Summary
Currently, gene sequence genealogies of the Oligotrichea Bütschli, 1889 comprise only few species. Therefore, a cladistic approach, especially to the Oligotrichida, was made, applying Hennig's method and computer programs. Twenty-three characters were selected and discussed, i.e., the morphology of the oral apparatus (five characters), the somatic ciliature (eight characters), special organelles (four characters), and ontogenetic particulars (six characters). Nine of these characters developed convergently twice. Although several new features were included into the analyses, the cladograms match other morphological trees in the monophyly of the Oligotrichea, Halteriia, Oligotrichia, Oligotrichida, and Choreotrichida. The main synapomorphies of the Oligotrichea are the enantiotropic division mode and the de novo-origin of the undulating membranes. Although the sister group relationship of the Halteriia and the Oligotrichia contradicts results obtained by gene sequence analyses, no morphologic, ontogenetic or ultrastructural features were found, which support a branching of Halteria grandinella within the Stichotrichida. The cladistic approaches suggest paraphyly of the family Strombidiidae probably due to the scarce knowledge. A revised classification of the Oligotrichea is suggested, including all sufficiently known families and genera.
Keywords: classification, computer programs, Halteria problem, Hennig's cladistic method, taxonomy
INTRODUCTION
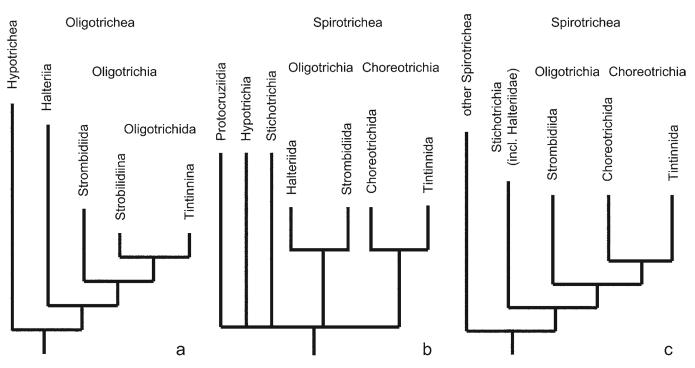
Since the Oligotrichea have not, except for the tintinnids, left fossil records, their phylogeny can only be reconstructed from the known features of extant species. In 1992, Petz and Foissner proposed the first cladistic system for the Oligotrichea on suprafamilial level, using morphologic and ontogenetic features. According to their genealogy and revised classification, the Halteriia are an adelphotaxon to the subclass Oligotrichia, which contains two orders, the Strombidiida and the Oligotrichida with the suborders Tintinnina and Strobilidiina (Fig. 1a). In earlier and even some recent classifications, however, the halteriids are a sister taxon to the strombidiids (Fig. 1b; Kahl 1932, Fauré-Fremiet 1970, Corliss 1979, Small and Lynn 1985, Maeda 1986, Montagnes and Lynn 1991, Laval-Peuto et al. 1994, Song et al. 1999, Lynn and Small 2002). Likewise, gene sequence analyses do not reflect the results of Petz and Foissner (1992) and of the other authors mentioned because Halteria grandinella clusters with the stichotrich Oxytricha granulifera (Fig. 1c; Baroin-Tourancheau et al. 1992, Hoffman and Prescott 1997, Shin et al. 2000, Bernhard et al. 2001, Snoeyenbos-West et al. 2002, Croft et al. 2003, Hewitt et al. 2003, Modeo et al. 2003, Strüder-Kypke and Lynn 2003, Agatha et al. 2004). On the other hand, the separation of the tintinnids and strobilidiids from the Oligotrichida, based on the shape of the membranellar zone (closed vs. C-shaped), is widely accepted and supported by gene sequence data (Small and Lynn 1985, Petz and Foissner 1992, Laval-Peuto et al. 1994, Lynn and Small 2002); only Song et al. (1999) followed Kahl's (1932) classification in assigning the aloricate Strobilidiidae, Strombidiidae, and Halteriidae to the same suborder Oligotrichina separated from the loricate tintinnids.
Figs 1a-c.
Cladograms showing different models of the phylogenetic relationships within the Spirotricha. a - according to Petz and Foissner (1992); b - according to Lynn and Small (2002); c - according to Strüder-Kypke and Lynn (2003).
Although molecular methods are frequently regarded as superior to traditional ones, using the subjective evaluation of morphologic characters, different molecules and methods often provide conflicting conclusions (Chen and Song 2002, Mayr and Bock 2002). Furthermore, currently too few gene sequences of Oligotrichea are available to elucidate their phylogenetic relationships at familial and generic level. Thus, a cladistic approach was made based on morphologic, ontogenetic, and ultra-structural data, and especially on the evolution of the ciliary patterns suggested by Agatha (2004).
MATERIALS AND METHODS
Cladistic analyses
The phylogenetic relationships within the Oligotrichea, with emphasis on the Oligotrichida, were elucidated by applying Hennig's argumentation method (Hennig 1982, Ax 1984, Sudhaus and Rehfeld 1992) and the computer programs PAUP 4.0b10 (Swofford 2002), HENNIG86, and FreeTree (http://www.natur.cuni.cz/~flegr/programs/freetree) with the Hypotrichea, i.e., the hypotrichs and stichotrichs, as out-group. The parsimony tree generated by the PAUP-program was founded on differently weighted features (for details, see Table 2), while equivalent weighting was used for the parsimony calculations with HENNIG86 and in the distance matrix cladogram produced with FreeTree (Jaccard index, UPGMA average linkage method, bootstrap re-sampled 1,000 times). The cladograms were printed by TreeView (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). Morphologic, ontogenetic, and ultrastructural data from the original literature were the basis for the analyses (Grim 1974, Mirabdullaev 1985, Foissner et al. 1988, Song 1993, Agatha and Riedel-Lorjé 1998, Agatha 2003b, as well as the papers cited in Maeda 1986 and Agatha 2004). However, only sufficiently known genera were considered, i.e., Cyrtostrombidium Lynn and Gilron, 1993; Laboea Lohmann, 1908; Limnostrombidium Krainer, 1995; Novistrombidium Song and Bradbury, 1998; Parallelostrombidium Agatha, 2004; Paratontonia Jankowski, 1978; Pelagostrombidium Krainer, 1991; Omegastrombidium Agatha, 2004; Pseudotontonia Agatha, 2004; Spirotontonia Agatha, 2004; Spirostrombidium Jankowski, 1978; Strombidium Claparède and Lachmann, 1859; and Tontonia FauréFremiet, 1914. Other genera, such as Echinostrombidium Jankowski, 1978, Lissostrombidium Jankowski, 1978, Metastrombidium FauréFremiet, 1924, Peristrombidium Jankowski, 1978, and Seravinella Alekperov and Mamajeva, 1992, were not taken into account because their type species are insufficiently known. Twenty-three characters were selected.
Table 2.
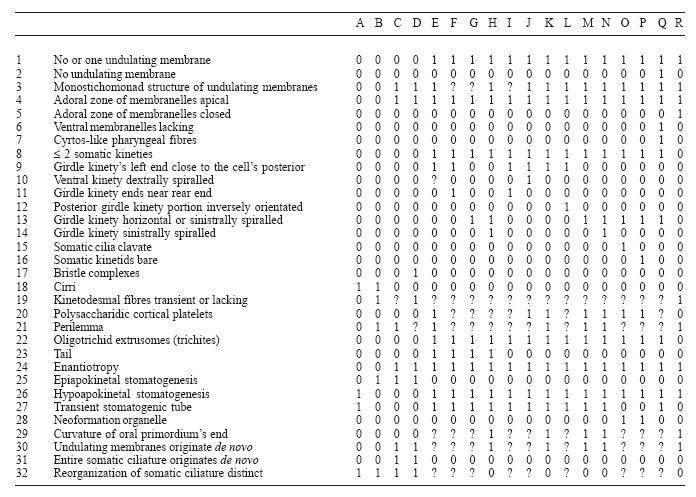
Distribution of none-hierarchical character states of the taxa cladistically analysed with the computer programs (Fig. 5). Note that multiple character states, such as the number of undulating membranes and the girdle kinety patterns (Table 1), have been separated and that (1) does not always mark an apomorphic state. In the PAUP program (Swofford 2002), the features 4, 6, 7, 20, and 28 have weight 2, features 5, 8, 17, 18, 22, 23, 24, 27, 30, 31 and 32 weight 3; the remaining features have weight 1. ? - character state unknown. A - Hypotrichida, B - Stichotrichida, C - Meseres, D - Halteria/Pelagohalteria, E - Tontonia, F - Paratontonia, G - Pseudotontonia, H - Spirotontonia, I - Omegastrombidium, J - Parallelostrombidium, K - Novistrombidium, L - Spirostrombidium, M - Strombidium, N - Laboea, O - Limnostrombidium, P - Pelagostrombidium, Q - Cyrtostrombidium, R - Choreotrichida.
Terminology
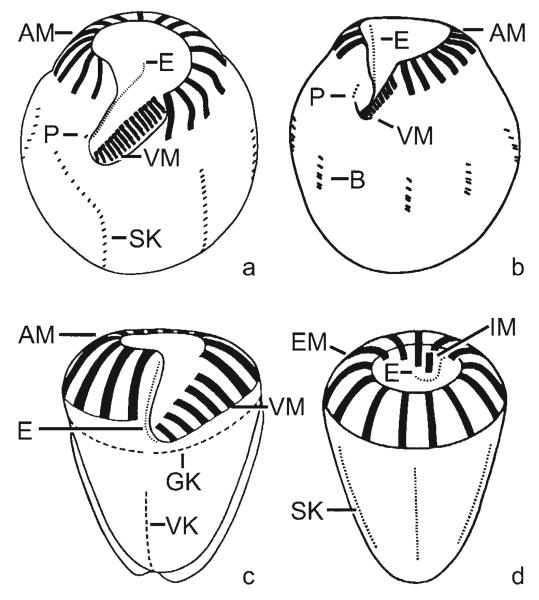
Halteriids have two undulating membranes (Figs 2a, b): the inner membrane is named endoral, while the outer membrane is called paroral; both are assumed to correspond to the endoral and paroral of the Stichotrichida (Szabó 1935). In long-term cultures of halteriids, the paroral is occasionally reduced (Foissner, pers. commun.), a process which probably happened also in the evolution of the Oligotrichia (see below). The homology of the inner membrane of halteriids and oligotrichids is indicated not only by the same position, but also by the de novo-origin, monostichomonad structure, and perilemma cover (Petz and Foissner 1992, Petz 1994, Song and Wang 1996, Agatha 2003a, Agatha et al. 2004). Thus, the inner membrane of the Oligotrichia should likewise be called endoral (Figs 2c, d). The direction of the spiral of the girdle kinety is determined in top view according to Montagnes and Taylor (1994).
Figs 2a-d.
Generalized ventral (a-c) and dorsal (d) views, illustrating some diagnostic features of the halteriid genera Meseres (a - modified from Petz and Foissner 1992) and Halteria (b - modified from Song 1993) as well as the oligotrichid genus Strombidium (c - from Agatha 2004) and the choreotrichid genus Rimostrombidium (d). The halteriids Meseres and Halteria have two undulating membranes, i.e., an outer paroral and an inner endoral, while the Oligotrichida and Choreotrichida possess only an endoral. AM - anterior polykinetids/membranelles, B - bristle complexes, E - endoral, EM - external polykinetids/membranelles, GK - girdle kinety, IM - internal polykinetids/membranelles; P - paroral, SK - somatic kineties, VK - ventral kinety, VM - ventral polykinetids/membranelles.
The taxonomic ranks used in the present paper follow the revised classification shown in Table 3.
Table 3.
Revised classification of the Oligotrichea (for further explanations, see “Classification of the Oligotrichea and diagnosis of some taxa”).
Superclass Spirotricha Bütschli, 1889
|
Again established by Small and Lynn (1985).
The paraphyly is indicated by quotation marks and the genera are, as far as possible, arranged according to the sequencing convention (Ax 1984).
RESULTS AND DISCUSSION
Characters, character states, and convergences considered
The Oligotrichea share several features with the Hypotrichea: a macronuclear replication band (Salvano 1975, Raikov 1982); an apokinetal development of the oral primordium (Foissner 1996); a conspicuous membranellar zone; and stichomonad undulating membranes on the right side of the buccal cavity (Grain 1972, Laval 1972, Grim 1987, Agatha 2003a). According to Corliss (1979), a stichomonad undulating membrane consists of a single row of identically orientated basal bodies. This is also shown in transmission electron micrographs of Strombidium and Novistrombidium provided by Modeo et al. (2003), although a dikinetidal structure of the endoral is described in the text.
In the Hypotrichea, and at least in dividing cells of Halteriia, the somatic kineties are composed of dikinetids, bearing a distinct cilium only at each anterior basal body (Szabó 1935, Grain 1972, Grim 1974, Ruffolo 1976, Petz and Foissner 1992); even the cirri of some stichotrichs show a dikinetidal composition (Wirnsberger-Aescht et al. 1989). In contrast to the Hypotrichea and Halteriia, most Oligotrichida have only a single longitudinal kinety, i.e., the ventral kinety. Nevertheless, its structure is identical to that of the hypotrich and halteriid kineties, and even the girdle dikinetids bear only a single distinct cilium at each left basal body (Fauré-Fremiet and Ganier 1970, Agatha 2003a, Modeo et al. 2003, Agatha et al. 2004). This peculiarity led to the evolution of the ciliary patterns discussed by Agatha (2004). In the Choreotrichida, however, the somatic kinetids are probably subject to several secondary modifications (Hedin 1976, Grim 1987, Lynn and Montagnes 1988, Montagnes and Lynn 1991, Agatha 2003b).
The cladistic analyses are founded on four groups of characters: the morphology of the oral apparatus (characters 1-5), the somatic ciliature (characters 6-13), special organelles (characters 14-17), and ontogenetic particulars (characters 18-23). The characters and their states are summarized in Table 1 and their distribution over the taxa is summarized in Table 2.
Table 1.
Character states and coding used for the construction of the traditional cladogram shown in Figure 4.
| Character states | ||
|---|---|---|
| Apomorphy | Plesiomorphy | |
| 1 | Paroral lacks (coded 1), paroral and endoral lack (coded 2) |
Endoral and paroral (coded 0) |
| 2 | Membranellar zone apical (coded 1) | Membranellar zone ventral (coded 0) |
| 3 | Membranellar zone closed (coded 1) | Membranellar zone C-shaped (coded 0) |
| 4 | Without ventral membranelles (coded 1) | Ventral membranelles (coded 0) |
| 5 | Cyrtos-like pharyngeal fibres (coded 1) | Common pharyngeal fibres (coded 0) |
| 6 | Reduction of somatic ciliature to ≤ 2 kineties (coded 1) |
Comprehensive somatic ciliature (coded 0) |
| 7 | Dextral spiral of somatic kineties (coded 1) | Longitudinal somatic kineties (coded 0) |
| 8 | Ventral kinety longitudinal (coded 1) | Ventral kinety dextrally spiralled (coded 0) |
| 9 | Ends of girdle kinety near posterior end of ventral side (coded 1), posterior portion of girdle kinety inversely orientated and parallel to ventral kinety (coded 2), girdle kinety horizontal (coded 3), girdle kinety sinistrally spiralled (coded 4) |
Girdle kinety dextrally spiralled (coded 0) |
| 10 | Somatic cilia clavate (coded 1) | Somatic cilia rod-shaped or fusiform (coded 0) |
| 11 | Somatic kinetids bare (coded 1) | Somatic kinetids ciliated (coded 0) |
| 12 | Somatic cilia arranged in bristle complexes (coded 1) | Somatic cilia arranged in ordinary rows (coded 0) |
| 13 | Kinetodesmal fibre of somatic kinetids lacking or transient (coded 1) |
Kinetodesmal fibres of somatic kinetids permanent (coded 0) |
| 14 | Polysaccharidic cortical platelets (coded 1) | No or other cortical platelets (coded 0) |
| 15 | Perilemma (coded 1) | Without perilemma (coded 0) |
| 16 | Oligotrichid extrusomes (trichites; coded 1) | No or other extrusomes (coded 0) |
| 17 | Tail (coded 1) | Without tail (coded 0) |
| 18 | Enantiotropy (coded 1) | Homeotropy (coded 0) |
| 19 | Stomatogenesis hypoapokinetal in temporary tube (coded 1) or pouch (coded 2) or permanent neoformation organelle (coded 3) |
Stomatogenesis epiapokinetal (coded 0) |
| 20 | Posterior end of oral primordium performs clockwise rotation (coded 1) |
Anterior end of oral primordium performs anticlockwise rotation (coded 0) |
| 21 | Undulating membranes originate de novo (coded 1) | Undulating membranes originate from oral primordium or cirral anlagen (coded 0) |
| 22 | Entire somatic ciliature originates de novo (coded 1) | At least parts of somatic ciliature originate by intrakinetal proliferation (coded 0) |
| 23 | Extensive reorganization of somatic ciliature (coded 1) | No or indistinct (intrakinetal) reorganization of somatic ciliature (coded 0) |
Character 1: Number of undulating membranes
Stichotrichs and some hypotrichs have two undulating membranes. Likewise, halteriids have an endoral and a minute paroral (Figs 2a, b; Szabó 1935, Grain 1972, Petz and Foissner 1992); the latter may be reduced in long-term cultures (Foissner, pers. commun.). Thus, it is assumed that the ancestor of the Hypotrichea (hypotrichs and stichotrichs) and Oligotrichea had two undulating membranes, of which the outer was lost in the Choreotrichida, the Oligotrichida, and convergently in some Hypotrichida, e.g., Euplotes (Grain 1972; Ruffolo 1976; Grim 1987; Petz and Foissner 1992; Agatha 2003a, b; Agatha et al. 2004). The Cyrtostrombidiidae lack any undulating membrane (own observ.; Lynn and Gilron 1993).
Character 2: Arrangement of membranellar zone
The adoral zone of membranelles is C-shaped and extends on the ventral side of the Hypotrichea. In the Halteriia and Oligotrichida, it is also C-shaped, but occupies the apical cell end. This arrangement is regarded as apomorphy.
Character 3: Shape of membranellar zone
In contrast to the Hypotrichea, Halteriia, and Oligotrichida, the adoral zone of membranelles of the Choreotrichida is circular and thus probably represents a derived state (Fig. 2d).
Character 4: Ventral membranelles
In the Oligotrichea and some stichotrichs, the adoral zone of membranelles is bipartited into large distal and small proximal membranelles. In three oligotrich genera, however, the ventral (proximal) portion is absent: Cyrtostrombidium Lynn and Gilron, 1993; Metastrombidium Fauré-Fremiet, 1924; and Seravinella Alekperov and Mamajeva, 1992. This is likely an apomorphy.
Character 5: Cyrtos
The pharyngeal fibres of Cyrtostrombidium Lynn and Gilron, 1993 are thick in protargol preparations, resembling the cyrtos (cytopharyngeal basket) of the Nassophorea, Phyllopharyngea, and Prostomatea (Lynn and Small 2002). Since the fibres of the other Spirotricha are distinctly finer, this feature is probably derived, especially, as it is accompanied by the lack of an endoral and ventral membranelles.
Character 6: Reduction of somatic ciliature
The ancestor of the Hypotrichea and Oligotrichea is supposed to have several longitudinal kineties, which were reduced to two ciliary rows in the Oligotrichida (Fig. 3b; Agatha 2004). The nature of the tail cilia in the tontoniids (Lynn and Gilron 1993, Suzuki and Song 2001) is uncertain; ontogenetic investigations are required.
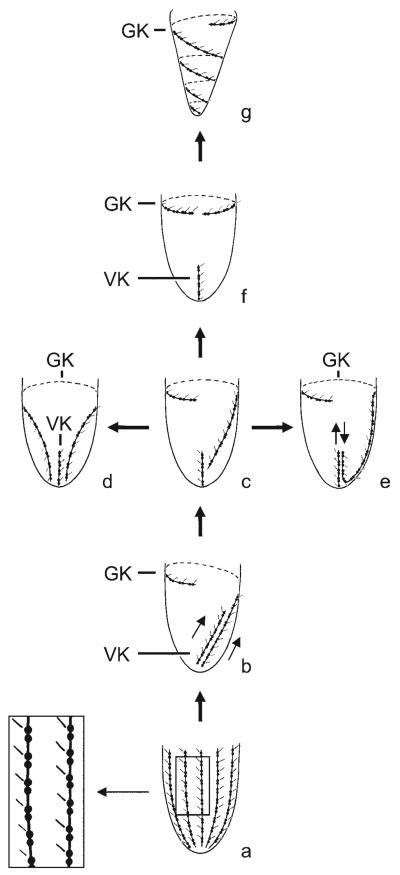
Figs 3a-g.
Evolution of the ciliary patterns in the Oligotrichida (from Agatha 2004). a - ancestor with many longitudinal somatic kineties, whose dikinetids bear a distinct cilium only at each anterior basal body (see detail); b - reduction in kinety number to two. The clockwise torsion of the proximal end of the membranellar zone and the cell proper caused the dextrally spiralled pattern of the girdle and ventral kinety; c - the ventral kinety orientated longitudinally; d - the right portion of the girdle kinety migrated posteriorly; both kinety ends are thus close to the cell's posterior on ventral side; e - the posterior portion of the girdle kinety curved anteriorly and is thus inversely orientated to the parallel ventral kinety; f - the left portion of the dextrally spiralled girdle kinety migrated anteriorly, causing a horizontal orientation; g - the right portion of the horizontal girdle kinety spiralled sinistrally to the rear end. The number of whorls performed by the girdle kinety is possibly positively correlated with the cell size because Tontonia turbinata with a length of 50-80 μm after protargol impregnation has ~ 1.5 whorls, while T. grandis with a size of up to 180 μm has 3-3.5 whorls (Song and Bradbury 1998, Suzuki and Han 2000, Agatha et al. 2004). Arrows indicate orientation of kineties. GK - girdle kinety, VK - ventral kinety.
Character 7: Dextral spiral of kineties
According to the proposed evolution of the ciliary patterns (Agatha 2004), the two remaining kineties (see Character 6) were located on the dorsal side and performed a dextral rotation parallel to the proximal portion of the adoral zone of membranelles (Fig. 3b). Further, the left kinety, i.e., the future ventral kinety, shortened anteriorly. Probably, this torsion of the oral apparatus is recapitulated during ontogenesis (see Character 20).
Character 8: Orientation of ventral kinety
Due to the dextral spiral of the posterior cell portion, both the ventral and girdle kinety were parallel to each other (Fig. 3b; Agatha 2004). Therefore, a longitudinal orientation of the ventral ciliary row is interpreted as an apomorphy (Fig. 3c).
Character 9: Girdle kinety patterns
Three patterns evolved from the dextrally spiralled course of the girdle kinety, as described by Agatha (2004) and briefly explained in the explanation of Fig. 3.
The lack of a ventral kinety in Pelagostrombidium, some Strombidium species, and probably also in Laboea strobila is difficult to interpret but is apparently only a species-specific feature and developed convergently several times.
Character 10: Shape of somatic cilia
Although detailed data are lacking for most Oligotrichia, the occurrence of clavate somatic cilia seems to be restricted to the freshwater genus Limnostrombidium (Kahl 1932; Krainer 1991, 1995; Foissner et al. 1999). Since cilia are usually rod-shaped or fusiform, clavate ones probably represent the derived state and developed convergently in the gymnostomatid ciliates.
Character 11: Lack of somatic cilia
Live observations, protargol impregnations, and ultrastructural studies show that the somatic kinetids are ciliated in the Hypotrichea, Halteriia, Choreotrichida, and Oligotrichida, except for those of Pelagostrombidium (Foissner et al. 1999). The latter state is therefore considered as an apomorphy.
Character 12: Bristle complexes
Separate cilia are the common state of the ciliature; accordingly, the bristles complexes of Halteria Dujardin, 1841 and Pelagohalteria Foissner, Skogstad and Pratt, 1988, that are composed of closely spaced dikinetids with one cilium each (Song and Wilbert 1989, Petz and Foissner 1992), likely represent the derived state.
Character 13: Fibrillar associates of somatic basal bodies
Hypotrichida have typical somatic dikinetids, i.e., with a kinetodesmal fibre, a transverse ribbon, and a postciliary ribbon, while the kinetodesmal fibres are resorbed during late ontogenetic stages in the Stichotrichida (Foissner 1996, Lynn and Small 2002). Data on the kinetid structure of Oligotrichia are only available for Halteria and three choreotrichids. While a kinetodesmal fibre is apparently lacking in Halteria grandinella (Grain 1972), Strobilidium velox (Grim 1987), and Petalotricha ampulla (Laval 1972), a short one occurs in Cyttarocylis brandti (Laval-Peuto 1994). In this cladistic approach, the lack of a kinetodesmal fibre is also assumed for morphostatic Oligotrichida and is regarded as the apomorphic state, that developed convergently in the Stichotrichida and Oligotrichea.
Character 14: Cortical platelets
Alveolata are characterized by cortical alveoli, which occasionally contain platelets. Polysaccharidic cortical platelets are restricted to the Oligotrichida (Kahl 1932, Laval-Peuto and Febvre 1986), the heterotrich family Sicuophoridae (Tuffrau 1994), and the dinoflagellates (Taylor 1987); likely, they developed convergently.
The distended cell surface in the posterior cell portion of the Oligotrichida is possibly correlated with the occurrence of the polysaccharidic cortical platelets.
Character 15: Perilemma
A perilemma, i.e., an additional layer probably covering the whole plasma membrane, was revealed by ultrastructural investigations of the Oligotrichida Strombidium, Novistrombidium, and Tontonia (Fauré-Fremiet and Ganier 1970, Laval-Peuto and Febvre 1986, Modeo et al. 2003), Tintinnina (Laval 1972, Laval-Peuto 1975, Hedin 1976), and several Stichotrichida (Bardele 1981, Wirnsberger-Aescht et al. 1989). A structure interpreted as perilemma was also recognized in TEM micrographs of Laboea strobila kindly provided by Per R. Jonsson (Tjärnö Marine Biological Laboratory, University of Göteborg, Sweden) and in the halteriid Meseres corlissi (Foissner; pers. commun.). Therefore, fixation problems might have caused the loss of the perilemma in Halteria grandinella (Grain 1972) and the choreotrichid ciliate Strobilidium velox where alveoli are also absent (Grim 1987). On the other hand, it is apparently lacking in the Hypotrichida (Bardele 1981). Bardele (1981) considered the perilemma as a temporary structure in stichotrichs, which is often renewed. Since the cyst wall is formed between the perilemma and the plasma membrane in stichotrichs, it might be a protection for the precursor of the cyst wall (Grimes 1973). Lynn and Corliss (1991) suggested that the perilemma might be a special preparation artifact of the glycocalyx. Nevertheless, its occurrence is apparently restricted to the Oligotrichea and Stichotrichida.
Character 16: Extrusomes
The trichites of strombidiids are extrusomes that differ distinctly in structure, size, and location from the extrusomes of hypotrichs, tintinnids, and strobilidiids (own observ.; Laval-Peuto and Barria de Cao 1987, Wirnsberger and Hausmann 1988, Modeo et al. 2001, Rosati and Modeo 2003, Agatha et al. 2004); thus, they are regarded as an autapomorphy.
Character 17: Tail
The contractile tail is an apomorphy of the tontoniids due to its complex and unique ultrastructure (Greuet et al. 1986, Agatha 2004).
Character 18: Division mode
The enantiotropic division mode is the most important autapomorphy of the Oligotrichea, although a modified (probably convergently developed) form is found in the prostomatid Pseudobalanion (Foissner et al. 1990, Petz and Foissner 1992, Foissner 1996). The Choreotrichida show a less pronounced kind of enantiotropy compared to the Halteriia and Oligotrichida (Petz and Foissner 1992, 1993; Dale and Lynn 1998; Agatha 2003b). This difference is probably correlated with the formation of the oral primordium within a pouch and the circular arrangement of almost all membranelles on the oral rim, a structure restricted to the choreotrichids (Fig. 2d).
Character 19: Stomatogenic mode
When Petz and Foissner (1992) established their phylogenetic system, the general validity of the hypoapokinetal stomatogenic mode in the Oligotrichia was uncertain. However, recent studies on Strombidium (Petz 1994, Song and Wang 1996, Agatha 2003a), Novistrombidium (Agatha 2003a), Laboea (Agatha et al. 2004), Strombidinopsis (Dale and Lynn 1998, Agatha 2003b), Pelagostrobilidium (own observ.), Spirotontonia (own observ.), and tintinnids from marine and freshwaters (own observ.; Petz and Foissner 1993) support their hypothesis. Thus, stomatogenesis takes place on the cell surface, except for the Oligotrichia (Anigstein 1913; Fauré-Fremiet 1912, 1953; Penard 1916, 1920, 1922; Buddenbrock 1922; Yagiu 1933; Kormos and Kormos 1958; Deroux 1974; Petz and Foissner 1992; Petz 1994; Song and Wang 1996; Agatha and Riedel-Lorjé 1997, 1998; Montagnes and Humphrey 1998; Suzuki and Song 2001), Hypotrichida (Ruffolo 1976, Song and Packroff 1993), and entodiniomorphids (Noirot-Timothée 1960); transitions to a subsurface development of the oral primordium are also found in some Stichotrichida (Foissner 1983). The hypoapokinetal stomatogenesis is therefore regarded as derived state and developed probably convergently in the taxa mentioned above, as other argumentations are less parsimonious (Petz and Foissner 1992). The assumption by Kahl (1932), that the subsurface development of the new oral apparatus became necessary when the membranelles undertook the cell's movement, cannot be supported; some data even indicate that this is not so: (i) in the related planktonic Halteriia, the new oral apparatus originates on the cell surface and (ii) a subsurface development of the new oral apparatus occurs in the benthic Hypotrichida and the endocommensalic Entodiniomorphida. The rigid cortex (polysaccharidic or proteinous cortical platelets in the Hypotrichida and Oligotrichida and skeletal plates in the Entodiniomorphida) possibly causes the special mode of stomatogenesis in these taxa.
The shape of the subsurface organelle, in which the oral primordium originates, probably depends on the shape of the adoral zone of membranelles, i.e., a C-shaped zone necessitates a tube, while a closed zone requires a pouch. Accordingly, it is reasonable to assume a parallel development of the closed zone and the subsurface pouch (cp. Character 3). In contrast to the suggestion by Petz and Foissner (1992), the pouch, not the tube, thus represents the derived state.
Since a temporary structure in which stomatogenesis occurs, as in the Hypotrichida and the Oligotrichia, is considered as plesiomorphic, a permanent one (neoformation organelle) is a strong synapomorphy of the genera Limnostrombidium Krainer, 1995 and Pelagostrombidium Krainer, 1991.
Character 20: Rotation of oral primordium
Although stomatogenesis of the Halteriia and Oligotrichia is similar at first glance, there is a difference, supporting a closer affiliation of the former with the Hypotrichea, viz., a pronounced anticlockwise rotation of the anterior end of the oral primordium (Fauré-Fremiet 1953, Ruffolo 1976, Petz and Foissner 1992, Song 1993, Berger 1999, Agatha 2004). This rotation is apparently lacking in the Oligotrichia or is, at least, less pronounced (Fauré-Fremiet 1953; Deroux 1974; Petz and Foissner 1992; Petz 1994; Song and Wang 1996; Dale and Lynn 1998; Agatha 2003a, b; Agatha et al. 2004). On the other hand, the posterior end of the oral primordium performs a distinct clockwise torsion, which is absent or less conspicuous in the Halteriia and the outgroup Hypotrichea. Accordingly, the distinct clockwise torsion is assumed to be apomorphic.
Character 21: Origin of undulating membranes
The undulating membranes of the outgroup Hypotrichea are generated by the oral primordium or cirral anlagen (Song and Packroff 1993, Berger 1999, Foissner et al. 2002, Song 2003), while they originate de novo in the Oligotrichea (Petz and Foissner 1992, 1993; Petz 1994; Song and Wang 1996; Dale and Lynn 1998; Agatha 2003a, b; Agatha et al. 2004). Since the oral anlage usually derives from the parental somatic or oral ciliature (Foissner 1996), the de novo-origin is regarded as apomorphy.
Character 22: Origin of somatic ciliature
The entire somatic ciliature of the Oligotrichia as well as the marginal and dorsal rows of the Hypotrichea are usually generated by intrakinetal proliferation of kinetids (Petz and Foissner 1992, 1993; Petz 1994; Song and Wang 1996; Dale and Lynn 1998; Berger 1999; Agatha 2003a, b; Agatha et al. 2004); only very rarely, de novo-formation occurs, e.g., in Engelmanniella (Wirnsberger-Aescht et al. 1989). Thus, the development of the girdle kinety within the neoformation organelle, as mentioned for Pelagostrombidium fallax (Petz and Foissner 1992), is considered to be a misobservation. In contrast to the intrakinetal proliferation, the de novo-generation of the entire somatic ciliature is regarded as the autapomorphy of the Halteriia.
Character 23: Reorganization of somatic ciliature
The somatic ciliature is usually not distinctly reconstructed during ontogenesis (Foissner 1996); thus, the extensive reorganization in the Hypotrichea is regarded as apomorphy, and the reorganization of the entire somatic ciliature in the Halteriia as a convergence (Petz and Foissner 1992, Song 1993). This explanation is more parsimonious than to assume a common ancestor of the Hypotrichea and Halteriia, which would require the assumption of several convergences in the Halteriia and the Oligotrichia (the enantiotropy, the de novo-origin of the undulating membranes, and the apical membranellar zone).
Characters not considered
Although occasionally mentioned in discussions, the following features were not included in this approach as they are plesiomorphies, convergences or require further investigations: structure of the membranellar zone, chromosomal fragmentation, arrangement of the extrusomes and their fibrillar associates, shape of the neoformation organelle, ontogenetic behaviour of the macronuclei, number of anlagen per somatic kinety, reorganization of the parental oral ciliature, arrangement of the cortical platelets, resting cysts, and fate of the somatic ciliature in encysted cells.
Comparison of morphological cladograms
There are few morphologic phylogenetic systems available for the oligotrichs, and all are confined to higher taxonomic levels (Puytorac et al. 1984, 1994; Petz and Foissner 1992). Although several new features (Characters 1, 2, 4-17, 20, 21, 23; Table 1) are included, the Hennigian tree matches that of Petz and Foissner (1992) very well (cp. Fig. 1a and Figs 4, 5). The monophyly of the Hypotrichea (hypotrichs and stichotrichs) and Oligotrichea bases on the macronuclear replication band; the apokinetal stomatogenesis is a newly introduced strong synapomorphy. Since the perilemma is apparently absent in the Hypotrichida, it is not a synapomorphy of the Hypotrichea and Oligotrichea, as suggested by Petz and Foissner (1992), but possibly developed convergently in the Stichotrichida and Oligotrichea. Otherwise, it is a synapomorphy of the Oligotrichea and Stichotrichida, and the cirri are either a convergence in the Hypotrichida and Stichotrichida or a symplesiomorphy which was lost in the Oligotrichea. However, there are no morphologic or ontogenetic data that support these two latter explanations. The Oligotrichea are mostly characterized by the enantiotropic division mode and the de novo-formation of the undulating membranes (a newly included character). With respect to the position of Halteria, the tree is supported by the parsimony analyses chiefly of ultrastructural data (Puytorac et al. 1984, 1994), in that the cluster of Halteria and the tintinnid Petalotricha ampulla forms a sister group with the monophyletic Hypotrichea. The unique feature of the Halteriia is the de novo-origin of the entire somatic ciliature, whereas the Oligotrichia are characterized by convergences (the hypoapokinetal stomatogenesis and the absence of a paroral), except for the rotation of the oral primordium, which is a potentially useful feature; more data are, however, required to support its significance. Since a concomitant development of the closed adoral zone of membranelles and the subsurface pouch is assumed (see Characters 2 and 19), two apomorphies characterize the Choreotrichida, instead of only one, as in the scheme of Petz and Foissner (1992).
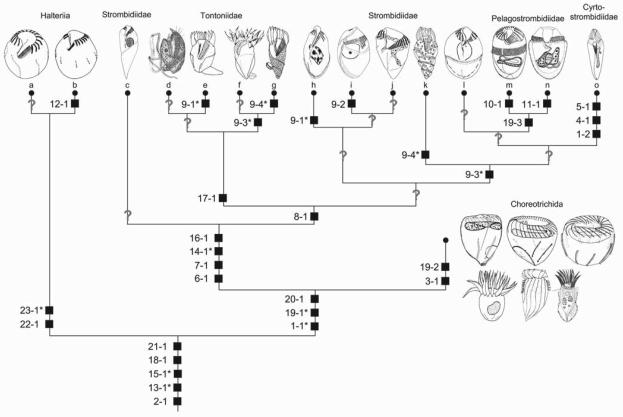
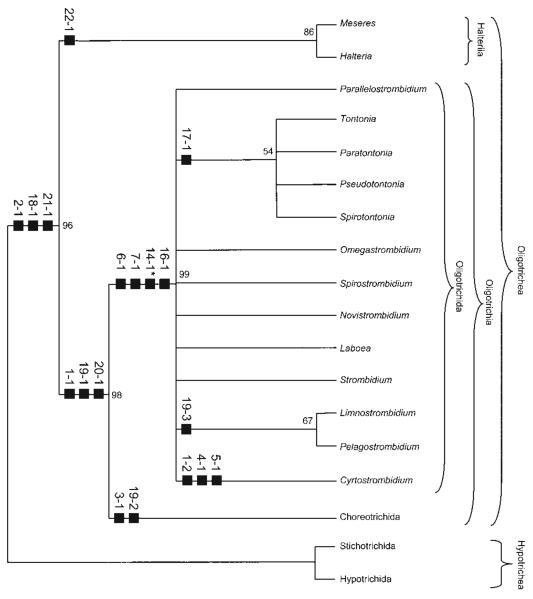
Fig. 4.
Cladistic scheme generated by Hennig's argumentation method. For character coding, see Table 1 and section on character states. Apomorphies are marked by black squares, convergences are starred. a - Meseres corlissi Petz and Foissner, 1992 (modified from Petz and Foissner 1992); b - Halteria grandinella (Müller, 1773) Dujardin, 1841 (modified from Song 1993); c - Parallelostrombidium rhyticollare (Corliss and Snyder, 1986) Agatha, 2004 (modified from Petz et al. 1995); d - Tontonia appendiculariformis Fauré-Fremiet, 1914 (from FauréFremiet 1924); e - Paratontonia gracillima (Fauré-Fremiet, 1924) Jankowski, 1978 (from Lynn et al. 1988); f - Pseudotontonia cornuta (Leegaard, 1915) Agatha, 2004 (modified from Suzuki and Song 2001); g - Spirotontonia grandis (Suzuki and Han, 2000) Agatha, 2004 (from Suzuki and Han 2000); h - Omegastrombidium elegans (Florentin, 1901) Agatha, 2004 (from Song et al. 2000); i - Spirostrombidium urceolare (Stein, 1867) Lei et al., 1999 (from Lei et al. 1999); j - Novistrombidium apsheronicum (Alekperov and Asadullayeva, 1997) Agatha, 2003 (from Agatha 2003a); k - Laboea strobila Lohmann, 1908 (from Montagnes et al. 1988); l - Strombidium sulcatum Claparède and Lachmann, 1859 (from Song et al. 2000); m - Limnostrombidium pelagicum (Kahl, 1932) Krainer, 1995 (from Krainer 1991); n - Pelagostrombidium mirabile (Penard, 1916) Krainer, 1991 (from Krainer 1991); o - Cyrtostrombidium longisomum Lynn and Gilron, 1993 (from Lynn and Gilron 1993).
Fig. 5.
50% majority-rule consensus tree (tree length = 74, retention index = 0.85, rescale consistency index = 0.68) computed with the maximum parsimony analysis of PAUP* version 4.0b10 (Swofford 2002), using the Hypotrichea, i.e., the hypotrichs and stichotrichs, as out-group (for feature matrix, see Table 2). The numbers represent the bootstrap values out of 100 re-samplings of the data set. The main apomorphies are marked by black squares; convergences are starred.
More detailed tree comparisons are impossible because the present cladistic approach is the first that investigated the genealogy of the families and genera of the Oligotrichida. Although the tail cilia in tontoniids might represent a third kinety besides the ventral and girdle kinety, the reduction of the somatic ciliature is distinct and represents together with the extrusomes (trichites) and the proposed dextral spiral of the kineties the main autapomorphy of the Oligotrichida. Like the neoformation organelle in the Pelagostrombidiidae and the oral structures of the Cyrtostrombidiidae (cyrtos-like pharyngeal fibres, no undulating membrane and ventral membranelles), the tontoniid tail is a good apomorphy due to its unique ultrastructure. The remaining apomorphies within the Oligotrichida, such as the ciliary patterns, are interpreted as convergences, or further ultrastructural data are needed to evaluate their importance and distribution. The cladistic relationships of the Oligotrichida on generic and familial level are mainly based on the evolution of the ciliary patterns proposed by Agatha (2004).
The attempt to reconstruct a phylogenetic tree for the Oligotrichida, using the Hennigian method, revealed that the family Strombidiidae is paraphyletic, which might be due to the scarce knowledge of the group (Fig. 4). The Hennigian scheme postulates that every split in the cladogram produces two new clades; diverging from each other and the parental phenotype multiple speciation and budding processes are excluded. Thus, the many unknown apomorphies in the cladistic scheme of the Oligotrichida are not only caused by the lack of data but also by the applied method. There are many good examples for the separation of new lineages, while the parental persists virtually/essentially unchanged (Mayr and Bock 2002). Furthermore, the Hennigian method uses the principle of parsimony as a methodological instrument, while the assumption of a parsimonious evolution is unfounded and many more than only the nine convergences in the evolution of the Oligotrichida might exist (Ax 1984, Moore and Willmer 1997). Therefore, phylogenetic trees do not represent reality, but can merely be a theorem of probability (Bachmann 1995, Haszprunar 1998).
All computer-generated cladograms support the monophyly of the Hypotrichea, Oligotrichea, Halteriia, Oligotrichia, Oligotrichida, and Choreotrichida found in the Hennigian scheme (Figs 4, 5; trees from HENNIG86 and FreeTree not shown). Likewise, they show a sister group relationship of the Halteriia and Oligotrichia. The classical and PAUP tree reveal a monophyly of the Tontoniidae and Pelagostrombidiidae due to the tail and the neoformation organelle, respectively. The cladograms generated with the unweighted data and the programs FreeTree and HENNIG86, however, place the genera mainly according to girdle kinety patterns, which have been developed convergently, according to the Hennigian scheme.
Comparison with cladograms inferred from gene sequence data
The Oligotrichia comprise at least 19 sufficiently known genera with about 180 species (Table 3; Fig. 4), while gene sequences are available from about twenty Choreotrichida and ten Oligotrichida species from the genera Strombidium, Laboea, Novistrombidium, and Spirostrombidium. Due to undersampling and unequal sampling, the gene trees are not comparable with the morphology based cladistic approach at familial and generic level (Agatha et al. 2004).
All molecular trees differ from the morphological cladograms in the position of the halteriids. They consistently reveal Halteria grandinella not as an early branch of the stichotrichs, but within this taxon as sister group to Oxytricha granulifera (Baroin-Tourancheau et al. 1992, Hoffman and Prescott 1997, Shin et al. 2000, Bernhard et al. 2001, Snoeyenbos-West et al. 2002, Croft et al. 2003, Hewitt et al. 2003, Modeo et al. 2003, Strüder-Kypke and Lynn 2003, Agatha et al. 2004). A placement of the Halteriia within the Stichotrichida is less parsimonious than the assumption presented above, as it requires several other convergences: (i) the diplo-/polystichomonad undulating membrane structure and the cirri in the Stichotrichida and Hypotrichida; and (ii) the enantiotropy, the apical adoral zone of membranelles, and the de novo-origin of the undulating membranes in the Halteriia and Oligotrichia. Shin et al. (2000) as well as Strüder-Kypke and Lynn (2003) argued that the enantiotropic division mode may be an adaptation to the planktonic lifestyle. Indeed, this might be true, although there are no evidences for this assumption (Foissner et al. 2004). Additionally, this argument does not favour an arrangement of the Halteriids within the Stichotrichida but also supports my cladistic scheme because it is more parsimonious to assume that the ancestor of the Oligotrichea was possibly a planktonic ciliate that developed enantiotropic division as an adaptation to this habitat.
Besides the sequence of the small subunit rRNA gene, there are morphological features suggesting a close relationship between the halteriids and stichotrichs: (i) the stomatogenesis on cell surface (plesiomorphic as it is present in most other ciliates); (ii) the four-rowed ventral membranelles (probably a plesiomorphy); (iii) the two undulating membranes (probably a plesiomorphy); (iv) the bristle complexes, which are absent in the halteriid Meseres (Fig. 2a; Petz and Foissner 1992) and whose homology to cirri has yet to be tested; and (v) the two distinct anlagen per somatic kinety in dividers, which also occur in some Hypotrichida, e.g., Diophrys (Song and Packroff 1993), and possibly the Oligotrichida. Finally, there are no derived morphologic, ontogenetic, or ultrastructural characters left, that support the position of Halteria within the stichotrichs. In agreement with Petz and Foissner (1992) and my results, the halteriids are, however, still the closest oligotrich relatives to the hypotrichs and stichotrichs. As discussed by Foissner et al. (2004), even the assumption that halteriids have developed from stichotrichs by an involution of the ventral and an extension of the dorsal side connected with a reduction of all cirri does not explain the enantiotropic division mode and the de novo-origin of the undulating membranes. Thus, the topologies of the gene and traditional trees concerning the position of the halteriids cannot be reconciled, especially, as nothing is known about the possible correlation between the evolution of the rRNA molecules and the selection of the phenotypes in ciliates (Puytorac et al. 1994). Accordingly, an exclusion of the Halteriia from the Oligotrichea, as suggested by Modeo et al. (2003) and Strüder-Kypke and Lynn (2003) seems to be unfounded, as genealogical analyses of the α-tubulin nucleotide sequences corroborate the cladistic scheme presented here by showing a closer relationship of H. grandinella to the Oligotrichia than to the stichotrichs (Snoeyenbos-West et al. 2002). Furthermore, molecular homologies are not always more accurate than morphological ones (Puytorac et al. 1994, Moore and Willmer 1997), and morphological characters, the product of a large number of genes, are usually quite reliable in phylogenetic analyses (Mayr and Bock 2002). However, the gene trees match the cladistic approach in other cases very well, e.g., in the close relationship between Meseres and Halteria (Katz and Foissner, pers. commun.) and between Novistrombidium and Spirostrombidium (both with a dextrally spiralled girdle kinety); the latter form a cluster separate from the Strombidiidae with a horizontal or sinistrally spiralled girdle kinety (Strüder-Kypke, pers. commun.). In summary, the SSrRNA trees alone do not solve all evolutionary problems, but together with other characters, such as morphologic, ontogenetic, and ultrastructural ones as well as other gene loci, they contribute to a better understanding of the phylogenetic relationships in ciliates (Moore and Willmer 1997, Hewitt et al. 2003).
Classification of the Oligotrichea and diagnosis of some taxa
The results obtained by the present cladistic approach match the findings of Petz and Foissner (1992). Accordingly, I follow mainly their classification and add all sufficiently known families and genera (Table 3). The permissibility of paraphyletic taxa in a classification is controversially discussed: it is supported by the evolutionary systematics, but rejected by the cladistic systematics (Sudhaus and Rehfeld 1992). In favour of simplicity and to provide a “user-friendly” classification, I follow the evolutionary systematics, i.e., the paraphyletic family Strombidiidae is not eliminated, however, it is marked as such. The members of the Strombidiidae are easily recognized, although the family is characterized only by plesiomorphies, as revealed by the cladistic analyses. The included genera are mostly arranged, following the sequencing convention (the first taxon represents the sister group to the remaining taxa and so on; Ax 1984). Additionally, no taxa have been established for the newly recognized sister groups, viz., for Tontonia and Paratontonia or Spirotontonia and Pseudotontonia.
The fact, that the ICZN (1999) does not govern the nomenclature above the familial level, causes some confusion within the Class Oligotrichea. Small and Lynn (1985) introduced the order Choreotrichida for taxa with a closed adoral zone of membranelles (strobilidiids and tintinnids) and assumed that the Halteriidae and Strombidiidae are adelphotaxa. The phylogenetic results of Petz and Foissner (1992), however, suggest a closer relationship of the strombidiids to the tintinnids and strobilidiids than to the halteriids; thus, the authors excluded the halteriids from the subclass Oligotrichia and established the subclass Halteriia (Fig. 1a). They summarized the Oligotrichia with a C-shaped membranellar zone in the order Strombidiida Jankowski, 1980. Petz and Foissner (1992) argued that the order Choreotrichida is superfluous and summarized the Oligotrichia with a closed membranellar zone in the order Oligotrichida. However, the terms “oligotrichs” for ciliates with a C-shape zone and “choreotrichs” for those with a closed zone have widely been accepted, not only in ecological papers but also in taxonomic publications. To avoid further confusion, and to be in accordance with the principle of an ascending and descending nomenclature used on subordinal levels (ICZN 1999), I reject the order Strombidiida Jankowski, 1980 and use the order Oligotrichida Bütschli, 1889. This seems especially justified as Jankowski (1980) only established a suborder Strombidiina, which is a junior synonymy to the suborder Oligotrichina Bütschli, 1889 used by Corliss (1979).
Based on the new characters, the diagnosis of some taxa are improved. Moreover, the lack of a type species in the genus Meseres, as recognized by Aescht (2001), is remedied by designating a type.
Class Oligotrichea Bütschli, 1889
Improved diagnosis
Cell usually globular to obconical. Macronucleus with replication band. Adoral zone of membranelles conspicuous, occupies apical cell end. Kinetodesmal fibres of somatic kinetids absent in at least morphostatic specimens. Undulating membranes monostichomonad, originate de novo. Stomatogenesis apokinetal, division enantiotropic. Mainly planktonic species.
Comparison with related taxa
The members of the class Hypotrichea, i.e., the hypotrichs and stichotrichs, are mainly dorsoventrally flattened benthic organisms, which can be distinguished from the Oligotrichea by the cirri, the division mode (homothetogenic vs. enantiotropic), the arrangement of the membranellar zone (mainly ventral vs. apical) as well as by the origin and structure of the undulating membranes (diplo-/polystichomonad structure and originating from the oral primordium or cirral anlagen vs. monostichomonad structure and originating de novo).
According to Lynn and Small (2002), the spirotrich ciliates comprise five subclasses (Fig. 1b): the Hypotrichia, Stichotrichia, Oligotrichia, Choreotrichia, and the Protocruziidia. The affiliation of the last of these with the spirotrichs is based only on gene sequence analyses as the morphologic and ontogenetic features (unusual nuclear complex, stichodyad undulating membrane, kinetid ultrastructure; Ammermann 1968, Ruthmann and Hauser 1974, Grolière et al. 1980, Song and Wilbert 1997) indicate rather a relationship to the heterotrichs; thus, a morphologic comparison of the Protocruziidia and the Oligotrichea is not necessary.
Subclass Halteriia Petz and Foissner, 1992
Improved diagnosis
Oligotrichea with endoral and minute paroral. Somatic ciliature comprises more than three kineties or bristle complexes, develops de novo, and reorganizes completely during ontogenesis. Oral primordium originates epiapokinetally and its anterior end rotates rightwards.
Comparison with related taxa
The Halteriia differ from the Hypotrichea and Oligotrichia in the origin of the somatic ciliature (entirely de novo vs. partially or completely by intrakinetal proliferation). The Oligotrichia are also distinguished by the stomatogenic mode (hypo- vs. epiapokinetal), the number of undulating membranes (one vs. two), the reorganization of the somatic ciliature (entire vs. none or indistinct), and the shaping of the new membranellar zone (rightwards rotation of proximal vs. distal end).
Order Halteriida Petz and Foissner, 1992
Improved diagnosis
With character of the subclass.
Type family
Halteriidae Claparède and Lachmann, 1859.
Family Halteriidae Claparède and Lachmann, 1859
Improved diagnosis
With characters of the order.
Type genus
Halteria Dujardin, 1841.
Genus Meseres Schewiakoff, 1892
Diagnosis
Halteriids with somatic kineties composed of dikinetids each with a cilium only at the anterior basal body. With perilemma.
Type species
Meseres cordiformis Schewiakoff, 1892.
Remarks
In 1892, Schewiakoff established the genus with M. cordiformis and M. stentor but did not fix any as type. Nevertheless, the genus name is available as it is accompanied by an indication, i.e., satisfies article 12.2.5. of the ICZN (1999). Following the recommendations of the Code, concerning the eligibility of species for type fixation (article 69A.10.), Meseres cordiformis is selected because it is the first species cited not only in Schewiakoff (1892) but also in Schewiakoff (1893), which includes drawings of the species.
Comparison with related genera
Meseres differs from Halteria Dujardin, 1841 and Pelagohalteria Foissner, Skogstad and Pratt, 1988 in the arrangement of the somatic ciliature (in long kineties vs. bristle complexes).
Subclass Oligotrichia Bütschli, 1889
Improved diagnosis
Endoral on inner wall of buccal lip in Oligotrichida or extending across peristomial field into oral funnel in Choreotrichida. With perilemma. Somatic ciliature entirely generated by intrakinetal proliferation, parental one not reorganized or without special anlagen. Oral primordium originates hypoapokinetally and its posterior end rotates rightwards.
Comparison with related subclass Halteriia
See discussion of Halteriia.
Order Oligotrichida Bütschli, 1889
Improved diagnosis
Adoral zone of membranelles C- shaped with ventral gap. Endoral on inner wall of buccal lip. Somatic ciliature reduced to usually a girdle kinety and a ventral kinety. Kineties composed of dikinetids each with a cilium only at the left, respectively, anterior basal body. Stomatogenesis in a subsurface tube. Polysaccharidic cortical platelets.
Comparison with order Choreotrichida
In contrast to the Oligotrichida, the Choreotrichida have a circular adoral zone of membranelles which originates in a subsurface pouch. Their endoral extends across the peristomial field into the oral funnel and their somatic ciliature usually comprises more than three kineties.
Acknowledgements
This study was supported by the Austrian Science Foundation (FWF, Project T 116). Special thanks to Prof. Dr. Wilhelm Foissner and Dr. David Montagnes for their constructive criticism.
REFERENCES
- Aescht E. Catalogue of the generic names of ciliates (Protozoa, Ciliophora) Denisia. 2001;1 [Google Scholar]
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Europ. J. Protistol. 2003a;39:245–266. [Google Scholar]
- Agatha S. Redescription of Strombidinopsis minima (Gruber, 1884) Lynn et al., 1991 (Protozoa, Ciliophora), with notes on its ontogenesis and distribution. Europ. J. Protistol. 2003b;39:233–244. [Google Scholar]
- Agatha S. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology. 2004;107:153–168. doi: 10.1016/j.zool.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of halteriids and strombidiids (Ciliophora, Oligotrichea) from coastal brackish water basins. Arch. Protistenk. 1997;148:445–459. [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of some strobilidiine ciliates (Ciliophora, Oligotrichea) from coastal brackish water basins of Germany. Europ. J. Protistol. 1998;34:10–17. [Google Scholar]
- Agatha S, Strüder-Kypke MC, Beran A. Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis. J. Euk. Microbiol. 2004;51:267–281. doi: 10.1111/j.1550-7408.2004.tb00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekperov I. Kh., Asadullayeva ES. New and little-known ciliates (orders Nassulida-Oligotrichida) from the Caspian Sea Apsheronian Coast Communication 2. Zool. Zh. 1997;76:1411–1417. (in Russian with English abstract) [Google Scholar]
- Alekperov IH, Mamajeva NV. Planktonic infusoria from the Chuckchee and the Bering Seas. Zool. Zh. 1992;71:5–14. (in Russian with English summary) [Google Scholar]
- Ammermann D. Die Kernverhältnisse des Ciliaten Protocrucia depressa n. sp. Arch. Protistenk. 1968;110:434–438. [Google Scholar]
- Anigstein L. Über Strombidium testaceum nov. spec. eine marine oligotriche Ciliate. Arch. Protistenk. 1913;32:79–110. + Plate 1, 2. [Google Scholar]
- Ax P. Das phylogenetische System. Systematisierung der lebenden Natur aufgrund ihrer Phylogenese. G. Fischer Verlag; Stuttgart: 1984. [Google Scholar]
- Bachmann K. Progress and pitfalls in systematics: cladistics, DNA and morphology. Acta Bot. Neerl. 1995;44:403–419. [Google Scholar]
- Bardele CF. Functional and phylogenetic aspects of the ciliary membrane: a comparative freeze-fracture study. Biosystems. 1981;14:403–421. doi: 10.1016/0303-2647(81)90046-0. [DOI] [PubMed] [Google Scholar]
- Baroin-Tourancheau A, Delgado P, Perasso R, Adoutte A. A broad molecular phylogeny of ciliates: identification of major evolutionary trends and radiations within the phylum. Proc. Nat. Acad. Sci. USA. 1992;89:9764–9768. doi: 10.1073/pnas.89.20.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) Kluwer Acad. Publ.; Dordrecht: 1999. (Monographiae Biologicae 78). [Google Scholar]
- Bernhard D, Stechmann A, Foissner W, Ammermann D, Hehn M, Schlegel M. Phylogenetic relationships within the class Spirotrichea (Ciliophora) inferred from small subunit rRNA gene sequences. Mol. Phylogen. Evol. 2001;21:86–92. doi: 10.1006/mpev.2001.0997. [DOI] [PubMed] [Google Scholar]
- Buddenbrock W. v. Über eine neue Strombidium-Art aus Helgoland (Str. clavellinae) Arch. Protistenk. 1922;45:129–132. [Google Scholar]
- Bütschli O. Erster Band. Protozoa. III. Abtheilung. Infusoria und System der Radiolaria. In: Bronn HG, editor. Klassen und Ordnungen des Thier-Reichs, wissenschaftlich dargestellt in Wort und Bild. C. F. Winter'sche Verlagshandlung; Leipzig: 1887-1889. pp. 1089–2035. + Plate 56-79 (1887: 1089-1280, 1888: 1281-1584, 1889: 1585-2035) [Google Scholar]
- Chen Z, Song W. Comparison of molecular biological techniques (RAPD, ARDRA and SSrRNA gene sequencing) on phylogenetic relationships of lower taxa within spirotrichous ciliates. High Tech. Letters. 2002;8:1–6. [Google Scholar]
- Claparède É, Lachmann J. Études sur les infusoires et les rhizopodes. Mém. Inst. natn. génev. 1859;6(year 1858):261–482. + Plate 14-24. [Google Scholar]
- Corliss JO. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 2nd ed. Pergamon Press; Oxford, New York, Toronto, Sydney, Paris, Frankfurt: 1979. [Google Scholar]
- Corliss JO, Snyder RA. A preliminary description of several new ciliates from the Antarctica, including Cohnilembus grassei n. sp. Protistologica. 1986;22:39–46. [Google Scholar]
- Croft KE, Dalby AB, Hogan DJ, Orr KE, Hewitt EA, Africa RJ, DuBois ML, Prescott DM. Macromolecular molecules encoding actins in spirotrichs. J. Mol. Evol. 2003;56:341–350. doi: 10.1007/s00239-002-2405-2. [DOI] [PubMed] [Google Scholar]
- Dale T, Lynn DH. Stomatogenesis of the ciliate genus Strombidinopsis with an improved description of S. spiniferum and S. acuminatum. J. Euk. Microbiol. 1998;45:210–217. [Google Scholar]
- Deroux G. Quelques précisions sur Strobilidium gyrans Schewiakoff. Cah. Biol. mar. 1974;15:571–588. [Google Scholar]
- Doflein F, Reichenow E. Lehrbuch der Protozoenkunde. 5. Auflage. G. Fischer Verlag; Jena: 1929. [Google Scholar]
- Dujardin F. Histoire naturelle des zoophytes. Infusoires, comprénant la physiologie et la classification de ces animaux, et la manière de les étudier a l'aide du microscope. Libraire Encyclopédique de Roret; Paris: 1841. [Google Scholar]
- Fauré-Fremiet E. Études cytologiques sur quelques infusoires des marais salants du Croisic. Arch. Anat. microsc. Morph. exp. 1912;13:401–479. + Plate 9, 10. [Google Scholar]
- Fauré-Fremiet E. Deux infusoires planctoniques Tontonia appendiculariformis (n. gen., n. sp.) et Climacostomum diedrum (n. sp.) Arch. Protistenk. 1914;34:95–107. [Google Scholar]
- Fauré-Fremiet E. Contribution a la connaissance des infusoires planktoniques. Bull. biol. Fr. Belg. 1924;6(Suppl.):1–171. [Google Scholar]
- Fauré-Fremiet E. La bipartition énantiotrope chez les ciliés oligotriches. Arch. Anat. microsc. Morph. exp. 1953;42:209–225. [Google Scholar]
- Fauré-Fremiet E. Remarques sur la morphologie comparée et la systématique des Ciliata Hypotrichida. C. r. hebd. Séanc. Acad. Sci., Paris. 1961;252:3515–3519. [Google Scholar]
- Fauré-Fremiet E. Remarques sur la systématique des ciliés Oligotrichida. Protistologica. 1970;5(year 1969):345–352. [Google Scholar]
- Fauré-Fremiet E, Ganier M.-Cl. Structure fine du Strombidium sulcatum Cl. et L. (Ciliata, Oligotrichida) Protistologica. 1970;6:207–223. [PubMed] [Google Scholar]
- Florentin R. Description de deux infusoires ciliés nouveaux des mares salées de Lorraine suivie de quelques considérations sur la faune des lacs salés. Annls Sci. nat. (Zool.) 1901;12:343–363. + Plate 15. [Google Scholar]
- Foissner W. Morphologie und Morphogenese von Psilotricha succisa (O. F. Müller, 1786) nov. comb. (Ciliophora, Hypotrichida) Protistologica. 1983;19:479–493. [Google Scholar]
- Foissner W. Ontogenesis in ciliated protozoa, with emphasis on stomatogenesis. In: Hausmann K, Bradbury PC, editors. Ciliates - Cells as Organisms. G. Fischer Verlag; Stuttgart: 1996. pp. 95–177. [Google Scholar]
- Foissner W, Skogstad A, Pratt JR. Morphology and infraciliature of Trochiliopsis australis n. sp., Pelagohalteria viridis (Fromentel, 1876) n. g., n. comb., and Strobilidium lacustris n. sp. (Protozoa, Ciliophora) J. Protozool. 1988;35:489–497. [Google Scholar]
- Foissner W, Oleksiv I, Müller H. Morphologie und Infraciliatur einiger Ciliaten (Protozoa: Ciliophora) aus stagnierenden Gewässern. Arch. Protistenk. 1990;138:191–206. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer; Landesamtes für Wasserwirtschaft: 1999. 3/99. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Part I: Text and line drawings. Part II: Photographs. Denisia. 2002;5 [Google Scholar]
- Foissner W, Moon-van der Staay SY, Moon-van der Staay GW, Hackstein JHP, Krautgartner W-D, Berger H. Reconciling classical and molecular phylogenies in the stichotrichines (Ciliophora, Spirotrichea), including new sequences from some rare species. Europ. J. Protistol. 2004 (in press) [Google Scholar]
- Grain J. Etude ultrastructurale d'Halteria grandinella O.F.M., (cilié oligotriche) et considérations phylogénétiques. Protistologica. 1972;8:179–197. [Google Scholar]
- Greuet C, Gayol P, Salvano P, Laval-Peuto M. Preliminary report on the ultrastructural organization of the contractile appendix of Tontonia appendiculariformis (Ciliophora Oligotrichina) Cell Motil. 1986;6:217–224. [Google Scholar]
- Grim JN. A protargol study of the fiber system of the ciliate Halteria. Trans. Am. microsc. Soc. 1974;93:421–425. [Google Scholar]
- Grim JN. The kinetid structures of the choreotrichous ciliate Strobilidium velox and an assessment of its evolutionary lineage. J. Protozool. 1987;34:117–123. [Google Scholar]
- Grimes GW. Differentiation during encystment and excystment in Oxytricha fallax. J. Protozool. 1973;20:92–104. doi: 10.1111/j.1550-7408.1973.tb06009.x. [DOI] [PubMed] [Google Scholar]
- Grolière CA, Puytorac P. de, Detcheva R. A propos d'observations sur la stomatogenèse et l'ultrastructure du cilié Protocruzia tuzeti Villeneuve-Brachon, 1940. Protistologica. 1980;16:453–466. [Google Scholar]
- Haszprunar G. Parsimony analysis as a specific kind of homology estimation and the implications for character weighting. Mol. Phylogenet. Evol. 1998;9:333–339. doi: 10.1006/mpev.1998.0496. [DOI] [PubMed] [Google Scholar]
- Hedin H. Microtubules and microfilaments in the tintinnid ciliate Ptychocylis minor Jörgensen. Zoon. 1976;4:3–10. [Google Scholar]
- Hennig W. Phylogenetische Systematik. Pareys Studientexte. 1982;34 [Google Scholar]
- Hewitt EA, Müller KM, Cannone J, Hogan DJ, Gutell R, Prescott DM. Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Mol. Phylogenet. Evol. 2003;29:258–267. doi: 10.1016/s1055-7903(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Prescott DM. Phylogenetic relationships among hypotrichous ciliates determined with the macronuclear gene encoding the large, catalytic subunit of DNA polymerase α. J. Mol. Evol. 1997;45:301–310. doi: 10.1007/pl00006234. [DOI] [PubMed] [Google Scholar]
- ICZN = International Commission on Zoological Nomenclature . International Code of Zoological Nomenclature. International Trust for Zoological Nomenclature; London: 1999. Fourth edition adopted by the International Union of Biological Sciences. Tipografia La Garangola, Padova. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski AW. Revision of a system of class Polyhymenophora (Spirotricha) Tezisky Dokl. zool. Inst. Akad. Nauk SSSR year. 1978;1978:39–40. (in Russian) [Google Scholar]
- Jankowski AW. Conspectus of a new system of the phylum Ciliophora. Trudy zool. Inst., Leningr. 1980;94:103–121. (in Russian with English translation) [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 3. Spirotricha. Tierwelt Dtl. 1932;25:399–650. [Google Scholar]
- Kent WS. A manual of the infusoria: including a description of all known flagellate, ciliate, and tentaculiferous Protozoa, British and foreign, and an account of the organization and affinities of the sponges. II. D. Bogue; London: pp. 473–913. + Plate 25-51. 1881-1882. [Google Scholar]
- Kofoid CA, Campbell AS. A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with descriptions of new species principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904-1905. Univ. Calif. Publs Zool. 1929;34 [Google Scholar]
- Kormos J, Kormos K. Die Zellteilungstypen der Protozoen. Acta Biol. hung. 1958;8:127–148. [Google Scholar]
- Krainer K-H. Contributions to the morphology, infraciliature and ecology of the planktonic ciliates Strombidium pelagicum n. sp., Pelagostrombidium mirabile (Penard, 1916) n. g., n. comb., and Pelagostrombidium fallax (Zacharias, 1896) n. g., n. comb. (Ciliophora, Oligotrichida) Europ. J. Protistol. 1991;27:60–70. doi: 10.1016/S0932-4739(11)80428-1. [DOI] [PubMed] [Google Scholar]
- Krainer K-H. Taxonomische Untersuchungen an neuen und wenig bekannten planktischen Ciliaten (Protozoa: Ciliophora) aus Baggerseen in Österreich. Lauterbornia. 1995;21:39–68. [Google Scholar]
- Laval M. Ultrastructure de Petalotricha ampulla (Fol). Comparaison avec d'autres tintinnides et avec les autres ordres de ciliés. Protistologica. 1972;8:369–386. [Google Scholar]
- Laval-Peuto M. Cortex, périlemme et réticulum vésiculeux de Cyttarocylis brandti (cilié tintinnide). Les ciliés a périlemme. Protistologica. 1975;11:83–98. [Google Scholar]
- Laval-Peuto M. Classe des Oligotrichea Bütschli, 1887. Ordre des Tintinnida Kofoid et Campbell, 1929. In: de Puytorac P, editor. Traité de Zoologie. Anatomie, systématique, biologie. II. Infusoires ciliés. 2. Systématique. Masson; Paris, Milan, Barcelona: 1994. pp. 181–219. [Google Scholar]
- Laval-Peuto M, Barria de Cao MS. Les capsules, extrusomes caracteristiques des Tintinnina (Ciliophora), permettent une classification evolutive des genres et des familles du sous-ordre. Ile Réun. Scientif. GRECO 88, Trav. C.R.M. 1987;8:53–59. [Google Scholar]
- Laval-Peuto M, Febvre M. On plastid symbiosis in Tontonia appendiculariformis (Ciliophora, Oligotrichina) Biosystems. 1986;19:137–158. doi: 10.1016/0303-2647(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Laval-Peuto M, Grain J, Deroux G. Classe des Oligotrichea Bütschli, 1887. Ordres des Oligotrichida Bütschli, 1887 et des Choreotrichida Small et Lynn, 1985. In: de Puytorac P, editor. Traité de Zoologie. Anatomie, systématique, biologie. II. Infusoires ciliés. 2. Systématique. Masson; Paris, Milan, Barcelona: 1994. pp. 153–179. [Google Scholar]
- Leegaard C. Untersuchungen über einige Planktonciliaten des Meeres. Nytt Mag. Naturvid. 1915;53:1–37. [Google Scholar]
- Lei Y, Xu K, Song W. Free living ciliates from marine farming ponds. In: Song W, editor. Progress in Protozoology. Qingdao Ocean Univ. Press; Qingdao: 1999. pp. 269–295. (in Chinese) [Google Scholar]
- Lohmann H. Untersuchungen zur Feststellung des vollständigen Gehaltes des Meeres an Plankton. Wiss. Meeresunters., Abt. Kiel. 1908;10:129–370. + Plate 9-17. [Google Scholar]
- Lynn DH, Corliss JO. Ciliophora. Microscopic Anatomy of Invertebrates. Protozoa. 1991;1:333–467. [Google Scholar]
- Lynn DH, Gilron GL. Strombidiid ciliates from coastal waters near Kingston Harbour, Jamaica (Ciliophora, Oligotrichia, Strombidiidae) J. mar. biol. Ass. U.K. 1993;73:47–65. [Google Scholar]
- Lynn DH, Montagnes DJS. Taxonomic descriptions of some conspicuous species of strobilidiine ciliates (Ciliophora: Choreotrichida) from the Isles of Shoals, Gulf of Maine. J. mar. biol. Ass. U.K. 1988;68:639–658. [Google Scholar]
- Lynn DH, Small EB. Phylum Ciliophora Doflein, 1901. In: An Illustrated Guide to the Protozoa. 2nd Edition. Organisms traditionally referred to as protozoa, or newly discovered groups. In: Lee JJ, Leedale GF, Bradbury P, editors. Society of Protozoologists, year 2000. Allen Press; Lawrence: 2002. pp. 371–656. [Google Scholar]
- Lynn DH, Montagnes DJS, Small EB. Taxonomic descriptions of some conspicuous species in the family Strombidiidae (Ciliophora: Oligotrichida) from the Isles of Shoals, Gulf of Maine. J. mar. biol. Ass. U.K. 1988;68:259–276. [Google Scholar]
- Maeda M. An illustrated guide to the species of the Families Halteriidae and Strobilidiidae (Oligotrichida, Ciliophora), free swimming protozoa common in the aquatic environment. Bull. Ocean Res. Inst., Univ. Tokyo. 1986;21:1–67. [Google Scholar]
- Mayr E, Bock WJ. Classifications and other ordering systems. J. Zool. Syst. Evol. Res. 2002;40:169–194. [Google Scholar]
- Mirabdullaev IM. Two new species of the oligociliated infusorians (Ciliophora, Oligotrichida) from water reservoirs of Uzbekistan. Zool. Zh. 1985;64:1892–1893. (in Russian with English summary) [Google Scholar]
- Modeo L, Petroni G, Bonaldi M, Rosati G. Trichites of Strombidium (Ciliophora, Oligotrichida) are extrusomes. J. Euk. Microbiol. 2001;48:95–101. doi: 10.1111/j.1550-7408.2001.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Modeo L, Petroni G, Rosati G, Montagnes DJS. A multidisciplinary approach to describe protists: redescriptions of Novistrombidium testaceum Anigstein 1914 and Strombidium inclinatum Montagnes, Taylor, and Lynn 1990 (Ciliophora, Oligotrichia) J. Euk. Microbiol. 2003;50:175–189. doi: 10.1111/j.1550-7408.2003.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Montagnes DJS, Humphrey E. A description of occurrence and morphology of a new species of red-water forming Strombidium (Spirotrichea, Oligotrichia) J. Euk. Microbiol. 1998;45:502–506. [Google Scholar]
- Montagnes DJS, Lynn DH. Taxonomy of choreotrichs, the major marine planktonic ciliates, with emphasis on the aloricate forms. Mar. Microb. Food Webs. 1991;5:59–74. [Google Scholar]
- Montagnes DJS, Taylor FJR. The salient features of five marine ciliates in the class Spirotrichea (Oligotrichia), with notes on their culturing and behaviour. J. Euk. Microbiol. 1994;41:569–586. [Google Scholar]
- Montagnes DJS, Lynn DH, Stoecker DK, Small EB. Taxonomic descriptions of one new species and redescription of four species in the family Strombidiidae (Ciliophora, Oligotrichida) J. Protozool. 1988;35:189–197. [Google Scholar]
- Moore J, Willmer P. Convergent evolution in invertebrates. Biol. Rev. 1997;72:1–60. doi: 10.1017/s0006323196004926. [DOI] [PubMed] [Google Scholar]
- Müller OF. Vermium terrestrium et fluviatilium, seu animalium infusorium helminthicorum et testaceorum, non marinorum, succincta historia. Heineck & Faber; Havniae & Lipsiae: 1773. [Google Scholar]
- Noirot-Timothée C. Étude d'une famille de ciliés: les “Ophryoscolecidae“. Structures et ultrastructures. Annls Sci. Nat. (Zool.) 1960;12:527–718. [Google Scholar]
- Penard E. Le Strombidium mirabile. Mém. Soc. Phys. Hist. nat. Genève. 1916;38:227–251. + Plate 8. [Google Scholar]
- Penard E. Observations sur le Strombidium viride Stein. Rev. Suisse Zool. 1920;28:1–9. [Google Scholar]
- Penard E. Études sur les infusoires d'eau douce. Georg & Cie; Genève: 1922. [Google Scholar]
- Petz W. Morphology and morphogenesis of Strombidium kryalis nov. spec. (Ciliophora, Strombidiida) from Antarctic sea ice. Arch. Protistenk. 1994;144:185–195. [Google Scholar]
- Petz W, Foissner W. Morphology and morphogenesis of Strobilidium caudatum (Fromentel), Meseres corlissi n. sp., Halteria grandinella (Müller), and Strombidium rehwaldi n. sp., and a proposed phylogenetic system for oligotrich ciliates (Protozoa, Ciliophora) J. Protozool. 1992;39:159–176. [Google Scholar]
- Petz W, Foissner W. Morphogenesis in some freshwater tintinnids (Ciliophora, Oligotrichida) Europ. J. Protistol. 1993;29:106–120. doi: 10.1016/S0932-4739(11)80303-2. [DOI] [PubMed] [Google Scholar]
- Petz W, Song W, Wilbert N. Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell Sea, Antarctica. Stapfia. 1995;40 [Google Scholar]
- Puytorac P. de, Grain J, Legendre P, Devaux J. Essai d'application de l'analyse phénétique à la classification du phylum des Ciliophora. J. Protozool. 1984;31:496–507. [Google Scholar]
- Puytorac P. de, Grain J, Legendre P. An attempt at reconstructing a phylogenetic tree of the Ciliophora using parsimony methods. Europ. J. Protistol. 1994;30:1–17. [Google Scholar]
- Raikov IB. The Protozoan Nucleus, Morphology and Evolution. Vol. 9. Springer Verlag; Wien: 1982. (Cell Biology Monographs). [Google Scholar]
- Rosati G, Modeo L. Extrusomes in ciliates: diversification, distribution, and phylogenetic implications. J. Euk. Microbiol. 2003;50:383–402. doi: 10.1111/j.1550-7408.2003.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Ruffolo JJ. Cortical morphogenesis during the cell division cycle in Euplotes: an integrated study using light optical, scanning electron and transmission electron microscopy. J. Morph. 1976;148:489–527. doi: 10.1002/jmor.1051480406. [DOI] [PubMed] [Google Scholar]
- Ruthmann A, Hauser M. Mitosis-like macronuclear division in a ciliate. Chromosoma. 1974;45:261–272. doi: 10.1007/BF00283410. [DOI] [PubMed] [Google Scholar]
- Salvano P. Comparaison du fonctionnement des bandes de réorganisation d'Euplotes crassus (Dujardin) et de Strombidium sulcatum Claparède et Linné après analyse microspectrographique en UV. J. Protozool. 1975;22:230–232. [Google Scholar]
- Schewiakoff W. Ueber die geographische Verbreitung der Süsswasser-Protozoën. Verh. naturh.-med. Ver. Heidelb. (N. S.) 1892;4(year 1891):544–567. [Google Scholar]
- Schewiakoff W. Über die geographische Verbreitung der Süsswasser- Protozoën. (Series 7).Zap. imp. Akad. Nauk. SSSR. 1893;41:1–201. [Google Scholar]
- Shin MK, Hwang UW, Kim W, Wright A-DG, Krawczyk C, Lynn DH. Phylogenetic position of the ciliates Phacodinium (Order Phacodiniida) and Protocruzia (Subclass Protocruziidia) and systematics of the spirotrich ciliates examined by small subunit ribosomal RNA gene sequences. Europ. J. Protistol. 2000;36:293–302. [Google Scholar]
- Small EB, Lynn DH. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Hutner SH, Bovee EC, editors. An Illustrated Guide to the Protozoa. Society of Protozoologists, Allen Press; Lawrence, Kansas: 1985. pp. 393–575. [Google Scholar]
- Snoeyenbos-West OLO, Salcedo T, McManus GB, Katz LA. Insights into the diversity of choreotrich and oligotrich ciliates (Class: Spirotrichea) based on genealogical analyses of multiple loci. Int. J. Syst. Evol. Microbiol. 2002;52:1901–1913. doi: 10.1099/00207713-52-5-1901. [DOI] [PubMed] [Google Scholar]
- Song W. Studies on the cortical morphogenesis during cell division in Halteria grandinella (Müller, 1773) (Ciliophora, Oligotrichida) Chin. J. Oceanol. Limnol. 1993;11:122–129. [Google Scholar]
- Song W. Reconsideration of the morphogenesis in the marine hypotrichous ciliate, Aspidisca leptaspis Fresenius, 1865 (Protozoa, Ciliophora) Europ. J. Protistol. 2003;39:53–61. [Google Scholar]
- Song W, Bradbury PC. Studies on some new and rare reported marine planktonic ciliates (Ciliophora: Oligotrichia) from coastal waters in North China. J. mar. biol. Ass. U.K. 1998;78:767–794. [Google Scholar]
- Song W, Packroff G. Beitrag zur Morphogenese des marinen Ciliaten Diophrys scutum (Dujardin, 1841) (Ciliophora, Hypotrichida) Zool. Jahrb. Abt. Anat. Ontog. Tiere. 1993;123:85–95. [Google Scholar]
- Song W, Wang M. Morphogenesis of Strombidium sulcatum during asexual binary division. Prog. nat. Sci. 1996;6:741–746. [Google Scholar]
- Song W, Wilbert N. Taxonomische Untersuchungen an Aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia. 1989;3:1–221. [Google Scholar]
- Song W, Wilbert N. Morphological investigations on some free living ciliates (Protozoa, Ciliophora) from China Sea with description of a new hypotrichous genus, Hemigastrostyla nov. gen. Arch. Protistenk. 1997;148:413–444. [Google Scholar]
- Song W, Zhu M, Chen Z. Updating the systematics of the planktonic oligotrichous ciliates (Ciliophora, Protozoa) with description of ciliature patterns at generic level. Yellow Sea. 1999;5:77–85. [Google Scholar]
- Song W, Wang M, Warren A. Redescriptions of three marine ciliates, Strombidium elegans Florentin, 1901, Strombidium sulcatum Claparède & Lachmann, 1859 and Heterostrombidium paracalkinsi Lei, Xu & Song, 1999 (Ciliophora, Oligotrichida) Europ. J. Protistol. 2000;36:327–342. [Google Scholar]
- Stein F. Der Organismus der Infusionsthiere nach eigenen Forschungen in systematischer Reihenfolge bearbeitet. I. Abtheilung. Allgemeiner Theil und Naturgeschichte der hypotrichen Infusionsthiere. W. Engelmann; Leipzig: 1859. [Google Scholar]
- Stein F. W. Engelmann; Leipzig: 1867. Der Organismus der Infusionsthiere nach eigenen Forschungen in systematischer Reihenfolge bearbeitet. II. Abtheilung. 1) Darstellung der neuesten Forschungsergebnisse über Bau, Fortpflanzung und Entwickelung der Infusionsthiere. 2) Naturgeschichte der heterotrichen Infusorien. [Google Scholar]
- Strüder-Kypke MC, Lynn DH. Sequence analyses of the small subunit rRNA gene confirm the paraphyly of oligotrich ciliates sensu lato and support the monophyly of the subclasses Oligotrichia and Choreotrichia (Ciliophora, Spirotrichea) J. Zool. (Lond.) 2003;260:87–97. [Google Scholar]
- Sudhaus W, Rehfeld K. Einführung in die Phylogenetik und Systematik. G. Fischer Verlag; Stuttgart: 1992. [Google Scholar]
- Suzuki T, Han M-S. A study on a new species of Tontonia (Ciliophora: Oligotrichida) from the East China Sea and adjacent sea areas. J. mar. biol. Ass. U.K. 2000;80:989–994. [Google Scholar]
- Suzuki T, Song W. A redescription of Tontonia cornuta (Leegaard, 1915) comb. nov., a planktonic oligotrichous ciliate (Ciliophora: Oligotrichia) from the northern Pacific Ocean. Hydrobiologia. 2001;457:119–123. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (* and other methods). Version 4.0b10. Sinauer Ass.; Sunderland, Massachusetts: 2002. [Google Scholar]
- Szabó M. Neuere Beiträge zur Kenntnis der Gattung Halteria (Protozoa, Ciliata) Arch. Protistenk. 1935;86:307–317. [Google Scholar]
- Taylor FJR. Dinoflagellate morphology. In: The Biology of Dinoflagellates. In: Taylor FJR, editor. Botanical Monographs. Vol. 21. Blackwell Sci. Publ.; Oxford: 1987. pp. 24–91. [Google Scholar]
- Tuffrau M. Classe des Heterotrichea Stein, 1859. In: de Puytorac P, editor. Traité de Zoologie. Anatomie, systématique, biologie. II. Infusoires ciliés. 2. Systématique. Masson; Paris, Milan, Barcelona: 1994. pp. 35–79. [Google Scholar]
- Wirnsberger E, Hausmann K. Fine structure of Pseudokeronopsis carnea (Ciliophora, Hypotrichida) J. Protozool. 1988;35:182–189. [Google Scholar]
- Wirnsberger-Aescht E, Foissner W, Foissner I. Morphogenesis and ultrastructure of the soil ciliate Engelmanniella mobilis (Ciliophora, Hypotrichida) Europ. J. Protistol. 1989;24:354–368. doi: 10.1016/S0932-4739(89)80006-9. [DOI] [PubMed] [Google Scholar]
- Yagiu R. Studies on the ciliates from the intestine of Anthocidaris crassispina (A. Agassiz) J. Sci. Hiroshima Univ., Ser. B., Div. 1. 1933;2:211–222. + Plate 1, 2. [Google Scholar]