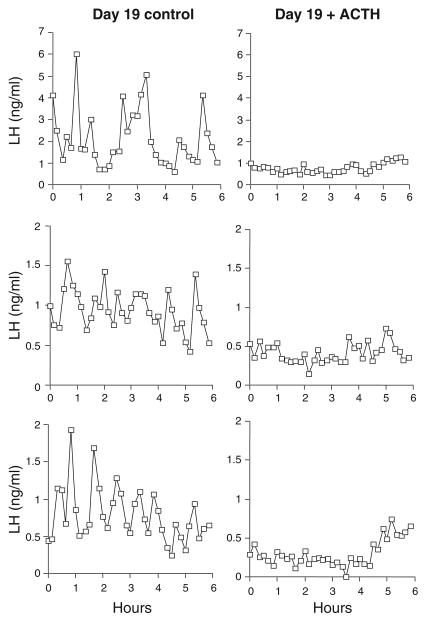
All our studies on the stress of lameness and mastitis have been observational; is there any supporting evidence from experimental studies in cows? There is none regarding long-term chronic activation of the hypothalamus-pituitary–adrenal axis. However, short-term administration of the synthetic corticoid, betamethasone, from day 10 to 19 of the oestrous cycle prevents the normal increase in oestradiol at the end of the cycle, thus inhibiting luteolysis that results in prolonged luteal phases and a 10-day delay in the occurrence of oestrus (Kanchev et al., 1976). In addition, road transport or betamethasone reduces the amount of LH released by exogenous GnRH; and road transport delays and attenuates the LH surge induced by exogenous oestradiol (Dobson, 1987; Dobson et al., 1987; Nanda et al., 1990). Furthermore, acute stimulation with adrenocorticotrophin hormone (ACTH) in the late follicular phase suppresses LH pulsatility, decreases oestradiol concentrations in peripheral plasma, eliminates the LH surge and results in very delayed or absent ovulation (Dobson et al., 2000; Figures 4 and 5). All these studies substantiate our hypothesis that events activating the hypothalamus–pituitary–adrenal axis (i.e. stressful situations such as lameness or mastitis) interfere at both the hypothalamus and the pituitary level to disrupt LH and oestradiol secretion, and thus the expression of oestrus behaviour.
Figure 4.
Peripheral plasma concentrations of LH in three control heifers on day 19 of the oestrous cycle (a) and in three heifers on day 19 during treatment with 100 IU ACTH every 12 h for 7 days from day 15 of the oestrous cycle (b). Dobson et al., 2000: reproduced with permission.
Figure 5.
Mean ± s.e. plasma oestradiol in the upper panel, FSH in the middle panel and follicle diameters in the lower panel for (○) six control heifers, (△) five heifers that formed a prolonged follicle and (□) six heifers that formed a persistent follicle after treatment with 100 IU ACTH every 12 h for 7 days from day 15 of the cycle. Stars indicate control oestradiol values different from prolonged and persistent follicle oestradiol values (P < 0.05). Dobson et al., 2000: reproduced with permission.
Solutions
Hence, there lies the problem; what can we do about it now? One solution may be to treat lame cows with progesterone prior to a chosen insemination period, but this is throwing drugs at the effect, rather than addressing the cause.
Prevention of production diseases
Extracting a variety of estimates concerning the incidence of lameness and mastitis in dairy herds throughout the world, there does appear to be an increasing trend (although not statistically significant) in spite of many attempts at prevention (Figure 6; Bigras-Poulin et al., 1990; Grohn et al., 1990; Kaneene and Hurd, 1990; Tranter and Morris, 1991; Bartlett et al., 1992; Lam et al., 1993; Oltenacu and Ekesbo, 1994; Chamberlain and Wassell, 1995; Hemsworth et al., 1995; Clarkson et al., 1996; Esslemont and Kossaibati, 1996; Etherington et al., 1996; Enting et al., 1997; Frei et al., 1997; Judge et al., 1997; Beckett et al., 1998; Shpigel et al., 1998; Duffield et al., 1999; Loeffler et al., 1999; Galindo et al., 2000; Stevenson, 2000; Heuer et al., 2001; Leonard et al., 2001; Hultgren, 2002; Regula et al., 2004; Haskell et al., 2006). Within these studies, the average incidence ± s.e. (and range) of lameness was 15.2 ± 2.2% (2–54), and for mastitis 27.4 ± 2.2% (6–48). Indeed, the overall incidence of clinical diseases recently reported for UK farms is alarming but it is clear that the ‘best’ 25% farmers are capable of reducing the impact (Table 5). No doubt preventative efforts are being made because these diseases are damaging both on welfare grounds and in financial terms; estimates per annum for the UK national herd are £160 million for lameness and £100 million for mastitis.
Figure 6.
Incidence of lameness (■) and mastitis (◇) in dairy cows reported over the last 50 years (references in text).
Table 5.
Annual incidence and mean % culling rates associated with the production diseases of dairy cows in >219 herds in UK 1998–2002 (derived from Whitaker et al., 2004)
| ‘Worst’ 25% farms | ‘Best’ 25% farms | Overall mean for all farms | |
|---|---|---|---|
| Annual incidence of clinical cases (%) | |||
| Fertility problems | 21.4 | ||
| Lameness | 41.5 | 2.4 | 20.7 |
| Mastitis | 76.4 | 8.2 | 38.2 |
| Involuntary culling (%) | |||
| Infertility | 7.6 | 2.9 | 5.5 |
| Lameness | 2.7 | 1.0 | 1.8 |
| Mastitis | 6.7 | 1.8 | 4.2 |
Prevention is better than cure, and one approach is to develop on-farm schemes to prevent production diseases. These schemes certainly have a positive short-term impact to lower the incidence (Kingwill et al., 1970; Green et al., 2007), but effects on fertility have not been reported.
As far as preventing the losses associated with heat detection is concerned, again all farmers and vets have sufficient information available now to make improvements but despite demonstrable technical efficacy and cost effectiveness, uptake is low. To improve the situation, there is a need for mutual encouragement to address motivation and specific barriers on each farm, without which progress will be limited (Garforth et al., 2006).
More appropriate use of genetics
Another solution to avoid the deleterious impact of production diseases on oestrus expression is to involve selective breeding. Regrettably, in 2003 across 15 countries, the average relative genetic emphasis for production, durability and health/reproduction was 59.5%, 28% and 12.5%, respectively (Miglior et al., 2005). Greater attempts should be made to redress this balance with respect to lameness and mastitis, and several countries now include fertility indices in selection traits. However, more forward-looking approaches also need to be explored. In dairy cattle, most clinical treatments, for example re-lameness or mastitis, take place from 1 week before to 10 weeks after calving (Zwald et al., 2004). Thus, calving is a period of great risk to a dairy cow and is to be avoided. Less frequent calving with more persistent lactations would be an advantage. Over a 3-year period (the UK average cow life-span after first calving), there really need only two high-risk periods (calvings), not the traditional three as at present. Persistent lactations achieve lower and later peak yields, but reasonable long-term milk production must be maintained to be financially acceptable. Progress through selective breeding should be possible as the heritability for persistent lactation has been estimated at 0.09 to 0.18 compared to 0.03 to 0.19 for fertility (Dekkers et al., 1998; Haile-Mariam et al., 2003; Muir et al., 2004).
Conclusion
The stresses and strains of high milk production have led animals onto a knife-edge; thus, anything (such as clinical disease, inadequate nutrition, poor housing) will tip cows off balance, thus disrupting hormonal equilibrium, reducing oestrus intensity, lowering LH that results in failure of ovulation and consequent sub-fertility. On-farm schemes to prevent lameness and mastitis, coupled with genetic approaches to improve persistency of lactation, are called for. Phenotypic trends during the last 20 years show that genetic improvement accounted for ~60% of the total increase in milk yield (JP Chenais and F Miglior, personal communication). Thus, production traits have contributed twice as much as durability/health traits, and in the Genotype–Environment interaction, the environment (how we keep animals, i.e. animal husbandry) has not kept pace with genetic ‘advances’. Our inability to appropriately feed and house high-yielding cows is leading to increased stress and thus a failure to meet genetic potential for yield and fertility. We must provide realistic solutions soon, if we want to use AI successfully to maintain a sustainable dairy industry in the future.
Footnotes
This invited paper was presented at BSAS meeting ‘Fertility in Dairy Cows – bridging the gaps’ 30–31 August 2007, Liverpool Hope University.
References
- At-Taras EE, Spahr SL. Detection and characterization of estrus in dairy cattle with an electronic heatmount detector and an electronic activity tag. Journal of Dairy Science. 2001;84:792–798. doi: 10.3168/jds.S0022-0302(01)74535-3. [DOI] [PubMed] [Google Scholar]
- Bartlett PC, Miller GY, Lance SE, Heider LE. Clinical mastitis and intramammary infections on Ohio dairy farms. Preventive Veterinary Medicine. 1992;12:59–71. [Google Scholar]
- Beckett S, Lean I, Dyson R, Tranter W, Wade L. Effects of monensin on the reproduction, health, and milk production of dairy cows. Journal of Dairy Science. 1998;81:1563–1573. doi: 10.3168/jds.S0022-0302(98)75722-4. [DOI] [PubMed] [Google Scholar]
- Bigras-Poulin M, Meek AH, Martin SW, McMillan I. Health-problems in selected Ontario Holstein cows – frequency of occurrences, time to first diagnosis and associations. Preventive Veterinary Medicine. 1990;10:79–89. [Google Scholar]
- Borsberry S, Dobson H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Veterinary Record. 1989;124:217–219. doi: 10.1136/vr.124.9.217. [DOI] [PubMed] [Google Scholar]
- Britt JH, Scott RG, Armstrong JD, Whitacre MD. Determinants of estrous behavior in lactating Holstein cows. Journal of Dairy Science. 1986;69:2195–2205. doi: 10.3168/jds.S0022-0302(86)80653-1. [DOI] [PubMed] [Google Scholar]
- Bulman DC, Wood PDP. Abnormal patterns of ovarian activity in dairy cows and their relationships with reproductive performance. Animal Production. 1980;30:177–188. [Google Scholar]
- Butler WR. Energy balance relationships with follicular development, ovulation and fertility in post partum dairy cows. Livestock Production Science. 2003;83:211–218. [Google Scholar]
- Cavalieri J, Flinker LR, Anderson GA, Macmillan KL. Characteristics of oestrus measured using visual observation and radiotelemetry. Animal Reproduction Science. 2003;76:1–12. doi: 10.1016/s0378-4320(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain AT, Wassell TR. The size and cost of the estimation error when using analysis of a sample of a dairy herd to assess whole herd performance. Preventive Veterinary Medicine. 1995;23:65–71. [Google Scholar]
- Clarkson MJ, Downham DY, Faull WB, Hughes JW, Manson FJ, Merritt JB, Murray RD, Russell WB, Sutherst JE, Ward WR. Incidence and prevalence of lameness in dairy cattle. Veterinary Record. 1996;138:563–567. doi: 10.1136/vr.138.23.563. [DOI] [PubMed] [Google Scholar]
- Coe BL, Allrich RD. Relationship between endogenous estradiol-17 beta and estrous behavior in heifers. Journal of Animal Science. 1989;67:1546–1551. doi: 10.2527/jas1989.6761546x. [DOI] [PubMed] [Google Scholar]
- Collick DW, Ward WR, Dobson H. Associations between types of lameness and fertility. Veterinary Record. 1989;125:103–106. doi: 10.1136/vr.125.5.103. [DOI] [PubMed] [Google Scholar]
- Dekkers JCM, Ten Hag JH, Weersink A. Economic aspects of persistency of lactation in dairy cattle. Livestock Production Science. 1998;53:237–252. [Google Scholar]
- Dobson H. Effect of transport stress on luteinizing hormone released by GnRH. Acta Endocrinologica. 1987;115:63–66. doi: 10.1530/acta.0.1150063. [DOI] [PubMed] [Google Scholar]
- Dobson H, Alam MGS, Kanchev LN. Effect of betamethasone treatment on luteal life-span and the response to GnRH in dairy cows. Journal of Reproduction and Fertility. 1987;80:25–30. doi: 10.1530/jrf.0.0800025. [DOI] [PubMed] [Google Scholar]
- Dobson H, Ribadu AY, Noble KM, Tebble JE, Ward WR. Ultrasound and hormone profiles of ACTH-induced persistent ovarian follicles (cysts) in cattle. Journal of Reproduction and Fertility. 2000;120:405–410. [PubMed] [Google Scholar]
- Dransfield MB, Nebel RL, Pearson RE, Warnick LD. Timing of insemination for dairy cows identified in estrus by a radiotelemetric estrus detection system. Journal of Dairy Science. 1998;81:1874–1882. doi: 10.3168/jds.S0022-0302(98)75758-3. [DOI] [PubMed] [Google Scholar]
- Duffield TF, Leslie KE, Sandals D, Lissemore K, McBride BW, Lumsden JH, Dick P, Bagg R. Effect of a monensin-controlled release capsule on cow health and reproductive performance. Journal of Dairy Science. 1999;82:2377–2378. doi: 10.3168/jds.S0022-0302(99)75488-3. [DOI] [PubMed] [Google Scholar]
- Enting H, Kooij D, Dijkhuizen AA, Huirne RBM, Noordhuizen GS, Stassen EN. Economic losses due to clinical lameness in dairy cattle. Livestock Production Science. 1997;49:259–267. [Google Scholar]
- Esslemont RJ, Bryant MJ. Oestrous behaviour in a herd of dairy cows. Veterinary Record. 1976;99:472–475. doi: 10.1136/vr.99.24.472. [DOI] [PubMed] [Google Scholar]
- Esslemont RJ, Kossaibati MA. Incidence of production diseases and other health problems in a group of dairy herds in England. Veterinary Record. 1996;139:486–490. doi: 10.1136/vr.139.20.486. [DOI] [PubMed] [Google Scholar]
- Etherington WG, Christie KA, Walton JS, Leslie KE, Wickstrom S, Johnson WH. Progesterone profiles in postpartum Holstein dairy-cows as an aid in the study of retained fetal membranes, pyometra and anestrus. Theriogenology. 1991;35:731–746. doi: 10.1016/0093-691x(91)90414-9. [DOI] [PubMed] [Google Scholar]
- Etherington WG, Kinsel ML, Marsh WE. Relationship of production to reproductive performance in Ontario dairy cows: herd level and individual animal descriptive statistics. Theriogenology. 1996;46:935–959. doi: 10.1016/s0093-691x(96)00259-2. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Martin GB. Hormonal control of proceptive and receptive sexual behavior and the preovulatory LH surge in the ewe: reassessment of the respective roles of estradiol, testosterone and progesterone. Hormones and Behavior. 1991;25:295–312. doi: 10.1016/0018-506x(91)90003-z. [DOI] [PubMed] [Google Scholar]
- Fonseca FA, Britt JH, McDaniel BT, Wilk JC, Rakes AH. Reproductive traits of Holsteins and Jerseys – effects of age, milk-yield, and clinical abnormalities on involution of cervix and uterus, ovulation, estrous cycles, detection of estrus, conception rate, and days open. Journal of Dairy Science. 1983;66:1128–1147. doi: 10.3168/jds.S0022-0302(83)81910-9. [DOI] [PubMed] [Google Scholar]
- Frei C, Frei PP, Stark KDC, Pfeiffer DU, Kihm U. The production system and disease incidence in a national random longitudinal study of Swiss dairy herds. Preventive Veterinary Medicine. 1997;32:1–21. doi: 10.1016/s0167-5877(97)00020-2. [DOI] [PubMed] [Google Scholar]
- Fulkerson WJ, Wilkins J, Dobos RC, Hough GM, Goddard ME, Davison T. Reproductive performance in Holstein-Friesian cows in relation to genetic merit and level of feeding when grazing pasture. Animal Science. 2001;73:397–406. [Google Scholar]
- Galindo F, Broom DM, Jackson PGG. A note on possible link between behaviour and the occurrence of lameness in dairy cows. Applied Animal Behaviour Science. 2000;67:335–341. doi: 10.1016/s0168-1591(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Garforth C, McKemy K, Rehman T, Tranter R, Cooke R, Park J, Dorward P, Yates C. Farmers' attitudes towards techniques for improving oestrus detection in dairy herds in South West England. Livestock Science. 2006;103:158–168. [Google Scholar]
- Garnsworthy PC. Body condition score in dairy cows: targets for production and fertility. In: Garnsworthy PC, Wiseman J, editors. Recent advances in animal nutrition – 2006. Nottingham University Press; Nottingham, UK: 2006. pp. 150–200. [Google Scholar]
- Glencross RG, Esslemont RJ, Bryant MJ, Pope G. Relationships between the incidence of pre-ovulatory behavior and the concentrations of oestradiol-17-beta and progesterone in bovine plasma. Applied Animal Ethology. 1981;7:141–148. [Google Scholar]
- Green MJ, Leach KA, Breen JE, Green LE, Bradley AJ. National intervention study of mastitis control in dairy herds in England and Wales. Veterinary Record. 2007;160:287–290. doi: 10.1136/vr.160.9.287. [DOI] [PubMed] [Google Scholar]
- Grohn YT, Erb HN, McCulloch CE, Salomiemi HS. Epidemiology of reproductive disorders in dairy cattle: associations among host characteristics, disease, and production. Preventive Veterinary Medicine. 1990;8:25–39. [Google Scholar]
- Hackett AJ, McAllister AJ. Onset of estrus in dairy-cows maintained indoors year-round. Journal of Dairy Science. 1984;67:1793–1797. doi: 10.3168/jds.S0022-0302(84)81506-4. [DOI] [PubMed] [Google Scholar]
- Haile-Mariam M, Bowman PJ, Goddard ME. Genetic and environmental relationship among calving interval, survival, persistency of milk yield and somatic cell count in dairy cattle. Livestock Production Science. 2003;80:189–200. [Google Scholar]
- Hall JG, Branton C, Stone EJ. Estrus, estrous cycles, ovulation time, time of service and fertility of dairy cattle in Louisiana. Journal of Dairy Science. 1959;42:1086–1094. [Google Scholar]
- Harrison RO, Ford SP, Young JW, Conely AJ, Freeman AE. Increased milk production versus reproductive and energy status in high-producing dairy cows. Journal of Dairy Science. 1990;73:2749–2758. doi: 10.3168/jds.S0022-0302(90)78960-6. [DOI] [PubMed] [Google Scholar]
- Haskell MJ, Rennie LJ, Bowell VA, Bell MJ, Lawrence AB. Housing system, milk production, and zero-grazing effects on lameness and leg injury in dairy cows. Journal of Dairy Science. 2006;89:4259–4266. doi: 10.3168/jds.S0022-0302(06)72472-9. [DOI] [PubMed] [Google Scholar]
- Hemsworth PH, Barnett JL, Beveridge L, Matthews LR. The welfare of extensively managed dairy-cattle – a review. Applied Animal Behaviour Science. 1995;42:161–182. [Google Scholar]
- Hernandez JA, Garbarino EJ, Shearer JK, Risco CA, Thatcher WW. Comparison of the calving-to-conception interval in dairy cows with different degrees of lameness during the prebreeding postpartum period. Journal of the American Veterinary Medical Association. 2005;227:1284–1291. doi: 10.2460/javma.2005.227.1284. [DOI] [PubMed] [Google Scholar]
- Heuer C, Schukken YH, Jonker LJ, Wilkinson JID, Noordhuizen JPTM. Effect of monensin on blood ketone bodies, incidence and recurrence of disease and fertility in dairy cows. Journal of Dairy Science. 2001;84:1085–1097. doi: 10.3168/jds.S0022-0302(01)74569-9. [DOI] [PubMed] [Google Scholar]
- Horan B, Mee JF, O'Connor P, Rath A, Dillon P. The effect of strain of Holstein-Friesian cow and feeding system on postpartum ovarian function, animal production and conception rate to first service. Theriogenology. 2005;63:950–971. doi: 10.1016/j.theriogenology.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Hultgren J. Foot/leg and udder health in relation to housing changes in Swedish dairy herds. Preventive Veterinary Medicine. 2002;53:167–189. doi: 10.1016/s0167-5877(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Hurnik JF, King GJ, Robertson HA. Estrus and related behavior in postpartum Holstein cows. Applied Animal Ethology. 1975;2:55–63. [Google Scholar]
- Huszenicza G, Janosi S, Kulcsar M, Korodi P, Reiczigel J, Katai L, Peters AR, de Rensis F. Effects of clinical mastitis on ovarian function in post-partum dairy cows. Reproduction in Domestic Animals. 2005;40:199–204. doi: 10.1111/j.1439-0531.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- Judge LJ, Erskine RJ, Bartlett PC. Recombinant bovine somatotropin and clinical mastitis: Incidence, discarded milk following therapy, and culling. Journal of Dairy Science. 1997;80:3212–3218. doi: 10.3168/jds.S0022-0302(97)76294-5. [DOI] [PubMed] [Google Scholar]
- Kanchev LN, Dobson H, Ward WR, Fitzpatrick RJ. Concentration of steroids in bovine peripheral plasma during the oestrous cycle and the effect of betamethasone treatment. Journal of Reproduction and Fertility. 1976;48:341–345. doi: 10.1530/jrf.0.0480341. [DOI] [PubMed] [Google Scholar]
- Kaneene JB, Hurd HS. The National Animal Health Monitoring System in Michigan. I. Design, data and frequencies of selected dairy cattle diseases. Preventive Veterinary Medicine. 1990;8:103–114. [Google Scholar]
- Kingwill RG, Neave FK, Dodd FH. Effect of a mastitis control system on levels of subclinical and clinical mastitis in 2 years. Veterinary Record. 1970;87:94–99. doi: 10.1136/vr.87.4.94. [DOI] [PubMed] [Google Scholar]
- Lam TJGM, Schukken YH, Grommers FJ, Smit JAH, Brand A. Within-herd and between-herd variation in diagnosis of clinical mastitis in cattle. Journal of the American Veterinary Medical Association. 1993;202:938–942. [PubMed] [Google Scholar]
- LeBlanc SJ, Leslie KE, Ceelen HJ, Kelton DF, Keefe GP. Measures of estrus detection and pregnancy in dairy cows after administration of gonadotropin-releasing hormone within an estrus synchronization program based on prostaglandin F-2 alpha. Journal of Dairy Science. 1998;81:375–381. doi: 10.3168/jds.S0022-0302(98)75587-0. [DOI] [PubMed] [Google Scholar]
- Leonard N, Egan J, Griffin J, Hanlon A, Poole D. A survey of some factors relevant to animal welfare on 249 dairy farms in the Republic of Ireland. Part 2: data on incidence of disease, culling and biosecurity measures. Irish Veterinary Journal. 2001;54:454–456. [Google Scholar]
- Loeffler SH, de Vries MJ, Schukken YH. The effects of time of disease occurrence, milk yield, and body condition on fertility of dairy cows. Journal of Dairy Science. 1999;82:2589–2604. doi: 10.3168/jds.S0022-0302(99)75514-1. [DOI] [PubMed] [Google Scholar]
- Lopez H, Bunch TD, Shipka MP. Estrogen concentrations in milk at estrus and ovulation in dairy cows. Animal Reproduction Science. 2002;72:37–46. doi: 10.1016/s0378-4320(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Gatius F, Yanis J, Madriles-Helm D. Effects of body condition score and score change on the reproductive performance of dairy cows: a meta-analysis. Theriogenology. 2003;59:801–812. doi: 10.1016/s0093-691x(02)01156-1. [DOI] [PubMed] [Google Scholar]
- Lyimo ZC, Nielen M, Ouweltjes W. Relationship among estradiol, cortisol and intensity of estrous behavior in dairy cattle. Theriogenology. 2000;53:1783–1795. doi: 10.1016/s0093-691x(00)00314-9. [DOI] [PubMed] [Google Scholar]
- McCoy MA, Lennox SD, Mayne CS, McCaughey WJ, Edgar HWJ, Catney DC, Verner M, Mackey DR, Gordon AW. Milk progesterone profiles and their relationship with fertility, production and disease in dairy cows in Northern Ireland. Animal Science. 2006;82:213–222. [Google Scholar]
- Melendez P, Bartolome J, Archbald LF, Donovan A. The association between lameness, ovarian cysts and fertility in lactating dairy cows. Theriogenology. 2003;59:927–937. doi: 10.1016/s0093-691x(02)01152-4. [DOI] [PubMed] [Google Scholar]
- Miglior F, Muir BL, van Doormaal BJ. Selection indices in Holstein cattle of various countries. Journal of Dairy Science. 2005;88:1255–1263. doi: 10.3168/jds.S0022-0302(05)72792-2. [DOI] [PubMed] [Google Scholar]
- Muir BL, Fateh J, Schaeffer LR. Genetic relationships between persistency and reproductive performance in first-lactation Canadian Holsteins. Journal of Dairy Science. 2004;87:3029–3037. doi: 10.3168/jds.S0022-0302(04)73435-9. [DOI] [PubMed] [Google Scholar]
- Nanda AS, Dobson H, Ward WR. Relationship between an increase in plasma cortisol during transport stress and the failure of oestradiol to induce an LH surge in dairy cows. Research in Veterinary Science. 1990;49:25–28. [PubMed] [Google Scholar]
- Oltenacu PA, Ekesbo I. Epidemiologic study of clinical mastitis in dairy-cattle. Veterinary Research. 1994;25:208–212. [PubMed] [Google Scholar]
- Opsomer G, Coryn M, Deluyker H, de Kruif A. An analysis of ovarian dysfunction in high yielding dairy cows after calving based on progesterone profiles. Reproduction in Domestic Animals. 1998;33:193–204. [Google Scholar]
- Parker AJC. Body condition score, milk fever and fertility in dairy cows. University of Liverpool; UK: 1992. DBR dissertation. [Google Scholar]
- Patton J, Kenny DA, McNamara S, Mee JF, O'Mara FP, Diskin MG, Murphy JJ. Relationships among milk production, energy balance, plasma analytes, and reproduction in Holstein-Friesian cows. Journal of Dairy Science. 2007;90:649–658. doi: 10.3168/jds.S0022-0302(07)71547-3. [DOI] [PubMed] [Google Scholar]
- Pennington JA, Albright JL, Callahan CJ. Relationships of sexual activities in estrous cows to different frequencies of observation and pedometer measurements. Journal of Dairy Science. 1986;69:2925–2934. doi: 10.3168/jds.S0022-0302(86)80748-2. [DOI] [PubMed] [Google Scholar]
- Petersson KJ, Gustafsson H, Strandberg E, Berglund B. A typical progesterone profiles and fertility in Swedish dairy cows. Journal of Dairy Science. 2006a;89:2529–2538. doi: 10.3168/jds.S0022-0302(06)72328-1. [DOI] [PubMed] [Google Scholar]
- Petersson KJ, Strandberg E, Gustafsson H, Berglund B. Environmental effects on progesterone profile measures of dairy cow fertility. Animal Reproduction Science. 2006b;91:201–214. doi: 10.1016/j.anireprosci.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy-cows using PGF(2-alpha), and GnRH. Theriogenology. 1995;44:915–923. doi: 10.1016/0093-691x(95)00279-h. [DOI] [PubMed] [Google Scholar]
- Regula G, Danuser J, Spycher B, Wechsler B. Health and welfare of dairy cows in different husbandry systems in Switzerland. Preventive Veterinary Medicine. 2004;66:247–264. doi: 10.1016/j.prevetmed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Roelofs JB, Bouwman EG, Dieleman SJ, Van Eerdenburg FJ, Kaal-Lansbergen LM, Soede NM, Kemp B. Influence of repeated rectal ultrasound examinations on hormone profiles and behaviour around oestrus and ovulation in dairy cattle. Theriogenology. 2004;62:1337–1352. doi: 10.1016/j.theriogenology.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Roelofs JB, van Eerdenburg FJCM, Soede NM, Kemp B. Pedometer readings for estrus detection and as predictor for time of ovulation in dairy cattle. Theriogenology. 2005a;64:1690–1703. doi: 10.1016/j.theriogenology.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Roelofs JB, van Eerdenburg FJCM, Soede NM, Kemp B. Various behavioral signs of estrus and their relationship with time of ovulation in dairy cattle. Theriogenology. 2005b;63:1366–1377. doi: 10.1016/j.theriogenology.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Royal MD, Darwash AO, Flint APF, Webb R, Wooliams JA, Lamming GE. Declining fertility in dairy cattle: changes in traditional and endocrine parameters of fertility. Animal Science. 2000;70:487–501. [Google Scholar]
- Schrick FN, Hockett ME, Saxton AM, Lewis MJ, Dowlen HH, Oliver SP. Influence of subclinical mastitis during early lactation on reproductive parameters. Journal of Dairy Science. 2001;84:1407–1412. doi: 10.3168/jds.S0022-0302(01)70172-5. [DOI] [PubMed] [Google Scholar]
- Shpigel NY, Winkler M, Ziv G, Saran A. Clinical, bacteriological and epidemiological aspects of clinical mastitis in Israeli dairy herds. Preventive Veterinary Medicine. 1998;35:1–9. doi: 10.1016/s0167-5877(98)00052-x. [DOI] [PubMed] [Google Scholar]
- Shrestha HK, Nakao T, Suzuki T, Akita M, Higaki T. Relationships between body condition score, body weight, and some nutritional parameters in plasma and resumption of ovarian cyclicity postpartum during pre-service period in high-producing dairy cows in a subtropical region in Japan. Theriogenology. 2005;64:855–866. doi: 10.1016/j.theriogenology.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sprecher DJ, Hostetler DE, Kaneene JB. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology. 1997;47:1179–1187. doi: 10.1016/s0093-691x(97)00098-8. [DOI] [PubMed] [Google Scholar]
- Stevenson MA. Disease incidence in dairy herds in the Southern highlands district of New South Wales, Australia. Preventive Veterinary Medicine. 2000;43:1–11. doi: 10.1016/s0167-5877(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Stevenson JS, Schmidt MK, Call EP. Factors affecting reproductive performance of dairy cows first inseminated after 5 weeks postpartum. Journal of Dairy Science. 1983;66:1148–1154. doi: 10.3168/jds.S0022-0302(83)81911-0. [DOI] [PubMed] [Google Scholar]
- Stevenson JS, Smith MW, Jaeger JR. Detection of estrus by visual observation and radiotelemetry in peripubertal, estrus-synchronized beef heifers. Journal of Animal Science. 1996;74:729–735. doi: 10.2527/1996.744729x. [DOI] [PubMed] [Google Scholar]
- Tranter WP, Morris RS. A case study of lameness in three dairy herds. New Zealand Veterinary Journal. 1991;39:88–91. doi: 10.1080/00480169.1991.35668. [DOI] [PubMed] [Google Scholar]
- Van Eerdenburg FJCM, Karthaus D, Taverne MAM, Merics I, Scenzi O. The relationship between estrous behavioral score and time of ovulation in dairy cattle. Journal of Dairy Science. 2002;85:1150–1156. doi: 10.3168/jds.s0022-0302(02)74177-5. [DOI] [PubMed] [Google Scholar]
- Van Vliet JH, Van Eerdenburg FJCM. Sexual activities and oestrus detection in lactating Holstein cows. Applied Animal Behaviour Science. 1996;50:57–69. [Google Scholar]
- Veerkamp RF, Oldenbroek JK, Van Der Gaast HJ, Van Der Werf JHJ. Genetic correlation between days until start of luteal activity and milk yield, energy balance, and live weights. Journal of Dairy Science. 2000;83:577–583. doi: 10.3168/jds.s0022-0302(00)74917-4. [DOI] [PubMed] [Google Scholar]
- Walker WL, Nebel RL, McGilliard ML. Time of ovulation relative to mounting activity in dairy cattle. Journal of Dairy Science. 1996;79:1555–1561. doi: 10.3168/jds.S0022-0302(96)76517-7. [DOI] [PubMed] [Google Scholar]
- Walker SL, Smith RF, Jones DN, Routly JE, Dobson H. Chronic stress, hormone profiles and estrus intensity in dairy cattle. Hormones and Behavior. 2008;53:493–501. doi: 10.1016/j.yhbeh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Whitaker DA, Macrae AI, Burrough E. Disposal and disease rates in British dairy herds between April 1998 and March 2002. Veterinary Record. 2004;155:43–50. doi: 10.1136/vr.155.2.43. [DOI] [PubMed] [Google Scholar]
- Williamson NB, Morris RS, Blood DC, Cannon CM, Wright PJ. Study of estrous behavior and estrus detection methods in a large commercial dairy-herd. 2. Estrous signs and behavior patterns. Veterinary Record. 1972;91:58–62. doi: 10.1136/vr.91.3.58. [DOI] [PubMed] [Google Scholar]
- Wishart DF. Observations on the oestrous cycle of the Friesian heifer. Veterinary Record. 1972;90:595–597. doi: 10.1136/vr.90.21.595. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, McKnight DJ, Vishwanath R. Estrus detection using radiotelemetry or visual observation and tail painting for dairy cows on pasture. Journal of Dairy Science. 1998;81:2890–2896. doi: 10.3168/jds.S0022-0302(98)75849-7. [DOI] [PubMed] [Google Scholar]
- Zwald NR, Weigal KA, Chang YM, Welper RD, Clay JS. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. Journal of Dairy Science. 2004;87:4287–4294. doi: 10.3168/jds.S0022-0302(04)73573-0. [DOI] [PubMed] [Google Scholar]