Abstract
While there is a general sense that lakes can act as sentinels of climate change, their efficacy has not been thoroughly analyzed. We identified the key response variables within a lake that act as indicators of the effects of climate change on both the lake and the catchment. These variables reflect a wide range of physical, chemical, and biological responses to climate. However, the efficacy of the different indicators is affected by regional response to climate change, characteristics of the catchment, and lake mixing regimes. Thus, particular indicators or combinations of indicators are more effective for different lake types and geographic regions. The extraction of climate signals can be further complicated by the influence of other environmental changes, such as eutrophication or acidification, and the equivalent reverse phenomena, in addition to other land-use influences. In many cases, however, confounding factors can be addressed through analytical tools such as detrending or filtering. Lakes are effective sentinels for climate change because they are sensitive to climate, respond rapidly to change, and integrate information about changes in the catchment.
Currently, climate change is considered to be one of the most severe threats to ecosystems around the globe (ACIA 2004; Rosenzweig et al. 2007). Monitoring and understanding the effects of climate change pose challenges because of the multitude of responses within an ecosystem and the spatial variation within the landscape. A substantial body of research demonstrates the sensitivity of lakes to climate and shows that physical, chemical, and biological lake properties respond rapidly to climate-related changes (ACIA 2004; Rosenzweig et al. 2007). Fast turnover times from organismal to ecosystem scales in lakes are prerequisite for detecting such rapid changes. Previous studies have suggested that lakes are good sentinels of global climate change because they are sensitive to environmental changes and can integrate changes in the surrounding landscape and atmosphere (Carpenter et al. 2007; Pham et al. 2008; Williamson et al. 2008). However, a more thorough analysis of the potential for lakes to act as sentinels for the rapid rates of current climate change is lacking.
Studies of lakes provided some of the early indications of the effects of current climate change on ecosystem structure and function (Schindler et al. 1996a; Magnuson et al. 2000; Verburg et al. 2003) and the consequences for ecosystem services (O’Reilly et al. 2003). Some climate-related signals are highly visible and easily measurable in lakes. For instance, climate-driven fluctuations in water level have been observed on a broad scale in North America (Williamson et al. 2009), and shifts in the timing of ice formation and thawing reflect climate warming at a global scale (Magnuson et al. 2000). Other signals may be more complex and difficult to detect in lakes, but they may be equally sensitive indicators of climate forcing or equally informative regarding effects on ecosystem services. Available long-term historical records and reconstructions from sediment cores have yielded insight into less visible climaterelated changes and provided us with an understanding of the mechanisms that give rise to these changes. Paleolimnological records, in particular, have been crucial in developing climate records over recent geologic timescales, allowing us to interpret current climate change and predict its effects (Smol 2008; Leavitt et al. 2009).
In many ways, lake ecosystems appear to be valid sentinels for current climate change. Lake ecosystems act as sentinels because they provide indicators of climate change either directly or indirectly through the influence of climate on the catchment. The indicators are measurable response variables, such as water temperature, dissolved organic carbon (DOC), or plankton composition. Specifically, lakes are likely to serve as good sentinels for current climate change because (1) lake ecosystems are well defined and are studied in a sustained fashion; (2) lakes respond directly to climate change and also incorporate the effects of climate-driven changes occurring within the catchment; (3) lakes integrate responses over time, which can filter out random noise; and (4) lakes are distributed worldwide and, as such, can act as sentinels in many different geographic locations and climatic regions, capturing different aspects of climate change (e.g., rising temperature, glacier retreats, permafrost melting). However, the large range in lake morphology, catchment characteristics, and geographic locations implies that we should be cautious about making broad statements about the ability of lakes to capture the effects of the current, rapidly changing climate.
Here, we discuss three critical issues related to the use of lakes as sentinels. The focus of this synthesis paper is to identify limnological variables that respond to climate forcing and to assess the complexity and difficulty associated with using these variables as indicators of climate change. Lake ecosystems are complex, they have many internal feedbacks, and the influence of the catchment can vary depending on geographic location, regional climate, and land use. Do lakes contain accurate indicators that are appropriate for current rates of change? How broadly can these indicators be applied? Can we detect climate-driven changes in the face of other environmental change? First, we identified key properties of lakes and the most appropriate variables that would indicate effects of climate change. To do this, we conducted a review analysis of studies of contemporary climate change and assessed the suitability of a response variable based on its relationship to primary climate drivers, possible confounding effects, and the basic utility (ease of measurement, advantages, and disadvantages) of that indicator. Second, we categorized the appropriateness of specific indicators for different lake types within different climate regions. Finally, we addressed the challenges associated with extracting climate signals from confounding effects. For the purpose of this paper, we excluded reservoirs because their hydrology is largely anthropogenically controlled, and thus their climate-related responses are influenced by specific features of individual systems.
Key response variables and their suitability as indicators of climate change
Clearly, there is a diverse set of variables responsive to climate that span a range of physical, chemical and biological properties in lakes (ACIA 2004; Rosenzweig et al. 2007). Some of the most effective indicators of climate change are listed in Table 1. The criteria for choosing those response variables were high synchronicity among lakes, ease of measurement, and their known relevance for ecosystem function. These response variables reflect either a direct influence of climate on the lake or an indirect change via the effect of climate on the catchment. It is beyond the scope of this synthesis paper to address all observed climate-driven changes in lakes around the globe; instead, our purpose is to highlight the advantages and limitations of effective indicators for key lake properties.
Table 1.
Summary of key response variables to climate change for physical, chemical, and biological lake characteristics, and major underlying processes, mechanisms, and climate drivers along with comments on confounding factors, advantages, and disadvantages. Abbreviations are as follows: PP, precipitation; AT, air temperature; WT, water temperature; WS, wind speed; CC, cloud cover; RH, relative humidity; TKE, turbulent kinetic energy; DOC, dissolved organic carbon; A, advantages; D, disadvantages.
| Lake properties | Response variables |
Processes or mechanisms |
Climate-related drivers |
Confounding factors and comments |
Advantage or disadvantage/ utility or limitation |
References |
|---|---|---|---|---|---|---|
| Hydrology | Water level | Runoff, dilution, and transport of elements, evapotranspiration |
PP, AT, WS, CC, RH |
Catchment characteristics and disturbances; already used as climate indicator by international assessments (Rosensweig et al. 2007) |
A: easily measured D: many lakes are regulated |
Rodionov (1994); ACIA (2004); Jöhnk et al. (2004) |
| Temperature | Epilimnetic temperature |
Radiative and nonradiative heat exchange across the air–water interface |
AT, WS, CC, RH | Turbidity, water color; already used as climate indicator by international assessments (Rosensweig et al. 2007) |
A: easily measured D: large short-term variations; does not always correlate highly with air temperatures in small lakes |
Livingstone and Dokulil (2001); O’Reilly et al. (2003); Keller (2007); Hampton et al. (2008) |
| Duration of summer stratification |
Shifts in the timing of spring and fall mixing events |
AT, WS, CC, RH | Can be related to ice phenology |
A: integrates climate signal over longer timescale (several months) D: requires high-resolution data both spatially and temporally |
McCormick and Fahnenstiel (1999); Livingstone (2003) |
|
| Depth of the thermocline |
As thermal stability: a product of the opposing effects of PE and TKE |
AT, WS, CC, RH | In small lakes dependent on transparency |
A: good estimate for certain habitat refuges D: dependent on lake morphometry; precise definition requires temperature profiles with high spatial resolution |
Schindler et al. (1996a); Coats et al. (2006); Tanentzap et al. (2008) |
|
| Ice phenology | Ice-out | Thawing in spring | AT, PP, WS, CC, RH |
Thawing of ice and snow are complex; PP as rain enhances thawing; PP as snow retards thawing |
A: directly influenced by meteorological forcing; spatially very coherent among different lakes over large areas; tightly related to air temperature D: definition of ice-off depends on the observer; ill-defined if the ice thaws and refreezes during winter |
Magnuson et al. (2000); Latifovic and Pouliot (2007) |
| Ice duration | Single or multiple occurrences of ice-on and ice-off |
AT, WS, CC, RH | Ice-on is critically dependent on individual lake morphometry and lake physics |
A: integrates climate signal over longer timescale; detectable by remote sensing on large spatial scale D: if intermittent, must be observed each day |
Magnuson et al. (2000); Latifovic and Pouliot (2007) |
|
| Transparency | DOC | Runoff, permafrost melting, changes in terrestrial C dynamics, shifts in vegetation patterns, glacier retreat |
AT, PP, CC, RH, WS, atmospheric deposition |
Modified by in-lake, catchment processes and recovery from acidification |
A: integrates multiple responses in lake processes and catchment processes D: site-specific; i.e., different lakes may display opposite patterns in response to climate change |
Yan et al. (1996); Williamson et al. (1999); Pienitz and Vincent (2000); Freeman et al. (2004); Frey and Smith (2005); Keller et al. (2005) |
| Turbidity | Scattering by living and nonliving particulates; glacial melting, changes in hydrology |
PP, AT, CC, glacier retreat |
Modified by runoff, physical disturbances, and trophic status |
A: easily measured D: site-specific |
Smith (1978); Leavitt et al. (2003) | |
| Secchi depth phenology |
Absorption by dissolved substances and particulates, scattering by particulates |
AT, WS, CC | Biological interactions i.e., grazing |
A: proved efficacy as indicator; easily measured; integrates a number of processes within the food web D: affected by trophic state |
Gerten and Adrian (2000); Straile (2002); Huber et al. (2008) |
|
| Chemistry | Oxygen | Ice, mixing, metabolism |
AT, WS | Confounded particularly by changes in trophic status |
A: hypolimnetic oxygen concentrations provide strong indicator for internal nutrient dynamics and habitat refuges D: site-specific; particularly trophic status |
Hanson et al. (2006); Jankowski et al. (2006); Wilhelm and Adrian (2008); Winder and Hunter (2008) |
| Conductivity or salinity, pH or alkalinity |
Dilution, evaporative concentration |
AT, PP, atmospheric deposition WS, CC, RH, |
Droughts can lead to subsequent reacidification; lakes dominated by surface-water or groundwater inputs may respond differently to changes in water supply |
A: easily measured D: site-specific |
Yan et al. (1996); Webster et al. (1996) | |
| Autecology | Phenology | WT, timing of ice-out, stratification onset |
AT | Trophic state; already used as climate indicator by international assessments (Rosensweig et al. 2007) |
A: applies to organisms with a distinct seasonality D: needs long-term data at high temporal resolution; sitespecific |
Adrian et al. (1999); Weyhenmeyer et al. (1999); Winder and Schindler (2004a,b) |
| Life history | Degree days, winter severity |
AT | Food availability, predation |
A: includes juvenile and adult stages D: not easily detectable; limited long-term records |
Adrian et al. (2006); Seebens et al. (2007) |
|
| Community structure |
Algal blooms | WT, stratification | AT, WS | Trophic state | A: applicable for specific algal taxa D: applies mostly to productive lakes |
Huisman et al. (2004); Jöhnk et al. (2008) |
| Changes in relative species composition |
WT, UV exposure, stratification intensity, transparency |
AT, WS | Trophic state, species interactions |
A: identifies winners of climate change D: ways in which species interactions will likely change with warming are incompletely understood; species-specific thermal tolerances in nature are often not known and may be modified by other factors |
Strecker et al. (2004); Adrian et al. (2006); Marinone et al. (2006); Rühland et al. (2008); Winder and Hunter (2008) |
|
| Primary productivity |
Stratification length and intensity, degree days, ice-free period |
AT, WS, CC | Trophic state, transparency, already used as indicator of climate change (Rosenzweig et al. 2007) |
A: particularly relevant for arctic, alpine, and subalpine lakes; detectable by remote sensing on large spatial scale D: site-specific; difficult to measure |
O’Reilly et al. (2003); Michelutti et al. (2005) |
|
| Invaders | Temperature, degree days, transparency |
AT, PP | Human activity, habitat modification, chemical environment |
A: documents change over large spatial scale D: only applicable for lakes with data of high taxonomic resolution |
Holzapfel and Vinebrooke (2005) | |
| Habitat structure |
Habitat refuge |
WT, vertical temperature and oxygen gradients; UV |
AT, WS | Lake morphometry, transparency |
A: if habitat diminishes, it is a far-reaching indicator D: species-specific |
De Stasio et al. (1996); Tartarotti et al. (2001); Jansen and Hesslein (2004) |
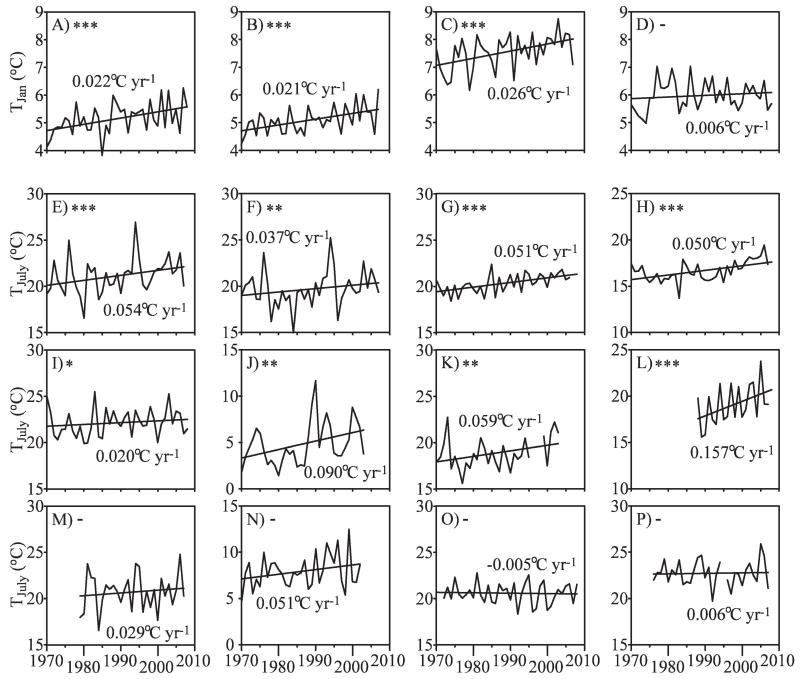
Water temperature: Surface and epilimnetic water temperatures, which can be highly correlated with regional-scale air temperatures, exhibit a rapid and direct response to climatic forcing, making epilimnetic temperature a useful indicator of climate change. In many (but no means all) lakes, the epilimnion has undergone recent warming (Fig. 1; Table 1). Epilimnetic temperatures are easy to monitor and reflect warming trends in air temperature in North America (Arhonditsis et al. 2004; Coats et al. 2006), Eurasia (Livingstone 2003; Hampton et al. 2008), and Africa (O’Reilly et al. 2003). In contrast, hypolimnetic temperatures exhibit a much more complex behavior, and they may undergo warming or cooling trends depending on lake morphometry (Gerten and Adrian 2001) and season (Robertson and Ragotzkie 1990; Livingstone and Lotter 1998; Straile et al. 2003). By influencing the density gradient in the water column, vertically heterogeneous changes in water temperature result in long-term changes in the intensity and duration of vertical mixing and stratification, thermal stability, and thermocline depth (Table 1). Long-term changes in thermal structure might in the future be responsible for mixing regimes shifting from polymictic to dimictic, dimictic to monomictic, or monomictic to oligomictic (Boehrer and Schultze 2008; Livingstone 2008). Although long-term changes in thermal structure and mixing regime may require detailed temperature measurements in the water column for their detection, they represent good indicators of climate change because of the directness and sensitivity of their response to climatic forcing. In addition, such long-term physical changes would have severe consequences for nutrient and oxygen concentrations, as well as for the vertical distribution and composition of the biota.
Fig. 1.
Long-term behavior of monthly mean near-surface temperatures since 1970 in January (TJan, A–D) and July (TJuly, E–P) in various Northern Hemisphere lakes. (A) and (E) Lake Zürich (Switzerland); (B) and (F) Lake Constance (Germany, Switzerland, Austria); (C) and (G) Lake Washington (Washington); (D) and (H) Lake Tahoe (California, Nevada); (I) Greifensee (Switzerland); (J) Lake Vättern, Edeskvarva basin (Sweden); (K) Lake Mäleren, Görvaln basin (Sweden); (L) Stensjön (Sweden); (M) Müggelsee (Germany); (N) Lake Baikal (Russia); (O) Lake Champlain (Vermont); (P) Blue Chalk Lake (Canada). Monthly means were obtained by spline-interpolating measured temperatures at daily intervals and averaging the results. For each lake, the significance level of the Mann–Kendall test for the existence of a monotonic trend is indicated by one, two, or three asterisks (p < 0.1, p < 0.05, and p < 0.01, respectively) or a dash (no monotonic trend at the p < 0.1 level). The mean rate of change of TJan or TJuly is also given, and the long-term linear trend is illustrated.
Water level: In nonregulated lakes, water level is a good indicator of climate change because it reflects the dynamic balance between water input (precipitation, runoff) and water loss (evaporation), and the timing of the ice-free season (ACIA 2004; Lenters et al. 2005; Van der Kamp et al. 2008) on timescales ranging from hours to centuries (Argyilan and Forman 2003; Ghanbari and Bravo 2008; Van der Kamp et al. 2008). Measurements of water level are especially useful in the case of closed-basin lakes, where long-term fluctuations in water level can be related to similar fluctuations in large-scale climate oscillations (Rodionov 1994). Possible confounding effects include the influence of groundwater level, along with changes in vegetation and land use in the catchment (Webster et al. 2000; Table 1).
Ice phenology: Although the processes governing the formation and thawing of lake ice depend on multiple interacting meteorological and limnological factors (Gu and Stefan 1990), air temperature is considered to be the dominant variable driving lake-ice phenology (Williams and Stefan 2006). Because individual physical lake properties influence freezing processes much more strongly than thawing processes, the timing of ice-off makes a better direct indicator of climate change than the timing of ice-on (Šporka et al. 2006; Table 1). The use of satellite data to study lake-ice phenology on large spatial scales enhances the utility of the timing of ice-off as a large-scale indicator of climate change (Wynne and Lillesand 1993; Latifovic and Pouliot 2007). However, the relationship between the timing of ice-off and air temperature is not necessarily linear and differs distinctly between colder and warmer geographical regions (Weyhenmeyer et al. 2004). Ice duration can be an appropriate climate-change indicator when ice cover is intermittent, for example, when ice cover forms and thaws several times during a warm winter (Livingstone and Adrian 2009).
Chemical variables: Many of a lake’s specific chemical properties represent the terrestrial landscape, and changes in these variables may serve as indicators of terrestrial processes that are otherwise difficult to detect in the more heterogeneous terrestrial ecosystem. Nutrient concentrations and ratios in lakes are likely to be altered as a consequence of changes in terrestrial export related to climatic influences on weathering rates, precipitation, run-off (Sommaruga-Wögrath et al. 1997; Rogora et al. 2003; Bergström and Jansson 2006), fire frequency (Kelly et al. 2006; Westerling et al. 2006), or terrestrial primary productivity (Boisvenue and Running 2006). Nutrient concentrations can also be affected by internal processes related to changes in thermal structure and/or primary productivity (Jeppesen et al. 2005; Wilhelm and Adrian 2008). The pH, ionic strength, ionic composition, and conductivity are very sensitive and easily measurable indicators of changes in weathering rate, as well as water balance. For many lakes, there can be challenges in disentangling the roles of internal and catchment changes with respect to water chemistry, which may be further complicated by confounding factors such as eutrophication, acidification, or atmospheric nitrogen deposition (Hessen et al. 2009). This is more likely to be the case for biologically reactive elements than for conservative elements (Na, Mg, and even Ca in lakes that do not experience weathering events).
Dissolved organic carbon (DOC) concentrations can also serve as an indicator, and they may be particularly appropriate for detecting changes within the terrestrial environment (Tranvik et al. 2009). DOC integrates multiple responses within the lake, such as water transparency (particularly in the ultraviolet range), heat absorption, and lake metabolism (Williamson et al. 1999), as well as changes observed in the catchment related to increased run-off, permafrost melting, shifts in vegetation, and changes in wetlands (Evans et al. 2006; Benoy et al. 2007; Keller et al. 2008), and increased CO2 concentrations (Freeman et al. 2004; Tables 1, 2). Allochthonous DOC concentrations can be a key indicator of catchment processes, particularly in boreal catchments, where DOC is generally dominated by allochthonous material, and increasing inputs associated with climate change are having strong effects on lake ecosystems (Parker et al. 2008). The disadvantages of DOC as an indicator of climate change are that it can also be influenced by atmospheric deposition (Monteith et al. 2007; Mladenov et al. 2008; Weyhenmeyer 2008a) and eutrophication (Nürnberg and Shaw 1998) and that long-term measurements are scarce.
Table 2.
Summary of indicators of climate change for lakes of various types. Lakes are defined by characteristics of their (A) climate and basin morphometry, and (B) location catchment characteristics. Arctic lakes are defined as those existing in regions with permafrost, or at higher latitudes; boreal lakes are high-latitude lakes with catchments composed mostly of coniferous forest; alpine lakes are those located above the tree line; temperate lakes and tropical lakes are only defined by their climate zones; arid lakes include those in Mediterranean and desert climates, or those located in grassland regions. Hypothesized or observed directions of changes in climate-related variables, indicators, and ecosystem services related to the response variables are indicated by + (positive) or − (negative). Indicators responding directly to the climate drivers are in line with those drivers. Indicators that are indirectly related to the climate driver are listed below the directly affected indicator. Ecosystem services are in line with the relevant indicators. Abbreviations: OM, organic matter; DOM, dissolved organic matter; strat, stratification; temp, temperature; TP, TN, total phosphorus and nitrogen, respectively; pCO2, partial pressure of carbon dioxide; Chl, chlorophyll a; UV-B, ultraviolet-B radiation (280–315 nm)
| Climate region | Catchment or mixing state |
External driving variables |
Indicators | Affected ecosystem services |
|---|---|---|---|---|
| Direct effects of climate across lake surface | ||||
| Arctic+alpine+ boreal+temperate |
+/−Snow fall | +/−Snow pack | −Albedo, water storage | |
| Air temp | +Ice-free period | +Productivity | ||
| Arctic | Monomictic, polymictic |
+Air temp | +Summer stability | |
| −Bottom O2 | −Habitat, +greenhouse gases (+CH4) |
|||
| Arctic+boreal+ temperate+arid |
Polymictic | +Air temp | +Surface temp | −Habitat, +climate indicator |
| +Strat frequency period | −Productivity, +nutrient retention |
|||
| Boreal+temperate | Dimictic | +Winter air temp | −Winter ice period | −Recreation, +/−habitat |
| +Winter bottom O2 | +Habitat | |||
| −Spring PO4 | −Productivity | |||
| Boreal+temperate+ arid |
Dimictic, monomictic | +Air temp | −Timing of stratification | −Fish production |
| +Stability | ||||
| +Cyanobacteria | −Water quality, −recreation | |||
| Temperate+arid+ tropical |
Dimictic, monomictic, oligomictic |
+Air temp | +Strat period, +stability | |
| −Surface TN, TP | −Productivity, +recreation | |||
| −Bottom O2, +H2S | −Habitat | |||
| +Deep NH4, PO4 | −Nutrient storage | |||
| Arid+tropical | Meromictic | +Evaporation, inflow | +Surface conductivity | |
| −Stability | ||||
| +Deep-water O2 | +Habitat | |||
| +Surface H2S, CO2 emissions |
−Human health | |||
| Tropical | +Air temp | +Surface temp | −Habitat | |
| Indirect effects of climate via catchment | ||||
| Arctic | Tundra | −Permafrost | −Water level | −Habitat, −regional heat capacity |
| +OM leaching from soil | +pCO2 | +Greenhouse gases | ||
| +Weathering of till | +Chl | +Productivity | ||
| −Benthic PPR | −Habitat | |||
| Alpine | Rock, meadow | +Air temp | +Ice-free period | +Productivity |
| +Annual UV-B | −Habitat, productivity | |||
| −DOM | −C storage | |||
| +Shifting tree line | +DOM | +Productivity | ||
| −Glaciers | −UV-B | +Habitat | ||
| Arid | Chaparral, desert, grassland |
−Regional water balance |
−Water level | −Water storage |
| +Conductivity, alkalinity | −Water quality | |||
| −Nutrient inputs | −Chl, +water clarity | −Productivity, +water quality | ||
| Boreal+temperate | Forested | +Soil decomposition, fire |
+DOM | −Recreation, −productivity, +carbon storage |
| +/−Precipitation (soil flushing) |
+/−DOM | +/−Recreation, +/−productivity, +/−carbon storage |
||
| Temperate | Urban, agricultural | +/−Precipitation | +/−Nutrients | −/+Water quality |
| +Air temp | +Stability | |||
| +Cyanobacteria | −Water quality, −recreation | |||
| −Bottom O2 | −Habitat, +greenhouse gases (N2O) |
|||
Oxygen concentrations in lakes can indicate climate shifts because oxygen levels are strongly influenced by temperature and thermal structure (Hanson et al. 2006; Table 1). For example, the extremely warm European summer of 2003 resulted in a long period of thermal stratification and increased hypolimnetic oxygen depletion in some Swiss lakes (Jankowski et al. 2006). When applicable, hypolimnetic oxygen concentrations have added value as indicators of climate change because they have widespread consequences for internal nutrient loading (Pettersson et al. 2003), habitat size, and refuge availability (De Stasio et al. 1996; Jansen and Hesslein 2004).
Biota
Interactions between climate change and lake biota are complex because other factors such as resource availability, density dependence, and predation strongly control the abundance, distribution, and size of the biota. In addition, responses are often species-specific and vary among ecosystems (Baines et al. 2000; Adrian et al. 2006). Despite these difficulties in using organisms as indicators for climate change, some widespread climate-related responses of lake biota have emerged, and the mechanisms involved in climate-related changes are becoming better understood. Planktonic organisms have been used widely as indicators of ecosystem change, and because these relatively short-lived organisms respond rapidly to subtle thermal changes, they are also useful indicators of climate change. Phytoplankton and zooplankton communities in many lakes have been relatively well-documented over extended time periods, and these records are useful for extracting climate-related responses.
Changes in spring and early summer phenology can provide a good reflection of climatic shifts (Straile 2002; Blenckner et al. 2007; Rusak et al. 2008; Table 1). For zooplankton, phenological shifts are usually restricted to fast-growing plankton (but see Winder and Schindler 2004a, Seebens et al. 2007), whereas longer-lived plankton may respond in more complex ways (Winder and Schindler 2004b; Adrian et al. 2006). For longer-lived organisms with a complex life cycle, climate warming can accelerate ontogenetic development, which can cause a shift in life history, as has been shown for fish and copepod species (Schindler et al. 2005; Adrian et al. 2006; Winder et al. 2009). Spring migrations for several anadromous fish species are happening earlier, and this timing can also be used as an indicator of climatic changes (Quinn and Adams 1996).
Growth rates, abundance, and species composition can each be considered an indicator of climate change. Given sufficient resource availability, increasing temperatures generally accelerate growth and development rates of individual organisms, although changes in absolute abundances tend to be species-specific (Adrian et al. 2006; Reist et al. 2006; Blenckner et al. 2007). Changes in species composition have been used as a climate indicator on longer geological timescales (Rühland et al. 2008), as well as in contemporary studies. For example, at higher temperatures, bloom-forming cyanobacteria have a competitive advantage over other phytoplankton groups (Jöhnk et al. 2008; Wagner and Adrian 2009b). Similarly, stronger vertical stratification can cause shifts in phytoplankton species composition (Verburg et al. 2003; Strecker et al. 2004; Winder and Hunter 2008). Shifts in phytoplankton species composition, especially among those taxonomic groups that are sensitive to temperature and mixing, such as cyanobacteria, diatoms, and flagellates, can be considered indicators of climate-induced enhancements in thermally stratified conditions (Table 1). Changes in fish-species distributions, abundance, and community structure are also indicative of climate effects, particularly as available habitat for cold-water species contracts (Gunn 2002; Reist et al. 2006).
Other climate-related responses of lake biota may be effective indicators for particular systems. These include responses such as changes in primary productivity (O’Reilly et al. 2003; Michelutti et al. 2005), zooplankton body size (Moore et al. 1996), increased bacterial cell densities (Rae and Vincent 1998), and benthic net photosynthesis and dark respiration rates (Baulch et al. 2005). Finally, climate may also affect species diversity and composition through the invasion of non-native species that expand their geographical range as water temperatures warm (Rahel and Olden 2008). Detailed knowledge of invasive species’ life-history requirements would be important for evaluating their efficacy as a climate indicator (Table 1). However, all of these responses are often lake-specific and can be difficult to predict on larger scales.
Geographic variation in the use of lakes as sentinels
Climate-driven changes will have different effects on lakes depending on geographic location, elevation, morphometry, climate, vegetation, and land use. For example, in forested lakes in temperate and boreal zones, DOC concentrations may best reflect climate-mediated changes in soil processes such as soil decomposition rates and the flushing of soil organic matter. In agricultural catchments, however, changes in precipitation and decomposition may primarily alter the import of nutrients into water bodies. In Arctic ponds, cation concentrations (and strontium isotopes) are sensitive indicators of melting permafrost, which exposes previously unweathered glacial till in the catchment to weathering (Hobbie et al. 1999; Prowse et al. 2006). The water levels of Arctic lakes have also proven to be a sensitive indicator of the northern extent of permafrost (Smith et al. 2005; Marsh et al. 2009). In contrast, lakes in arid and tropical environments experience shifts in water level and conductivity related to swings in water balance and evapo-concentration (Awange et al. 2008; Croley and Lewis 2008; Van der Kamp et al. 2008).
The utility of a specific indicator depends on the characteristics of the lake in which it is measured (Table 2). At the simplest level, characteristics of the lake and the catchment influence both the sensitivity of the indicator variable to climate change and the amount of background variability to which the indicator is subject. For example, in very small lakes with low heat capacity, surface-water temperature responds quickly to radiative and evaporative heat exchange and solar irradiance (Gerten and Adrian 2001). While high sensitivity is a useful characteristic for an indicator, it also leads to more of the same high-frequency “noise” that masks long-term trends in climate data. One may postulate, therefore, that there is a lake size for which the combination of noise reduction and sensitivity is optimal. A similar line of argument could be constructed for an optimal hydrologic residence time when using water chemistry to detect climate-mediated changes that result from alterations in catchment inputs. Certainly there appears to be an optimal combination of landscape position and residence time when choosing lakes to use for assessing the effect of climate on groundwater flows in response to drought (Webster et al. 1996; Baines et al. 2000).
A lake’s internal physical structure, morphometry, hydrological setting, and biota can alter the primary mechanisms by which climate affects a given indicator variable. As a result, a variable that shows one response to climate change in one lake may exhibit a very different response in another (Weyhenmeyer 2008b). For example, in short-residence-time boreal lakes, DOC may most closely reflect variations in inputs related to the production of DOC in catchment soils and its subsequent export to streams or groundwater. Melting permafrost, longer growing seasons, increased soil temperatures, and greater precipitation in boreal regions might lead to increases in DOC for such lakes. In lakes with longer residence times, loss processes such as bacterial degradation (Tranvik 1992), photo-oxidation (Lindell et al. 1995; Bertilsson and Tranvik 2000), aggregation, and sedimentation (Von Wachenfeldt et al. 2008) become more important. To the extent that water temperature and the duration of the icefree period affect these loss processes (Sommaruga et al. 1999; Reche et al. 2000), the expected increase in DOC with climate change may be dampened or even reversed in these longer-residence-time lakes. Trends in mineral nutrient levels may also be expected to differ among lakes based on the processes driving them. In stratified lakes, warming is likely to decrease productivity by increasing the intensity, extent, or frequency of stratification and thereby reducing internal loading (O’Reilly et al. 2003; Coats et al. 2006). In other lakes, the effects of climate change may result in the delivery of new nutrients from the catchment or enhanced internal recycling (McKee et al. 2003; Wilhelm and Adrian 2008; Wagner and Adrian 2009b).
Differences among lakes, as outlined in Table 2, provide both complications and opportunities with respect to the use of lakes as sentinels of climate change. At one level, the effective use of indicator variables will depend critically on identifying the lake–variable combinations that are most sensitive and least affected by other confounding processes. This identification depends to some extent on a sound quantitative knowledge of the mechanistic linkages between climate variables and lake response variables and suggests that multiple indicator variables may be necessary (Table 2). Linking changes in climate to changes in variables such as DOC, nutrient concentrations, and algal productivity may be demonstrable, but the relationship is not always well understood for some lakes. In these situations, the existence of variability among lakes represents a significant opportunity to understand the linkages between climate and these indicator variables, as well as the ways in which these linkages are altered by catchment and basin characteristics. Comparisons of a single indicator variable across a spectrum of different lake types affected by a common climate signal can clarify the sensitivity of an indicator variable to climate and highlight contextual variables that influence that sensitivity (Baines et al. 2000; Webster et al. 2000; Pace and Cole 2002).
Attributing changes to climate effects
Lakes are commonly affected by multiple interacting stressors (Christensen et al. 2006; Yan et al. 2008), which could confound the signals from climate change. Even lake surface temperature, which is usually tightly linked to air temperatures, may not always reflect the direct effects of climate warming (Fig. 1). For example, reduced wind speed as a consequence of tree planting combined with an increase in DOC concentration due to deacidification resulted in a decrease in the whole-lake temperature of Clearwater Lake, Ontario, despite a regional increase in air temperatures (Tanentzap et al. 2008). In many relatively small lakes, air temperatures alone often explain only a relatively small portion of the observed variation in lake surface temperatures (Fee et al. 1996; Keller 2007). The problem of confounding factors increases when considering chemical or biological response variables. Lakes in various regions around the globe have been subject to changes in land-use patterns (Pham et al. 2008), nutrient inputs (eutrophication and oligotrophication; Jeppesen et al. 2005; Van Donk et al. 2008), and the deposition of sulfur and nitrogen oxides (acidification and deacidification; Schindler et al. 1996b). These processes may interact in a nonlinear fashion with climate change or may neutralize climate effects (Wagner and Adrian 2009a).
Eutrophication
The effects of eutrophication and oligotrophication on lakes will diminish their utility as sentinels of climate change. Both the direct and indirect effects of warmer water temperatures and increased nutrient availability due to eutrophication may enhance process rates and complicate the use of oxygen concentrations (Matzinger et al. 2007), phytoplankton phenology (Thackeray et al. 2008), phytoplankton community composition (Paerl and Huisman 2008), zooplankton community composition (Straile 2005), and overall community composition as indicators of climate change. Additive effects of eutrophication with climate can be highly complex when food-web interactions are involved (Seebens et al. 2007; Van Donk et al. 2008), and the processes involved in responses to climate change and eutrophication are intertwined. For example, changes in phytoplankton density due to eutrophication and oligotrophication will alter water transparency, thereby modifying the thermal structure of lakes (Jones et al. 2005). Furthermore, the effects of climate change may be partially based on processes similar to those associated with eutrophication because warming might increase phosphorus concentrations through increased internal loading (Jensen and Andersen 1992), especially in concert with extended anoxic conditions (Wilhelm and Adrian 2008).
Acidification
Climate change also interacts with acidification and recovery from acidification (Sommaruga-Wögrath et al. 1997). Acidification reduces DOC concentrations in lakes (Schindler et al. 1996b; Yan et al. 1996), thereby altering transparency of the water column. Hence, acidification (or deacidification) will affect thermal structure, especially in small lakes (Fee et al. 1996), through changes in the depth of the mixed layer (Yan 1983; Mazumder and Taylor 1994) and changes in thermal gradients, which affect the stability of the water column (Gunn et al. 2001; Persson and Jones 2008). In addition, changes in DOC concentration due to changes in acidification status will have an important effect on ultraviolet (UV) penetration (Schindler et al. 1996b; Yan et al. 1996). Hence, acidification and deacidification may interfere with the use of physical, chemical, as well as biological variables as indicators of climatic change. In particular, in regions affected by historically high acid deposition, the recovery of lakes from acidification will need to be considered in assessments of climate-change effects (Keller et al. 2005; Monteith et al. 2007).
Dealing with confounding factors
Confounding factors such as eutrophication, acidification, nitrification, and the equivalent reverse phenomena generally fluctuate less rapidly than climatic variables. This difference in behavior can be utilized to distinguish between climatic forcing and confounding factors by removing trends and low-frequency fluctuations from relevant time series by detrending or high-pass filtering, and analyzing only the remaining, higher-frequency fluctuations that are driven mainly by external physical forcing. A detrending approach has been used successfully in studying the influence of climate on the population dynamics of a calanoid copepod (Seebens et al. 2007) and on the influx of nutrients in lakes of the English Lake District (George et al. 2004). Likewise, the effect of climate on nitrate concentrations in lakes was revealed after accounting for the effects of a long-term decrease of atmospheric nitrate deposition in a multiple regression model (Weyhenmeyer et al. 2007). Jankowski et al. (2005) used high-pass filtering to remove long-term nonlinear anthropogenic effects from nitrate time series in order to focus on the effects of comparatively high-frequency climatic forcing. Detrending is also a useful option when separating the effects of nonclimate stressors from climate effects due to climate oscillations such as El Niño–Southern Oscillation, the North Atlantic Oscillation, or the Pacific Decadal Oscillation, thus allowing these large-scale climatic phenomena to be used as proxies for the effects of climate change (George et al. 2004; Jankowski et al. 2005; Blenckner et al. 2007). Detrending or high-pass filtering will also remove any long-term trend in the data that might result from climate warming, which suggests that in lakes with ongoing changes in other stressors, many chemical and biological state variables will be of only limited use as indicators of climate change. When this is the case, mechanistic simulation models may help to disentangle the effects of warming from other stressors (Elliott and May 2008; Huber et al. 2008). Hydrodynamic models can also be used to distinguish between the effects of changes in air temperature and changes in wind speed and light attenuation on the thermal regimes of lakes (Tanentzap et al. 2008). Simulation models can quantify the effect sizes of confounding variables on response variables. For example, Schalau et al. (2008) showed that a 10-fold increase in algal carrying capacity mimicking eutrophication had only a minor effect on the timing of maximum Daphnia abundances and the clear-water phase as compared to changes in the onset of vernal warming.
Overall, our synthesis indicates that lakes have a strong potential as sentinels of current climate change and, as such, can contribute to our understanding of global climate effects. We identified a suite of response variables that can act as effective indicators of climate change. These indicators have response times that allow them to reflect the rapid rates of current changes in climate and to reflect changes in many different properties of lake ecosystems (Table 1). Not all indicators can be used broadly across all lakes; there are certain indicators that are particularly suitable for different lake types and regions (Table 2). Even so, the global distribution of lakes contributes substantially to their utility as sentinels and allows them to stand out from many other current indicators of climate change that are typically biome-specific, such as the retreat of alpine glaciers, the melting of permafrost, or the reduction in sea ice. As sentinels, lakes provide a way to detect and monitor the effects of climate change at the ecosystem scale in locations that are under-represented in climate studies or are influenced by other environmental changes.
Part of the strength of using lakes as sentinels comes from the fact that lakes are able to provide information about the effects of climate change on the terrestrial landscape as well as the lake itself. By using combinations of indicators and by comparing responses among lakes, it is possible to tease apart the effects of climate on the catchment from those on the lake. The value of lakes for detecting changes within the terrestrial landscape enhances their use as sentinels, particularly for cases when it would otherwise be difficult to determine these changes in the catchment, i.e., weathering rates. By using lakes as sentinels of catchment changes, we may be able to increase our mechanistic understanding of terrestrial–aquatic linkages.
The efficacy of lakes as sentinels of climate change depends upon our understanding of internal lake processes. For the key indicators, listed in Table 1, we have a reasonably solid understanding of the mechanistic link between climate and lake response that allows us to use these indicator variables effectively. The application of these indicators to different lakes depends on our ability to distinguish the signal from noise, which can be affected by interannual lake variability, terrestrial–aquatic linkages, species life histories, and other factors. Therefore, the use of lakes as sentinels not only allows us to detect global effects of climate change, but it can also further our understanding of ecosystem processes in lakes.
Acknowledgments
The manuscript greatly profited from the input provided by the participants of the Chapman Conference on “Lakes and Reservoirs as Sentinels, Integrators, and Regulators of Climate Change” in general, but particularly from the specific contributions provided by the members of the Sentinel group: B. Boehrer, R. N. Coats, D. Coenen, S. L. Cooke, I. Droscher, M. D. Graham, A. W. Groeger, S. E. Hampton, J. E. Hobbie, L. R. Imest’eva, J. Karlsson, Y. A. Kontar, A. Korhola, T. K. Kratz, S. Larsen, J. D. Lenters, M. E. Llames, M. M. Manca, B. Matthews, J. M. Melack, N. Mladenov, M. Moore, D. R. Mueller, P. J. Neale, M. H. Olson, J. Oris, A. M. Paterson, S. Pham, K. C. Rose, C. R. Salm, R. L. Smyth, A. Tucker, P. Verburg, A. M. Verschoor, R. D. Vinebrooke, C. Wagner, R. P. Weidman, as well as the conveners C. E. Williamson and J. Saros. For contributing the data used in Figure 1 and for providing the financial support necessary for their measurement, we thank Wasserversorgung Zürich, Switzerland; the Institute of Lake Research, Langenargen, Germany; the Andrew W. Mellon Foundation; Lake Tahoe Interagency Monitoring Program; Amt für Abfall, Wasser, Energie und Luft, Canton of Zürich, Switzerland; Swedish University of Agricultural Sciences, Uppsala, Sweden; Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, Germany; the Lake Champlain Long-term Water Quality and Biological Monitoring Project (implemented by the Vermont Department of Environmental Conservation and the New York Department of Environmental Conservation, in conjunction with the Lake Champlain Basin Program); the Dorset Environmental Science Centre, Ontario, Canada; and M. Moore for contributing part of data set 2005620028 registered with the government of Russia. We thank the guest editors J. P. Smol and W. F. Vincent for their strong support during the review process and two reviewers for their very helpful advice and recommendations, which substantially improved the manuscript.
References
- ACIA . Impacts of a warming Arctic: Arctic climate impact assessment. Cambridge Univ. Press; 2004. [Google Scholar]
- Adrian R, Walz N, Hintze T, Hoeg S, Rusche R. Effects of ice duration on the plankton succession during spring in a shallow polymictic lake. Freshwater Biol. 1999;41:621–623. [Google Scholar]
- Adrian R, Wilhelm S, Gerten D. Life history traits of lake plankton species may govern their phenological response to climate warming. Glob. Change Biol. 2006;12:652–661. [Google Scholar]
- Argyilan EP, Forman SL. Lake level response to seasonal climatic variability in the Lake Michigan–Huron system from 1920 to 1995. J. Great Lakes Res. 2003;29:488–500. [Google Scholar]
- Arhonditsis GB, Brett MT, DeGasperi CL, Schindler DE. Effects of climatic variability on the thermal properties of Lake Washington. Limnol. Oceanogr. 2004;49:256–270. [Google Scholar]
- Awange JL, Ogalo L, Bae KH, Were P, Omondi P, Omute P, Omullo M. Falling Lake Victoria water levels: Is climate a contributing factor? Clim. Change. 2008;89:281–297. [Google Scholar]
- Baines SB, Webster KE, Kratz TK, Carpenter SR, Magnuson JJ. Synchronous behavior of temperature, calcium and chlorophyll in lakes of northern Wisconsin. Ecology. 2000;81:815–825. [Google Scholar]
- Baulch HM, Schindler DW, Turner MA, Findlay DL, Paterson MJ, Vinebrooke RD. Effects of warming on benthic communities in a boreal lake: Implications of climate change. Limnol. Oceanogr. 2005;50:1377–1392. [Google Scholar]
- Benoy G, Cash K, McCauley E, Wrona F. Carbon dynamics in lakes of the boreal forest under a changing climate. Environ. Rev. 2007;15:175–189. [Google Scholar]
- Bergström A-K, Jansson M. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob. Change Biol. 2006;12:1–9. [Google Scholar]
- Bertilsson S, Tranvik LJ. Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 2000;45:753–762. [Google Scholar]
- Blenckner T, others Large-scale climatic signatures in lakes across Europe: A meta-analysis. Glob. Change Biol. 2007;13:1314–1326. [Google Scholar]
- Boehrer B, Schultze M. Stratification of lakes. Rev. Geophys. 2008;46:RG2005. doi:10.1029/2006RG000210. [Google Scholar]
- Boisvenue C, Running SW. Impacts of climate change on natural forest productivity—evidence since the middle of the 20th century. Glob. Change Biol. 2006;12:862–882. [Google Scholar]
- Carpenter SR, others Understanding regional change: A comparison of two lake districts. Bioscience. 2007;57:323–335. [Google Scholar]
- Christensen MR, Graham MD, Vinebrooke RD, Findlay DL, Paterson MJ, Turner MA. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Change Biol. 2006;12:2316–2322. [Google Scholar]
- Coats R, Perez-Losada J, Schladow G, Richards R, Goldman C. The warming of Lake Tahoe. Clim. Change. 2006;76:121–148. [Google Scholar]
- Croley TE, Lewis CFM. Warmer and drier climates that make Lake Huron into a terminal lake. Aquat. Ecosyst. Health. 2008;11:153–160. [Google Scholar]
- De Stasio BT, Hill DK, Kleinhans JM, Nibbelink NP, Magnuson JJ. Potential effects of global climate change on small north-temperate lakes: Physics, fish, and plankton. Limnol. Oceanogr. 1996;41:1136–1149. [Google Scholar]
- Elliott JA, May L. The sensitivity of phytoplankton in Loch Leven (UK) to changes in nutrient load and water temperature. Freshwater Biol. 2008;53:32–41. [Google Scholar]
- Evans CD, Chapman PJ, Clark JM, Monteith DT, Cresser MS. Alternative explanations for rising dissolved organic carbon export from organic soils. Glob. Change Biol. 2006;12:2044–2053. [Google Scholar]
- Fee EJ, Hecky RE, Kasian SEM, Cruikshank DR. Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol. Oceanogr. 1996;41:912–920. [Google Scholar]
- Freeman C, others Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature. 2004;430:195–198. doi: 10.1038/nature02707. [DOI] [PubMed] [Google Scholar]
- Frey KE, Smith LC. Amplified carbon release from vast West Siberian peatlands by 2100. Geophys. Res. Lett. 2005;32:L09401. doi:10.1029/2004GL022025. [Google Scholar]
- George DG, Maberly SC, Hewitt DP. The influence of the North Atlantic Oscillation on the physical, chemical and biological characteristics of four lakes in the English Lake District. Freshwater Biol. 2004;49:760–774. [Google Scholar]
- Gerten D, Adrian R. Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation. Limnol. Oceanogr. 2000;45:1058–1066. [Google Scholar]
- Gerten D, Adrian R. Differences in the persistency of the North Atlantic Oscillation signal among lakes. Limnol. Oceanogr. 2001;46:448–455. [Google Scholar]
- Ghanbari RN, Bravo HR. Coherence between atmospheric teleconnections, Great Lakes water levels, and regional climate. Adv. Water Res. 2008;31:1284–1298. [Google Scholar]
- Gu R, Stefan HG. Year-round temperature simulation of cold climate lakes. Cold Reg. Sci. Tech. 1990;18:147–160. [Google Scholar]
- Gunn JM. Impact of the 1998 El Niño event on a lake charr, Salvelinus namaycush population recovering from acidification. Environ. Biol. Fishes. 2002;64:343–351. [Google Scholar]
- Gunn JM, Snucins E, Yan ND, Arts MT. Use of water clarity to monitor the effects of climate change and other stressors on oligotrophic lakes. Environ. Monit. Assess. 2001;67:69–88. doi: 10.1023/a:1006435721636. [DOI] [PubMed] [Google Scholar]
- Hampton SE, Izmest’eva LR, Moore MV, Katz SL, Dennis B, Silow EA. Sixty years of environmental change in the world’s largest freshwater lake—Lake Baikal. Siberia. Glob. Change Biol. 2008;14:1947–1958. [Google Scholar]
- Hanson PC, Carpenter SR, Armstrong DE, Stanley EH, Kratz TK. Lake dissolved inorganic carbon and dissolved oxygen: Changing drivers from days to decades. Ecol. Monogr. 2006;76:343–363. [Google Scholar]
- Hessen DO, Andersen T, Larsen S, Skjelkvale BL, de Wit HA. Nitrogen deposition, catchment productivity and climate as determinants of lake stoichiometry. Limnol. Oceanogr. 2009;54:2520–2528. [Google Scholar]
- Hobbie JE, others Impact of global change on the biogeochemistry and ecology of an Arctic freshwater system. Polar Res. 1999;18:207–214. [Google Scholar]
- Holzapfel AM, Vinebrooke RD. Environmental warming increases invasion potential of alpine lake communities by imported species. Glob. Change Biol. 2005;11:2009–2015. [Google Scholar]
- Huber V, Adrian R, Gerten D. Phytoplankton response to climate warming modified by trophic state. Limnol. Oceanogr. 2008;53:1–13. [Google Scholar]
- Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH, Sommeijer B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology. 2004;85:2960–2970. [Google Scholar]
- Jankowski T, Livingstone DM, Forster R, Bührer H. Long-term nitrate concentrations in five perialpine lakes: Regional coherence. Verh. Internat. Verein. Limnol. 2005;29:927–931. [Google Scholar]
- Jankowski T, Livingstone DM, Forster R, Bührer H, Niederhauser P. Consequences of the 2003 European heat wave for lakes: Implications for a warmer world. Limnol. Oceanogr. 2006;51:815–819. [Google Scholar]
- Jansen W, Hesslein RH. Potential effects of climate warming on fish habitats in temperate zone lakes with special reference to Lake 239 of the experimental lakes area (ELA), north-western Ontario. Environ. Biol. Fish. 2004;70:1–22. [Google Scholar]
- Jensen HS, Andersen FO. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of 4 shallow, eutrophic lakes. Limnol. Oceanogr. 1992;37:577–589. [Google Scholar]
- Jeppesen E, others Lake responses to reduced nutrient loading—an analysis of contemporary long-term data from 35 case studies. Freshwater Biol. 2005;50:1747–1771. [Google Scholar]
- Jöhnk K, Huisman J, Sharples J, Sommeijer B, Visser PM, Stroom JM. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Change Biol. 2008;14:495–512. [Google Scholar]
- Jöhnk K, Straile D, Ostendorp W. Water level variability and trends in Lake Constance in the light of the 1999 centennial flood. Limnologica. 2004;34:15–21. [Google Scholar]
- Jones I, George G, Reynolds C. Quantifying effects of phytoplankton on the heat budgets of two large limnetic enclosures. Freshwater Biol. 2005;50:1239–1247. [Google Scholar]
- Keller W. Implications of climate warming for Boreal Shield lakes: A review and synthesis. Environ. Reviews. 2007;15:99–112. [Google Scholar]
- Keller W, Heneberry J, Leduc J. Linkages between weather, dissolved organic carbon, and cold-water habitat in a Boreal Shield lake recovering from acidification. Can. J. Fish. Aquat. Sci. 2005;62:341–347. [Google Scholar]
- Keller W, Paterson AM, Somers KM, Dillon PJ, Heneberry J, Ford A. Relationships between dissolved organic carbon concentrations, weather, and acidification in small Boreal Shield lakes. Can. J. Fish. Aquat. Sci. 2008;65:786–795. [Google Scholar]
- Kelly EN, Schindler DW, St. Louis VL, Donald DB, Vlaclicka KE. Forest fire increases mercury accumulation by fishes via food web restructuring and increased mercury inputs. Proc. Nat. Acad. Sci. USA. 2006;103:19380–19385. doi: 10.1073/pnas.0609798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifovic R, Pouliot D. Analysis of climate change impacts on lake ice phenology in Canada using the historical satellite data record. Rem. Sens. Environ. 2007;106:492–507. [Google Scholar]
- Leavitt PR, Cummings BF, Smol JP, Reasoner M, Pienitz R, Hodgson DA. Climate control of ultraviolet radiation effects on lakes. Limnol. Oceanogr. 2003;48:2062–2069. [Google Scholar]
- Leavitt PR, others Paleolimnological evidence of the effects on lakes of energy and mass transfer from climate and humans. Limnol. Oceanogr. 2009;54:2330–2348. [Google Scholar]
- Lenters JD, Kratz TK, Bowser CJ. Effects of climate variability on lake evaporation: Results from a long-term energy budget study of Sparkling Lake, northern Wisconsin (USA) J. Hydrology. 2005;308:168–195. [Google Scholar]
- Lindell MJ, Graneli W, Tranvik LJ. Enhanced bacterial-growth in response to photochemical transformation of dissolved organic-matter. Limnol. Oceanogr. 1995;40:195–199. [Google Scholar]
- Livingstone DM. Impact of secular climate change on the thermal structure of a large temperate central European lake. Clim. Change. 2003;57:205–225. [Google Scholar]
- Livingstone DM. A change of climate provokes a change of paradigm: Taking leave of two tacit assumptions about physical lake forcing. Internat. Rev. Hydrobiol. 2008;93:404–414. [Google Scholar]
- Livingstone DM, Adrian R. Modeling the duration of intermittent ice cover on a lake for climate-change studies. Limnol. Oceanogr. 2009;54:1709–1722. doi: 10.4319/lo.2009.54.6_part_2.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone DM, Dokulil M. Eighty years of spatially coherent Austrian lake surface temperatures and their relationship to regional air temperature and the North Atlantic Oscillation. Limnol. Oceanogr. 2001;46:1220–1227. [Google Scholar]
- Livingstone DM, Lotter AF. The relationship between air and water temperatures in lakes of the Swiss Plateau: A case study with palæolimnological implications. J. Paleolimnol. 1998;19:181–198. [Google Scholar]
- Magnuson JJ, others Historical trends in lake and river ice cover in the Northern Hemisphere. Science. 2000;289:1743–1746. doi: 10.1126/science.289.5485.1743. and Errata 2001, Science 291: 254. [DOI] [PubMed] [Google Scholar]
- Marinone MC, others UVR radiation as a potential driving force for zooplankton community structure in Patagonian lakes. Photochem. Photobiol. 2006;82:962–971. doi: 10.1562/2005-09-09-RA-680. [DOI] [PubMed] [Google Scholar]
- Marsh P, Russell M, Pohl S, Haywood H, Onclin C. Changes in thaw lake drainage in the Western Canadian Arctic from 1950 to 2000. Hydrol. Proc. 2009;23:145–158. [Google Scholar]
- Matzinger A, others Eutrophication of ancient Lake Ohrid: Global warming amplifies detrimental effects of increased nutrient inputs. Limnol. Oceanogr. 2007;52:338–353. [Google Scholar]
- Mazumder A, Taylor WD. Thermal structure of lakes varying in size and water clarify. Limnol. Oceanogr. 1994;39:968–976. [Google Scholar]
- McCormick MJ, Fahnenstiel GL. Recent climatic trends in nearshore water temperatures in the St. Lawrence Great Lakes. Limnol. Oceanogr. 1999;44:530–540. [Google Scholar]
- McKee D, others Response of freshwater microcosm communities to nutrients, fish, and elevated temperature during winter and summer. Limnol. Oceanogr. 2003;48:707–722. [Google Scholar]
- Michelutti N, Wolfe AP, Vinebrooke RD, Rivard B, Briner JP. Recent primary production increases in arctic lakes. Geophys. Res. Lett. 2005;32:L19715. doi:10.1029/2005GL023693. [Google Scholar]
- Mladenov N, Pulido-Villena E, Morales-Baquero R, Ortega-Retuerta E, Sommaruga R, Reche I. Spatiotemporal drivers of dissolved organic matter in high alpine lakes: The role of Saharan dust inputs and bacterial activity. J. Geophys. Res. 2008;113:G00D01. doi: 10.1029/2008JG000699. doi:10.1029/2008JG000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith DT, others Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature. 2007;450:537–541. doi: 10.1038/nature06316. [DOI] [PubMed] [Google Scholar]
- Moore MV, Folt CL, Stemberger RS. Consequences of elevated temperatures for zooplankton assemblages in temperate lakes. Arch. Hydrobiol. 1996;135:289–319. [Google Scholar]
- Nürnberg GK, Shaw M. Productivity of clear and humic lakes: Nutrients, phytoplankton, bacteria. Hydrobiologia. 1998;382:97–112. [Google Scholar]
- O’Reilly CM, Alin SR, Plisnier P-D, Cohen AS, McKee BA. Climate change decreases aquatic ecosystem productivity in Lake Tanganyika, Africa. Nature. 2003;424:766–768. doi: 10.1038/nature01833. [DOI] [PubMed] [Google Scholar]
- Pace ML, Cole JJ. Synchronous variation of dissolved organic carbon and color in lakes. Limnol. Oceanogr. 2002;47:333–342. [Google Scholar]
- Paerl HW, Huisman J. Climate—blooms like it hot. Science. 2008;320:57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- Parker BR, Vinebrooke RD, Schindler DW. Recent climate extremes alter alpine lake ecosystems. Proc. Nat. Acad. Sci. USA. 2008;105:12927–12931. doi: 10.1073/pnas.0806481105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson I, Jones ID. The effect of water colour on lake hydrodynamics: A modelling study. Freshwater Biol. 2008;53:2345–2355. [Google Scholar]
- Pettersson K, Grust K, Weyhenmeyer G, Blenckner T. Seasonality of chlorophyll and nutrients in Lake Erken—effects of weather conditions. Hydrobiologia. 2003;506:75–81. [Google Scholar]
- Pham SV, Leavitt PR, McGowan S, Peres-Nato P. Spatial variability of climate and land-use effects on lakes of the northern Great Plains. Limnol. Oceanogr. 2008;53:728–742. [Google Scholar]
- Pienitz R, Vincent WF. Effect of climate change relative to ozone depletion on UV exposure in subarctic lakes. Nature. 2000;404:484–487. doi: 10.1038/35006616. [DOI] [PubMed] [Google Scholar]
- Prowse TD, Wrona FJ, Reist JD, Hobbie JE, Levesque LMJ, Vincent WF. General features of the Arctic relevant to climate change in freshwater ecosystems. Ambio. 2006;35:330–338. doi: 10.1579/0044-7447(2006)35[330:gfotar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Adams DJ. Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology. 1996;77:1151–1162. [Google Scholar]
- Rae R, Vincent WF. Effects of temperature and ultraviolet radiation on microbial foodweb structure: Potential responses to global change. Freshwater Biol. 1998;40:747–758. [Google Scholar]
- Rahel FJ, Olden JD. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 2008;22:521–533. doi: 10.1111/j.1523-1739.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- Reche I, Pace ML, Cole JJ. Modeled effects of dissolved organic carbon and solar spectra on photobleaching in lake ecosystems. Ecosystems. 2000;3:419–432. [Google Scholar]
- Reist JD, others General effects of climate change on Arctic fishes and fish populations. Ambio. 2006;35:370–380. doi: 10.1579/0044-7447(2006)35[370:geocco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Ragotzkie RA. Changes in the thermal structure of moderate to large sized lakes in response to changes in air temperature. Aquat. Sci. 1990;52:360–380. [Google Scholar]
- Rodionov SN. Global and regional climate interaction: The Caspian Sea experience. Springer; 1994. [Google Scholar]
- Rogora M, Mosello R, Arisci S. The effect of climate warming on the hydrochemistry of alpine lakes. Water, Air, Soil Poll. 2003;148:347–361. [Google Scholar]
- Rosenzweig C. Assessment of observed changes and responses in natural and managed systems. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, others, editors. Climate change 2007—impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ. Press; 2007. pp. 79–131. [Google Scholar]
- Rühland K, Paterson AM, Smol JP. Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Glob. Change Biol. 2008;14:2740–2754. [Google Scholar]
- Rusak JA, Yan ND, Somers KM. Regional climatic drivers of synchronous zooplankton dynamics in north-temperate lakes. Can. J. Fish. Aquat. Sci. 2008;65:878–889. [Google Scholar]
- Schalau K, Rinke K, Straile D, Peeters F. Temperature is the key factor explaining interannual variability of Daphnia development in spring: A modelling study. Oecologia. 2008;157:531–543. doi: 10.1007/s00442-008-1081-3. [DOI] [PubMed] [Google Scholar]
- Schindler DW, Bayley SE, Parker BR. The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnol. Oceanogr. 1996a;41:1004–1017. [Google Scholar]
- Schindler DW, Curtis PJ, Parker BR, Stainton MP. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature. 1996b;379:705–708. [Google Scholar]
- Schindler DW, Rogers DE, Scheuerell MD, Abrey CA. Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology. 2005;86:198–209. [Google Scholar]
- Seebens H, Straile D, Hoegg R, Stich HB, Einsle U. Population dynamics of a freshwater calanoid copepod: Complex responses to changes in trophic status and climate variability. Limnol. Oceanogr. 2007;52:2364–2372. [Google Scholar]
- Smith LC, Sheng Y, MacDonald GM, Hinzman LD. Disappearing arctic lakes. Science. 2005;308:1429. doi: 10.1126/science.1108142. doi:10.1126/science.1108142. [DOI] [PubMed] [Google Scholar]
- Smith ND. Sedimentation processes and patterns in a glacier-fed lake with low sediment input. Can. J. Earth Sci. 1978;15:741–756. [Google Scholar]
- Smol JP. Pollution of lakes and rivers: A paleoenvironmental perspective. 2nd ed. Blackwell; 2008. [Google Scholar]
- Sommaruga R, Psenner R, Schaferer E, Koinig K, Sommaruga-Wögrath S. Dissolved organic carbon concentration and phytoplankton biomass in high-mountain lakes of the Austrian Alps: Potential effect of climatic warming on UV underwater attenuation. Arct. Antarct. Alp. Res. 1999;31:247–254. [Google Scholar]
- Sommaruga-Wögrath S, Koinig K, Schmidt R, Tessadri R, Sommaruga R, Psenner R. Temperature effects on the acidity of remote alpine lakes. Nature. 1997;387:64–67. [Google Scholar]
- Šporka F, Livingstone DM, Stuchlík E, Turek J, Galas J. Water temperatures and ice cover in the lakes of the Tatra Mountains. Biologia. 2006;61:77–90. [Google Scholar]
- Straile D. The North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc. Royal Soc. B Bio. 2002;269:391–395. doi: 10.1098/rspb.2001.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straile D. Food webs in lakes—seasonal dynamics and the impact of climate variability. In: Belgrano A, Scharler U, Dunne J, Ulanowicz RE, editors. Aquatic food webs: An ecosystem approach. Oxford Univ. Press; 2005. pp. 41–50. [Google Scholar]
- Straile D, Jöhnk K, Rossknecht H. Complex effects of winter warming on the physiochemical characteristics of a deep lake. Limnol. Oceanogr. 2003;48:1432–1438. [Google Scholar]
- Strecker AL, Cobb TP, Vinebrooke RD. Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnol. Oceanogr. 2004;49:1182–1190. [Google Scholar]
- Tanentzap AJ, others Cooling lakes while the world warms: Effects of forest re-growth and increased dissolved organic matter on the thermal regime of a temperate urban lake. Limnol. Oceanogr. 2008;53:404–410. [Google Scholar]
- Tartarotti B, Laurion I, Sommaruga R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnol. Oceanogr. 2001;46:1546–1552. [Google Scholar]
- Thackeray SJ, Jones ID, Maberly SC. Long-term change in the phenology of spring phytoplankton: Species-specific responses to nutrient enrichment and climatic change. J. Ecol. 2008;96:523–535. [Google Scholar]
- Tranvik LJ. Allochthonous dissolved organic-matter as an energy-source for pelagic bacteria and the concept of the microbial loop. Hydrobiologia. 1992;229:107–114. [Google Scholar]
- Tranvik LJ, others Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009;54:2283–2297. doi: 10.4319/lo.2009.54.6_part_2.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kamp G, Keir D, Evans MS. Long-term water level changes in closed-basin lakes of the Canadian prairies. Can. Water Res. J. 2008;33:23–38. [Google Scholar]
- Van Donk E, Hessen DO, Verschoor AM, Gulati RD. Re-oligotrophication by phosphorus reduction and effects on seston quality in lakes. Limnologica. 2008;38:189–202. [Google Scholar]
- Verburg P, Hecky RE, Kling H. Ecological consequences of a century of warming in Lake Tanganyika. Science. 2003;301:505–507. doi: 10.1126/science.1084846. [DOI] [PubMed] [Google Scholar]
- Von Wachenfeldt E, Sobek S, Bastviken D, Tranvik LJ. Linking allochthonous dissolved organic matter and boreal lake sediment carbon sequestration: The role of light-mediated flocculation. Limnol. Oceanogr. 2008;53:2416–2426. [Google Scholar]
- Wagner C, Adrian R. Exploring lake ecosystems: Hierarchy responses to long-term change. Glob. Change Biol. 2009a;15:1104–1115. [Google Scholar]
- Wagner C, Adrian R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009b;54:2460–2468. [Google Scholar]
- Webster KE, Kratz TK, Bowser CJ, Magnuson JJ, Rose WJ. The influence of landscape position on lake chemical responses to drought in northern Wisconsin. Limnol. Oceanogr. 1996;41:977–984. [Google Scholar]
- Webster KE, others Structuring features of lake districts: Landscape controls on lake chemical responses to drought. Freshwater Biol. 2000;43:499–515. [Google Scholar]
- Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Warming and earlier spring increases western US forest wildfire activity. Science. 2006;313:940–943. doi: 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- Weyhenmeyer GA. Water chemical changes along a latitudinal gradient in relation to climate and atmospheric deposition. Climatic Change. 2008a;88:199–208. [Google Scholar]
- Weyhenmeyer GA. Rates of change in physical and chemical lake variables—are they comparable between large and small lakes. Hydrobiologia. 2008b;599:105–110. [Google Scholar]
- Weyhenmeyer GA, Blenckner T, Pettersson K. Changes of the plankton spring outburst related to the North Atlantic Oscillation. Limnol. Oceanogr. 1999;44:1788–1792. [Google Scholar]
- Weyhenmeyer GA, Meili M, Livingstone DM. Nonlinear temperature response of lake ice breakup. Geophys. Res. Lett. 2004;31:L07203. doi:10.1029/2004GL019530. [Google Scholar]
- Weyhenmeyer GA, others Nitrate-depleted conditions on the increase in shallow northern European lakes. Limnol. Oceanogr. 2007;52:1346–1353. [Google Scholar]
- Wilhelm S, Adrian R. Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshwater Biol. 2008;53:226–237. [Google Scholar]
- Williams SG, Stefan HG. Modeling of lake ice characteristics in North America using climate, geography, and lake bathymetry. J. Cold Reg. Eng. 2006;20:140–167. [Google Scholar]
- Williamson CE, Dodds W, Kratz TK, Palmer M. Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front. Ecol. Environ. 2008;6:247–254. [Google Scholar]
- Williamson CE, Morris DP, Pace ML, Olson OG. Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol. Oceanogr. 1999;44:795–803. [Google Scholar]
- Williamson CE, Saros JE, Schindler DW. Sentinels of change. Science. 2009;323:887–889. doi: 10.1126/science.1169443. [DOI] [PubMed] [Google Scholar]
- Winder M, Hunter DA. Temporal organization of phytoplankton communities linked to physical forcing. Oecologia. 2008;156:179–192. doi: 10.1007/s00442-008-0964-7. [DOI] [PubMed] [Google Scholar]
- Winder M, Schindler DE. Climate change uncouples trophic interactions in a lake ecosystem. Ecology. 2004a;85:2100–2106. [Google Scholar]
- Winder M, Schindler DE. Climatic effects on the phenology of lake processes. Glob. Change Biol. 2004b;10:1844–1856. [Google Scholar]
- Winder M, Schindler DE, Essington TE, Litt AH. Disrupted seasonal clockwork in the population dynamics of a freshwater copepod by climate warming. Limnol. Oceanogr. 2009;54:2493–2505. [Google Scholar]
- Wynne RH, Lillesand TM. Satellite observations of lake ice as a climate indicator—initial results from statewide monitoring in Wisconsin. Photogramm. Eng. Rem. S. 1993;59:1023–1031. [Google Scholar]
- Yan ND. Effects of changes in pH on transparency and thermal regimes of Lohi Lake, near Sudbury, Ontario. Can. J. Fish. Aquat. Sci. 1983;40:621–626. [Google Scholar]
- Yan ND, Keller W, Scully NM, Lean DRS, Dillon PJ. Increased UV-B penetration in a lake owing to drought-induced acidification. Nature. 1996;381:141–143. [Google Scholar]
- Yan ND, others Long-term trends in zooplankton of Dorset, Ontario, lakes: The probable interactive effects of changes in pH, TP, DOC and predators. Can. J. Fish. Aquat. Sci. 2008;65:862–877. [Google Scholar]